Abstract
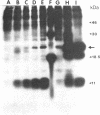
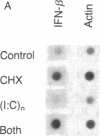
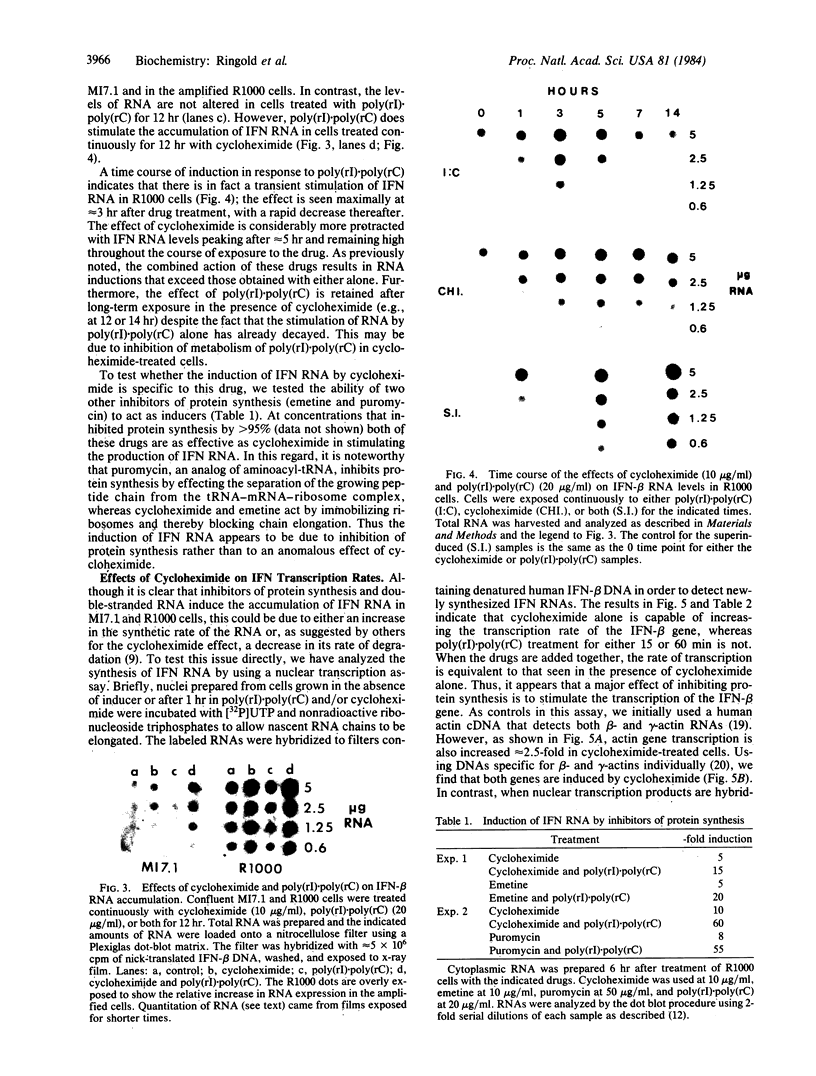
Using Chinese hamster ovary (CHO) cells transfected with a plasmid carrying the human beta-interferon gene, we find that inhibitors of protein synthesis, in the absence of any other inducer, stimulate the production of interferon RNA; this effect is maintained in cells in which the plasmid sequences have been amplified 25- to 50-fold. Nuclear transcription assays show that a major effect of cycloheximide is to increase the rate of transcription of the interferon gene. This contradicts the generally accepted explanation that inhibitors of protein synthesis augment interferon production by stabilizing interferon mRNA. In addition, we have studied the effects of double stranded RNA [poly(rI) X poly(rC)] on the induction of interferon RNA in the presence and absence of cycloheximide. Our results indicate that poly(rI) X poly(rC) by itself causes a transient increase in interferon RNA; however, in the presence of cycloheximide this effect is prolonged. We do not, however, find an increase in transcription of the interferon gene(s) as an early response to poly(rI) X poly(rC). Finally, we have found that cells treated with cycloheximide or infected with Newcastle disease virus induce large amounts of a secreted 11-kDa protein. This cellular protein is not inducible by poly(rI) X poly(rC). We propose that both interferon and this 11-kDa protein belong to a family of proteins in which production is regulated in a coordinate fashion during viral inhibition of cellular protein synthesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Darnell J. E., Jr Cycloheximide stimulates early adenovirus transcription if early gene expression is allowed before treatment. J Virol. 1983 Feb;45(2):683–692. doi: 10.1128/jvi.45.2.683-692.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr Variety in the level of gene control in eukaryotic cells. Nature. 1982 Jun 3;297(5865):365–371. doi: 10.1038/297365a0. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Derynck R., Content J., DeClercq E., Volckaert G., Tavernier J., Devos R., Fiers W. Isolation and structure of a human fibroblast interferon gene. Nature. 1980 Jun 19;285(5766):542–547. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- Goeddel D. V., Yelverton E., Ullrich A., Heyneker H. L., Miozzari G., Holmes W., Seeburg P. H., Dull T., May L., Stebbing N. Human leukocyte interferon produced by E. coli is biologically active. Nature. 1980 Oct 2;287(5781):411–416. doi: 10.1038/287411a0. [DOI] [PubMed] [Google Scholar]
- Gunning P., Ponte P., Okayama H., Engel J., Blau H., Kedes L. Isolation and characterization of full-length cDNA clones for human alpha-, beta-, and gamma-actin mRNAs: skeletal but not cytoplasmic actins have an amino-terminal cysteine that is subsequently removed. Mol Cell Biol. 1983 May;3(5):787–795. doi: 10.1128/mcb.3.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton M., Stewart A. G., Doel S. M., Emtage J. S., Eaton M. A., Smith J. C., Patel T. P., Lewis H. M., Porter A. G., Birch J. R. The amino-terminal sequence of human fibroblast interferon as deduced from reverse transcripts obtained using synthetic oligonucleotide primers. Nucleic Acids Res. 1980 May 10;8(9):1913–1931. doi: 10.1093/nar/8.9.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maeda S., McCandliss R., Gross M., Sloma A., Familletti P. C., Tabor J. M., Evinger M., Levy W. P., Pestka S. Construction and identification of bacterial plasmids containing nucleotide sequence for human leukocyte interferon. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7010–7013. doi: 10.1073/pnas.77.12.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroteaux L., Chen L., Mitrani-Rosenbaum S., Howley P. M., Revel M. Cycloheximide induces expression of the human interferon beta 1 gene in mouse cells transformed by bovine papillomavirus-interferon beta 1 recombinants. J Virol. 1983 Jul;47(1):89–95. doi: 10.1128/jvi.47.1.89-95.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick F., Trahey M., Innis M., Dieckmann B., Ringold G. Inducible expression of amplified human beta interferon genes in CHO cells. Mol Cell Biol. 1984 Jan;4(1):166–172. doi: 10.1128/mcb.4.1.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata S., Taira H., Hall A., Johnsrud L., Streuli M., Ecsödi J., Boll W., Cantell K., Weissmann C. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980 Mar 27;284(5754):316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Pestka S., Baron S. Definition and classification of the interferons. Methods Enzymol. 1981;78(Pt A):3–14. doi: 10.1016/0076-6879(81)78091-1. [DOI] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Analysis of interferon mRNA in human fibroblast cells induced to produce interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7426–7430. doi: 10.1073/pnas.78.12.7426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj N. B., Pitha P. M. Two levels of regulation of beta-interferon gene expression in human cells. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3923–3927. doi: 10.1073/pnas.80.13.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Regulation of mouse mammary tumor virus gene expression by glucocorticoid hormones. Curr Top Microbiol Immunol. 1983;106:79–103. doi: 10.1007/978-3-642-69357-1_4. [DOI] [PubMed] [Google Scholar]
- Sehgal P. B., Lyles D. S., Tamm I. Superinduction of human fibroblast interferon production: further evidence for increased stability of interferon mRNA. Virology. 1978 Aug;89(1):186–198. doi: 10.1016/0042-6822(78)90051-x. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Reeve A. E., Huang R. C. Analysis of RNA initiated in isolated mouse myeloma nuclei using purine nucleoside 5'[gamma-S]triphosphates as affinity probes. Cell. 1978 Oct;15(2):615–626. doi: 10.1016/0092-8674(78)90030-2. [DOI] [PubMed] [Google Scholar]
- Stallcup M. R., Washington L. D. Region-specific initiation of mouse mammary tumor virus RNA synthesis by endogenous RNA polymerase II in preparations of cell nuclei. J Biol Chem. 1983 Mar 10;258(5):2802–2807. [PubMed] [Google Scholar]
- Tan Y. H., Armstrong J. A., Ke Y. H., Ho M. Regulation of cellular interferon production: enhancement by antimetabolites. Proc Natl Acad Sci U S A. 1970 Sep;67(1):464–471. doi: 10.1073/pnas.67.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavernier J., Gheysen D., Duerinck F., Van der Heyden J., Fiers W. Deletion mapping of the inducible promoter of human IFN-beta gene. Nature. 1983 Feb 17;301(5901):634–636. doi: 10.1038/301634a0. [DOI] [PubMed] [Google Scholar]
- Vilcek J., Ng M. H. Post-transcriptional control of interferon synthesis. J Virol. 1971 May;7(5):588–594. doi: 10.1128/jvi.7.5.588-594.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinn K., DiMaio D., Maniatis T. Identification of two distinct regulatory regions adjacent to the human beta-interferon gene. Cell. 1983 Oct;34(3):865–879. doi: 10.1016/0092-8674(83)90544-5. [DOI] [PubMed] [Google Scholar]