Abstract
Background
Although a number of medicines are available for the management of hypertension, the organ damage induced by hypertension is not resolved. The aim of this study was to investigate the protection of ginsenoside Rg1 (Rg1) against vascular remodeling and organ damage in spontaneously hypertensive rats (SHR).
Methods
Male SHR were treated with 5, 10 or 20 mg/kg Rg1 through intraperitoneal injection per day for 1 month. SHR or Wistar-Kyoto rats (WKY) receiving vehicle (saline) was used as control. Blood pressure detection and pathological stain, transmission electron microscope, immunohistochemical assay were used to elucidate the protection of Rg1.
Results
Blood pressures were not different between control SHR rats and Rg1 treated SHR rats, but Rg1 improved the aortic outward remodeling by lowering the lumen diameter and reducing the media thickness according the histopathological and ultrastructural detections. Rg1 also protected the retinal vessels against inward remodeling detected by immunohistochemical assay. Furthermore, Rg1 attenuated the target heart and kidney damage with improvement on cardiac and glomerular structure.
Conclusions
These results suggested that Rg1 held beneficial effects on vascular structure and further protected against the organ-damage induced by hypertension. These findings also paved a novel and promising approach to the treatment of hypertensive complications.
Keywords: Hypertension, Hypertensive complication, Ginsenoside Rg1, Vascular remodeling
Background
Recent evidence suggests that the average blood pressure (BP) in children and adults is rising in the last two decades [1]. Hypertension is becoming one of the main risk factor for cardiovascular and renal vascular disease, and also for mortality around the world [2]. Although the blockers of calcium channel and inhibitors of rennin-angiotensin system are widely applied for clinical therapy, the target-organ damage accompanied by hypertension is still not solved. Therefore, new therapeutic strategies and new medicines to attenuate hypertension-complications are urgently needed [3].
Panax notoginseng, one of the most frequently used traditional Chinese medicine, is well known for its efficacy in promoting blood circulation, ameliorating pathological hemostasis, alleviating pain [4-7]. The main active components of Panax notoginseng include more than 30 different types of saponins, among which Rg1and Rb1 are found in the highest content. A number of clinical and physiological effects of Rg1 have been described recently, such as inhibition of tubular epithelial to myofibroblast transition [8], improvement of myocardial dysfunction in rats with burn injuries [9], amelioration of hepatic microcirculatory disturbances [10], anti-hyperglycemic activity [11], and improvement of endothelial cell function [12]. Recently, Rg1 has been identified to be an angiogenic factor, which can induce neovascularizaton in vivo and promote proliferation and tubulogenesis of endothelial cells in vitro [13-15]. Further mechanism research revealed that Rg1 could activate phosphatidylinositol-3 kinase Akt pathway, inhibit P38 MARK pathway [16]. Recent studies have demonstrated the beneficial effects of Rg1 on improvement of cardial and renal function [17].
Thus, we carried out the present study in SHR rats to test our hypothesis that Rg1 may inhibit the vascular remodeling and targeted-organ damage induced by hypertension.
Methods
Animals and Rg1 treatment
2 month old male Wistar-Kyoto rats (WKY, 280–300 g) and spontaneous hypertension rats (SHR, 280–300 g) were purchased from Shanghai Center of Experimental Animals, Chinese Academy of Sciences. Rats were acclimatized in temperature and humidity-controlled rooms with a 12-h dark/light cycle throughout the study. After 8 week high salt diet, WKY rats that treated by saline were used as normal control (WKY, n = 10). SHR rats were randomly divided into four groups (10 per group): rats that treated by saline were used as hypertension model (SHR); rats in the other three groups were treated by 5 mg/kg Rg1 (SHR-Rg1(5)), 10 mg/kg Rg1 (SHR-Rg1(10)) or 20 mg/kg Rg1 (SHR-Rg1(20)). Saline or Rg 1 (dissolved in saline) were given once a day intraperitoneally. The purity of Rg1 that purchased from Shanghai Yousi Bio-Tech Co., Ltd. was more than 99% evaluated by high-performance liquid chromatography ( Additional file 1: Figure S1) and the chemical structure of Rg1 was elucidated by 13 C NMR ( Additional file 2: Figure S2Additional file 3: Figure S3), which is in agreement with those previous report [18]. 8.0% high salt diet was fed during the whole research, and saline or Rg1 was given from the ninth week of experiment for 4 weeks. The whole experiment protocol was shown in Additional file 4 Figure S4. Experimental procedures were approved by the institute animal ethics committee (SIMM-AE-GDA-2010-05) and were in accordance with the National Institute of Health guidelines.
Measurement of blood pressure in conscious rats
Systolic blood pressure (SBP) and diastolic pressure (DBP) were measured 0.5 h after the administration of Rg1 at the indicated time ( Additional file 4: Figure S4) using the tail-cuff method. Briefly, the rats were placed in a restrainer with heating pad. The blood pressure was continuously recorded by a tail-cuff apparatus (ALC-NIBP, Shanghai Alcott Biotech Co., China) that was controlled with a computer after stabilizing at 37°C for at least 10 min.
Measurements of hemodynamic parameters
The rats were anesthetized, and a Mikro-tipped SPR-320 catheter (Millar Instruments Inc) was inserted through the right carotid artery into left ventricle. Heart rate, mean arterial pressure (MAP), left ventricular systolic pressure (LVSP), end-diastolic pressure (EDP) of rats were recorded by PowerLab 8/30 instrument (ADInstruments, Australia). Maximal rate of pressure development for contraction (+dP/dtmax) and maximal rate of pressure development for relaxation (−dP/dtmax) were all calculated from the continuously collected pressure signal.
Histopathological detection
After the treatment of Rg1, ascending aorta, heart and kidney of each rat were weighed, and then fixed by 4% neutral-buffered paraformaldehyde for 24 h. Heart index was calculated as that the heart weight was divided by body weight. All the specimens were paraffin-embedded, cut at 5 μm and were stained with haematoxylin and eosin. Photomicrographs were taken using an Olympus BX51 microscope plus Olympus DP71 CCD camera (Olympus Corporation, Japan). Software Image-Pro Plus version 6.0 was used to detect lumen diameter and media thickness of aorta. The aortic lumen diameter was calculated as the mean of the inner diameters through the center of vascular circle, and the media thickness defined as the distance between the internal and external elastic lamina. At least 16 values were measured from the points distributed evenly on every aorta. Paraffin-embedded slices were also stained with 0.1% picric sirius red (Sigma-Aldrich Inc, St Louis, USA) for fibrosis detection.
Immunohistochemical detection on retinal vessels
Rat eyes were fixed in 4% neutral-buffered paraformaldehyde and the solution was replaced every two days. Before retinal dissection, the eyes were washed by running water for 20 mins. Retinas were dissected, flattened on slides, washed by PBS, then incubated in PBS containing 0.5% Triton X-100 and 10% normal goat serum for 1 h at room temperature. After a short rinse with PBS, retinas were incubated with the α-SMA-Cy3 (Sigma-Aldrich, Germany) and lectin-FITC (Sigma-Aldrich, Germany) at 1000 times dilution at 37°C for 1 h. Retinal artery could be stained by both α-SMA-Cy3 and lectin-FITC positively. Photomicrographs were taken with Olympus BX51 fluorescence microscope and retinal vessel diameter was directly measured at the point of 0.5 mm from the middle of the arteriole.
Transmission electron microscopy
Aorta, heart, kidney samples were dissected, cut into small pieces and fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer for 2 h in 4°C. The specimens were then rinsed, post-fixed in cacodylate -buffered 2% osmium tetroxide, and embedded as monolayers in LX-112 (Ladd Research Industries, USA). Ultrathin sections were contrasted with uranyl acetate followed by lead citrate and observed with a Tecnai 12 BioTwin transmission electron microscope (Philips Electronic Instruments, USA). Random sections were selected for analysis by an electron microscopy technician blinded to the treatments.
Data analysis
All quantitative values are given as mean ± S.E. Mean values of data from different treatment groups were compared using one-way ANOVA. After confirming the equal variances, unpaired Student’s t-test was used to compare difference between means of two groups using SigmaPlot software. P < 0.05 was considered to be statistically significant.
Results
No influence of Rg1 on blood pressure
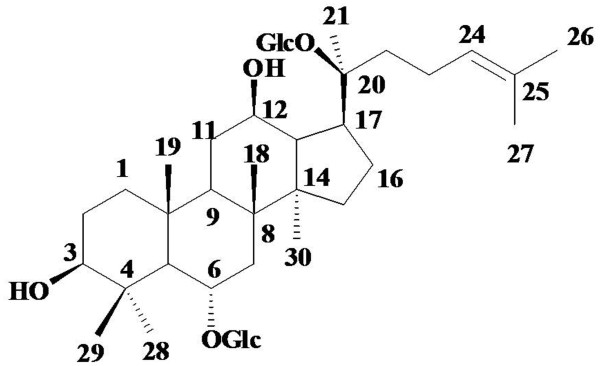
The structure of Rg1 was shown in Figure 1. Hypertension is well established as a stimulus for cardiovascular remodeling. It was therefore important to determine whether Rg1 regulated blood pressure or not. Throughout the experiments, the blood pressure in the four SHR groups were significantly higher than in the WKY group, but no regulation was found for Rg1 on blood pressure during the whole experiment using tail-cuff method. Furthermore, Rg1 did not show considerable regulation on left ventricular function demonstrated by HR, MAP, +dP/dtmax, -dP/dtmax and LVSP detected by Millar catheter (Table 1).
Figure 1.
Chemical structure of Rg1.
Table 1.
Effects of Rg1 on blood pressure
| WKY | SHR | SHR-Rg1(5) | SHR-Rg1(10) | SHR-Rg1(20) | |
|---|---|---|---|---|---|
| SBP(mmHg) |
133.7 ± 2.2 |
184.5 ± 3.4### |
186.0 ± 2.4### |
183.5 ± 3.3### |
183.0 ± 4.2### |
| DBP(mmHg) |
97.5 ± 2.0 |
139.0 ± 3.0### |
138.0 ± 2.4### |
139.1 ± 2.8### |
133.8 ± 3.9### |
| HR (bpm) |
360.6 ± 14.0 |
393.6 ± 12.5# |
366.7 ± 13.7 |
412.6 ± 8.9 |
433.5 ± 9.3* |
| MAP(mmHg) |
111.5 ± 6.3 |
170.1 ± 16.7### |
181.6 ± 14.0### |
175.4 ± 37.4### |
193.7 ± 10.4### |
| EDP(mmHg) |
6.1 ± 1.7 |
7.5 ± 3.0 |
3.1 ± 1.0 |
4.8 ± 3.9 |
5.5 ± 1.8 |
| LVSP(mmHg) |
120.9 ± 7.1 |
207.1 ± 17.9### |
213.7 ± 21.1### |
224.3 ± 35.4### |
217.5 ± 16.8### |
| +dP/dtmax (mm HgS-1) |
8661.1 ± 717.7 |
11735.7 ± 635.8## |
13063.6 ± 470.8### |
12651.9 ± 803.0## |
12636.7 ± 514.7### |
| -dP/dtmax (mm HgS-1) |
−7514.6 ± 626.0 |
−11272.1 ± 872.8## |
−12410.0 ± 595.7### |
−11330.2 ± 722.8### |
−11464.1 ± 534.8### |
| HW/BW(mg/g) | 2.9 ± 0.2 | 3.9 ± 0.05# | 3.8 ± 0.08# | 3.8 ± 0.07# | 3.6 ± 0.06# ** |
All the values are expressed as mean ± S.E. Systolic pressure (SBP), diastolic pressure (DBP), heart rate (HR), mean arterial pressure (MAP), maximum rate of pressure development for relaxation (−dP/dtmax), maximum rate of pressure development for contraction (+dP/dtmax), left ventricular systolic pressure (LVSP) were demonstrated. Heart weight divided by body weight (HW/BW) is also calculated. MAP, EDP, LVSP, +dp/dtmax, -dp/dtmax were acquired using Millar catheter and recorded by PowerLab 8/30 instrument (ADInstruments, Australia). Heart rate, SBP and DBP were acquired by tail-cuff apparatus. Mean values of data from different treatment groups were compared using one-way ANOVA. After confirming the equal variances, unpaired Student’s t-test was used to compare difference between means of two groups using SigmaPlot software. #P < 0.05, ###P < 0.001 versus WKY; **P < 0.01, ***P < 0.001 versus SHR. n = 10 for each group.
Rg1 reduces aortic remodeling
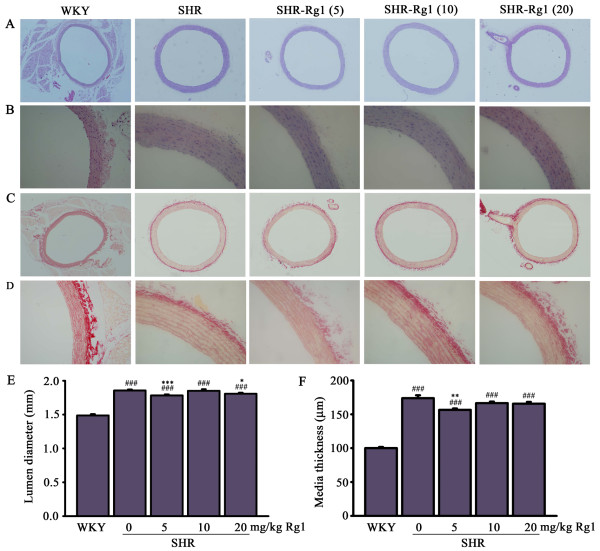
Aortic structure was investigated by histopathological analysis using haematoxylin and eosin stain and shown in Figure 2A and Figure 2B. The cell alignment was un-regular and cell density was reduced in SHR relative to WKY, and Rg1 treatment markedly attenuated this phenomenon (Figure 2B). Vascular fibrosis that impairs vascular contractility was detected by picro sirus stain. No significant improvement of Rg1 on vascular fibrosis was found (Figure 2C, D). It is well known that long term hypertension could induce vascular remodeling including greater values of lumen diameter and media thickness. The lumen diameter was 1.86 ± 0.02 mm in SHR rats compared with 1.49 ± 0.02 mm in WKY rats (P < 0.001). Rg1 decreased this outward remodeling significantly (P < 0.001 for 5 mg/kg Rg1; P < 0.05 for 20 mg/kg Rg1; Figure 2E). Furthermore, the thickening of aortic media was clearly observed in the arteries of SHR comparing with WKY (173.7 ± 4.3 μm versus 100.0 ± 1.7 μm; P < 0.001), and the media thickness in 5 mg/kg Rg1 treated SHR decreased to 156.6 ± 2.1 μm (P < 0.01; Figure 2F).
Figure 2.
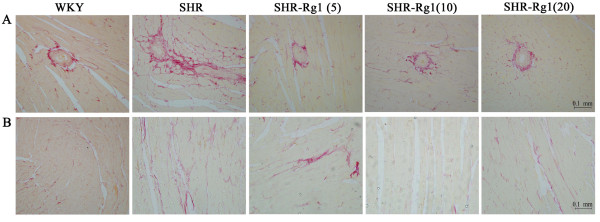
Effects of Rg1 on aorta structure. (A) Representative sections of the aortas staining with hematoxylin-eosin (magnification 40X). (B) Representative sections of the aortas staining with hematoxylin-eosin (magnification 200X) (C) Representative sections of the aortas staining with Sirius red ((magnification 40X) (D) Representative sections of the aortas staining with Sirius red (magnification 200X) (E) Quantitative data of lumen diameter. (F) Quantitative data of media thickness. ###p < 0.001 compared with WKY, ***p < 0.001 compared with SHR. n = 4 for each group.
Rg1 reduces retinal vascular remodeling
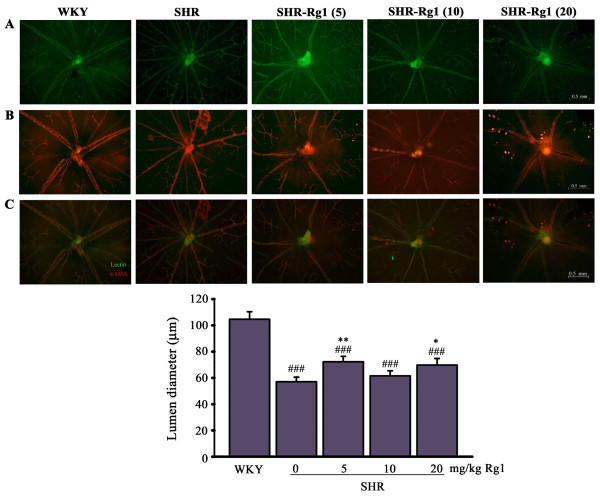
Hypertension is a strong stimulus not only on conductance vessel such as aorta, but also on resistance vessel such as retinal arteries [19]. In the present study, we investigated effects of Rg1 on the remodeling of small retinal arteries (Figure 3). Arteries and veins were distinguished by double-staining of the retinal blood vessels with lectin-FITC and an anti-α-SMA-Cy3 antibody, which resulted in a more pronounced α-SMA staining of small arteries and arterioles. The remodeling of retinal arteries was evaluated by arteries diameter at the point 0.5 mm to the middle of the arteriole. Hypertension induced considerable decrease on retinal arterial diameter in SHR (57.06 ± 3.47 μm) compared with WKY (104.65 ± 5.695 μm; P < 0.001). While Rg1 inhibited this inward remodeling induced by hypertension, the diameter values were 72.2 ± 4.2 μm (SHR-Rg1(5)), 61.5 ± 3.9 μm (SHR-Rg1(10)) and 69.8 ± 5.0 μm (SHR-Rg1(20)), respectively.
Figure 3.
Effects of Rg1 on retinal remodeling induced by hypertension. Whole-mount double-staining of retinal vessels with FITC-coupled lectin (green) and α-SMA antibody (red). (A) lectin positive arteries. (B) α-SMA–positive arteries. (C) The merged picture for both (A) and (B). (D) Quantitative data of lumen diameter for retinal arteries. ###p < 0.001 compared with WKY; **p < 0.01, **p < 0.01 compared with SHR. n = 4 for each group.
Rg1 protects the ultra-structural integrity of aorta
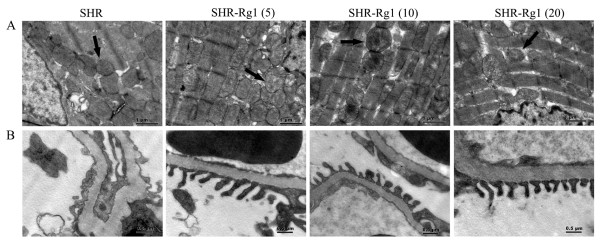
The ultra-structure of aorta was examined using transmission electron microscopy (Figure 4). Vascular smooth muscle cell is the main cell type of aorta and play important role on vascular function. In the present study, we detected the mitochondrial ultra-structure of smooth muscle cells of aorta. The mitochondria of SHR showed irregularly and spherical shaped, especially swollen with electron-lucent matrix. With Rg1 treatment, although the matrix of mitochondria was partial disruption, but no-swollen cristae was detected.
Figure 4.
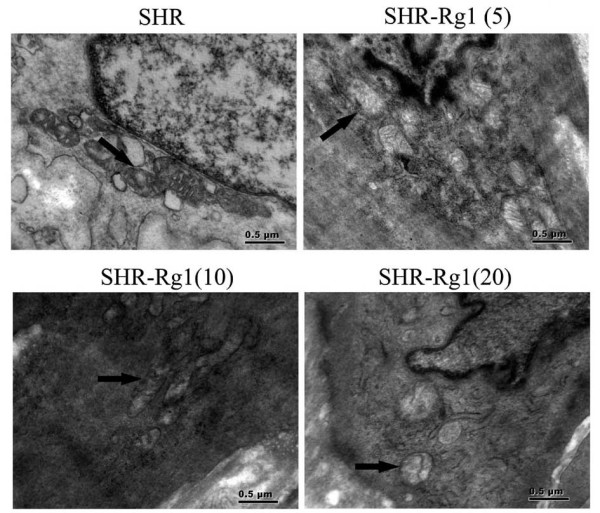
Representative photographs of transmission electron microscopy for vascular smooth muscle cells. Arrows indicate mitochondria. n = 3 for each group. The representative figures were shown.
Rg1 protects against the impairment of hypertensive heart and kidney
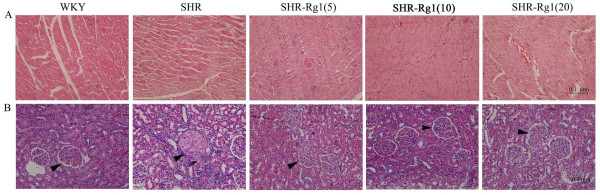
Cardial and renal deterioration are both complications in patients with hypertension. Histopathological detection was performed in order to investigate the protective effects of Rg1 on structure of heart and kidney in SHR. Cardiomyocyte hypertrophy was a significant characteristic in SHR compared with WKY, and this impairment was attenuated in SHR-Rg1 groups (Figure 5A). This finding is consistence with the result of heart index, the values were 2.9 ± 0.2 mg/kg (WKY), 3.9 ± 0.05 mg/kg (SHR), 3.8 ± 0.08 mg/kg (SHR-Rg1(5)), 3.8 ± 0.07 mg/kg (SHR-Rg1(10)), 3.6 ± 0.06 mg/kg (SHR-Rg1(20)), respectively (Table 1). The typical photomicrographs of glomeruli were shown in Figure 5B. Glomeruli of SHR showed prominent hyalinization (mesangial thickening) comparing with WKY rats. SHR treated with Rg1 showed the reduction in the development of hyalinization.
Figure 5 .
Rg1 improved heart and kidney structure of SHR detected by hematoxylin-eosin stain. (A) Representative photomicrographs from heart tissue of WKY, SHR, SHR-Rg1(5), SHR-Rg1(10), SHR-Rg1(20) with 200 X magnification. (B) Representative photomicrographs from kidney tissue of WKY, SHR, SHR-Rg1(5), SHR-Rg1(10), SHR-Rg1(20) with 200 X magnification. n = 4 for each group.
Rg1 protects against cardio-fibrosis induced by hypertension
Myocardial fibrosis, the common end point of hypertensive heart disease, has been linked to the development of contractile dysfunction and cardiac failure. Picro sirus stain showed the fibrotic area was larger in SHR compared with WKY at the perivascular area. With Rg1 treatment, the fibrotic areas were considerably smaller than SHR (Figure 6A). Thicker and darker stain of collagen fiber was seen on SHR compared with WKY at the few-vascular area; while collagen fiber became thinner, weaker and more discontinuous with Rg1 treatment (Figure 6B). No significant improvement of Rg1 on renal fibrosis was detected (data not shown).
Figure 6.
Rg1 decreased heart fibrosis induced by hypertension. (A) Representative perivascular area of whole heart stained by Sirius red. (B) Representative few-vascular area of whole heart stained by Sirius red. The position of collagen deposition was stained in red. n = 4 for each group.
Rg1 protects the ultra-structure integrity of heart and kidney of SHR
The damage on mitochondria of cardiomyocyte included swelling, disorganization or even disappearance of cristae in SHR group. While, no overt ultra-structural abnormality was observed in ventricular samples from Rg1 treated groups, with relative organized mitochondria (Figure 7A). Electron microscopy also demonstrated a heterogeneous thickening of the glomerular basement membrane, accompanying irregular arrangement or inter-adhesion of the podocytes in SHR group. With Rg1 treatment, podocytes were in very regular array (Figure 7B).
Figure 7.
Rg1 improved ultra-structure of heart and kidney. (A) Electron micrograph of mitochondria marked by arrow in heart. (B) Electron micrograph of glomerular basement membrane. n = 3 for each group. The representative figures were shown.
Discussion
This study assessed the protective effects of Rg1 on hypertension target-organ damage in SHRs. The main findings are as follows: (i) Rg1 attenuated the outward remodeling of aorta. (ii) Rg1 decreased the inward remodeling of small artery. (iii) Rg1 improved the cardial hypertrophy and fibrosis. (iv) Rg1 maintained the normal structure of kidney.
Numerous studies have demonstrated that high salt intake causes adverse structural and functional effects in the cardiovascular system [20,21]. Excessive salt intake is often associated with an increase in arterial pressure and, consequently, increases in arterial pressure may partially mediate salt-related adverse effects [22]. In addition to the well-admitted effect of sodium on blood pressure, several clinical and experimental observations are in favour of non-pressure-related effects of salt that could contribute to its influence on cardiovascular outcome [23]. High salt intake caused hypertrophic response, then concentric cardiac-remodeling [24]. High dietary salt led to widespread fibrosis and increased TGF-β1 in the heart and kidney in normotensive and hypertensive rats, suggesting that excessive salt intake may be an important direct pathogenic factor for cardiovascular disease [21].
Up to now, cardiovascular disease is still the most important factor that affects people’s life, especially the hypertension [1,2,25]. To delay or prevent hypertension target-organ, containing heart failure and renal failure, is essential to improve patient’s quality of life [26]. In agreement with previous reports that the most common change found in large arteries in hypertension is an “outward hypertrophic remodeling”, we detected the increased lumen diameter and wall thickness in SHR compared with WKY. In hypertensive large arteries, these changes appear to be the consequence of cellular hypertrophy, cell hyperplasia, increased deposition of fibrillar or nonfibrillar matrix, or from a combination of these events [27-30]. Remodeling of the small resistance arteries may involve an inward or an outward remodeling [19,31]. In the present study, we confirmed an inward remodeling in small retinal arteries of SHR compared with WKY. Rg1 treatment not only reduced the outward remodeling of large conductance arteries but also attenuated the inward remodeling of small resistance arteries, although no regulation of Rg1 on blood pressure was found. These results agree with those of other reports that ACE inhibitors are effective in controlling or reversing vascular remodeling, not depending on the anti-hypertensive effects [32,33].
The final goal of novel therapy of hypertension is not only to normalize the blood pressure level but also to prevent end-organ damage, such as cardiac hypertrophy and renal dysfunction [26,34]. Altered retinal arteries diameter has also been demonstrated to be associated with heart failure, suggesting that evaluation of the retinal microvasculature may be a useful predictor of target organ damage [35,36]. Collagen deposition is the risk factor which plays an important role in development of organ failure, such as heart failure and renal failure [37]. The myocardial matrix becomes less distensible, as the formation of the adducts in collagen resists normal turnover. Therefore, monitoring cardiac fibrosis and use of medicines that reverse collagen accumulation might represent a novel opportunity to alter the natural history of hypertensive heart disease.
In the previous report, the presence of myocardial hypertrophy and fibrosis in SHR was obvious at early stage, but the diastolic and systolic dysfunction did not occur until 13 to 18 months of age of SHR [38]. This finding is in consistence with our present study. It is hopeful that the improvement of Rg1 on cardiac function could be detected by elongation of Rg1 treatment or selection of SHR more than 13 month old, basing on the anti-fibrotic effects of Rg1 at the early stage of hypertension.
We detected heart rate on conscious SHR during the experiment using tail-cuff apparatus, and up-regulation of Rg1 on heart rate was found. Increase of heart rate induced by Rg1 should be considered as a side effect in cardiovascular disease. Shen et al. have reported that Rg1 down-regulated heart rate in anesthetized mice treated by glutamate [39]. The difference between our report and Shen et al. maybe derive from the different species used, suggesting the increase of Rg1 on heart rate may not occur if animal model other than SHR was used. Different biological response induced by extract from herbs between species was also reported [40,41]. Further study was necessary to clarify the effects of Rg1 on heart rate on different species and the underlying mechanism.
Conclusion
In summary, Rg1 performed cardiac and renal protection with inhibition of vascular remodeling not only on large conductance artery but also on small resistance artery. The unique structure and efficiency of Rg1 afford a great deal of potential for further optimization of this natural compound into therapeutics for hypertension related abnormalities.
Competing interest
The authors declare that they have no competing interests.
Authors’ contributions
Hui Chen, Yangping Deng, Lingling Xu and Tingting Zhang performed animal experiments. Min Yang, Wanying Wu and Shuhong Guan performed Chemical analysis, Fukang Teng, Defang Li, Yufan Cheng, Sha Liu, Dong Wang, Xuan Liu performed histological and immunohistochemical detection. Jun Yin, Baohong Jiang and De-an Guo designed, analyzed experiments and prepared the manuscript. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Supplementary Material
Figure S1. The representative chromatogram of high-performance liquid chromatography for Rg1. Agilent 1100 HPLC system was used and the detection wavelength was set at 280 nm. The mobile phase consisted of (A) acetonitrile and (B) 0.05% aqueous trifluoroacetic acid (V/V), using a gradient elution of 2%-10% A at 0–7 min, 10%–30% A at 7–20 min, 23%–27% A at 20–35 min, and 27%–60% A at 35–50 min .
Figure S2.13C NMR spectrum of Rg1 detected by Bruker AM-400 spectrometer.
Figure S3. Structure elucidation of Rg1. (A) Chemical structure of Rg1. (B)13C NMR (100 MHz) spectral data for Rg1.
Figure S4. Experimental protocol. Rg1 treatment was performed from week 9 to week 12.
Contributor Information
Hui Chen, Email: chenhui_luo@163.com.
Jun Yin, Email: yinjun826@sina.com.
Yanpin Deng, Email: shimbiro@sina.com.
Min Yang, Email: m_yang@mail.shcnc.ac.cn.
Lingling Xu, Email: candyxll@163.com.
Fukang Teng, Email: fineskypig1983@163.com.
Defang Li, Email: lidefang@163.com.
Yufan Cheng, Email: chengyufan2009@163.com.
Sha Liu, Email: drliusha@163.com.
Dong Wang, Email: dongwang@syphu.edu.cn.
Tingting Zhang, Email: tingyu3160@163.com.
Wanying Wu, Email: wanyingwu@mail.shcnc.ac.cn.
Xuan Liu, Email: xuanliu@mail.shcnc.ac.cn.
Shuhong Guan, Email: sh_guan@mail.shcnc.ac.cn.
Baohong Jiang, Email: jiangbh@mail.shcnc.ac.cn.
Dean Guo, Email: daguo@mail.shcnc.ac.cn.
Acknowledgements
This work was supported by National Natural Science Foundation of China grant (81173587), partly supported by the Major Projects of Knowledge Innovation Program of the Chinese Academy of Sciences (KSCX2-YW-R-166).
References
- Cutler JA, Sorlie PD, Wolz M, Thom T, Fields LE, Roccella EJ. Trends in hypertension prevalence, awareness, treatment, and control rates in United States adults between 1988–1994 and 1999–2004. Hypertension. 2008;52(5):818–827. doi: 10.1161/HYPERTENSIONAHA.108.113357. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopex AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360(9343):1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- Yamori Y. Implication of hypertensive rat models for primordial nutritional prevention of cardiovascular diseases. Clin Exp Pharmacol Physiol. 1999;26(7):568–572. doi: 10.1046/j.1440-1681.1999.03085.x. [DOI] [PubMed] [Google Scholar]
- Dong TT, Cui XM, Song ZH, Zhao KJ, Ji ZN, Lo CK, Tsim KW. Chemical assessment of roots of Panax notoginseng in China: regional and seasonal variations in its active constituents. J Agric Food Chem. 2003;51(16):4617–4623. doi: 10.1021/jf034229k. [DOI] [PubMed] [Google Scholar]
- Ng TB. Pharmacological activity of sanchi ginseng (Panax notoginseng) J Pharm Pharmacol. 2006;58(8):1007–1019. doi: 10.1211/jpp.58.8.0001. [DOI] [PubMed] [Google Scholar]
- Sun HX, Pan HJ, Pan YJ. Haemolytic activities and immunologic adjuvant effect of Panax notoginseng saponins. Acta Pharmacol Sin. 2003;24(11):1150–1154. [PubMed] [Google Scholar]
- Gillis CN. Panax ginseng pharmacology: A nitric oxide link? Biochem Pharmacol. 1997;54(1):1–8. doi: 10.1016/S0006-2952(97)00193-7. [DOI] [PubMed] [Google Scholar]
- Xie XS, Yang M, Liu HC, Zuo C, Li HJ, Fan JM. Ginsenoside Rg1, a major active component isolated from Panax notoginseng, restrains tubular epithelial to myofibroblast transition in vitro. J Ethnopharmacol. 2009;122(1):35–41. doi: 10.1016/j.jep.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Zhang HG, Li XH, Yang ZC. Effects of Panax notoginseng saponins on myocardial Gsalpha mRNA expression and ATPase activity after severe scald in rats. Burns. 2003;29(6):541–546. doi: 10.1016/S0305-4179(03)00143-8. [DOI] [PubMed] [Google Scholar]
- Park WH, Lee SK, Kim CH. A Korean herbal medicine, Panax notoginseng, prevents liver fibrosis and hepatic microvascular dysfunction in rats. Life Sci. 2005;76(15):1675–1690. doi: 10.1016/j.lfs.2004.07.030. [DOI] [PubMed] [Google Scholar]
- Yang CY, Wang J, Zhao Y, Shen L, Jiang X, Xie ZG, Liang N, Zhang L, Chen ZH. Anti-diabetic effects of Panax notoginseng saponins and its major anti-hyperglycemic components. J Ethnopharmacol. 2010;130(2):231–236. doi: 10.1016/j.jep.2010.04.039. [DOI] [PubMed] [Google Scholar]
- Chen SW, Li XH, Ye KH, Jiang ZF, Ren XD. Total saponins of Panax notoginseng protected rabbit iliac artery against balloon endothelial denudation injury. Acta Pharmacol Sin. 2004;25(9):1151–1156. [PubMed] [Google Scholar]
- Sengupta S, Toh SA, Sellers LA, Skepper JN, Koolwijk P, Leung HW, Yeung HW, Wong RN, Sasisekharan R, Fan TP. Modulating angiogenesis: The yin and the yang in ginseng. Circulation. 2004;110(10):1219–1225. doi: 10.1161/01.CIR.0000140676.88412.CF. [DOI] [PubMed] [Google Scholar]
- Lu MC, Lai TY, Hwang JM, Chen HT, Chang SH, Tsai FJ, Wang HL, Lin CC, Kuo WW, Huang CY. Proliferation-and migration-enhancing effects of ginseng and ginsenoside Rg1 through IGF-I- and FGF-2-signaling pathways on RSC96 Schwann cells. Cell Biochem Funct. 2009;27(4):186–192. doi: 10.1002/cbf.1554. [DOI] [PubMed] [Google Scholar]
- Ma ZC, Gao Y, Wang YG, Tan HL, Xiao CR, Wang SQ. Ginsenoside Rg1 inhibits proliferation of vascular smooth muscle cells stimulated by tumor necrosis factor-alpha. Acta Pharmacol Sin. 2006;27(8):1000–1006. doi: 10.1111/j.1745-7254.2006.00331.x. [DOI] [PubMed] [Google Scholar]
- Leung KW, Pon YL, Wong RN, Wong AS. Ginsenoside-Rg1 induces vascular endothelial growth factor expression through the glucocorticoid receptor-related phosphatidylinositol 3-kinase/Akt and beta-catenin/T-cell factor-dependent pathway in human endothelial cells. J Biol Chem. 2006;281(47):36280–36288. doi: 10.1074/jbc.M606698200. [DOI] [PubMed] [Google Scholar]
- Yin H, Liu Z, Li F, Ni M, Wang B, Qiao Y, Xu X, Zhang M, Zhang J, Lu H, Zhang Y. Ginsenoside-Rg1 enhances angiogenesis and ameliorates ventricular remodeling in a rat model of myocardial infarction. J Mol Med. 2011;89(4):363–375. doi: 10.1007/s00109-011-0723-9. [DOI] [PubMed] [Google Scholar]
- Kim YH, Lee YG, Choi KJ, Uchida K, Suzuki Y. Transglycosylation to ginseng saponins by cyclomaltodextrin glucanotransferases. Biosci Biotechnol Biochem. 2001;65(4):875–883. doi: 10.1271/bbb.65.875. [DOI] [PubMed] [Google Scholar]
- Rizzoni D, Porteri E, Boari GE, De Ciuceis C, Sleiman I, Muiesan ML, Castellano M, Miclini M, Agabiti-Rosei E. Prognostic significance of small-artery structure in hypertension. Circulation. 2003;108(18):2230–2235. doi: 10.1161/01.CIR.0000095031.51492.C5. [DOI] [PubMed] [Google Scholar]
- Mercier N, Labat C, Louis H, Cattan V, Benetos A, Safar ME, Lacolley P. Sodium, arterial stiffness, and cardiovascular mortality in hypertensive rats. Am J Hypertens. 2007;20(3):319–325. doi: 10.1016/j.amjhyper.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Yu CM, Burrell LM, Black MJ, Wu LL, Dilley RJ, Cooper ME, Johnston CI. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation. 1998;98(23):2621–2628. doi: 10.1161/01.CIR.98.23.2621. [DOI] [PubMed] [Google Scholar]
- Susic D, Frohlich ED, Kobori H, Shao W, Seth D, Navar LG. Salt-induced renal injury in SHRs is mediated by AT1 receptor activation. J Hypertens. 2011;29(4):716–723. doi: 10.1097/HJH.0b013e3283440683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimran A, du Cailar G. Dietary sodium: the dark horse amongst cardiovascular and renal risk factors. Nephrol Dial Transplant. 2008;23(7):2138–2141. doi: 10.1093/ndt/gfn160. [DOI] [PubMed] [Google Scholar]
- Leenen FH, Yuan B. Dietary-sodium-induced cardiac remodeling in spontaneously hypertensive rat versus Wistar-Kyoto rat. J Hypertens. 1998;16(6):885–892. doi: 10.1097/00004872-199816060-00020. [DOI] [PubMed] [Google Scholar]
- Vilela-Martin JF, Vaz-de-Melo RO, Kuniyoshi CH, Abdo AN, Yugar-Toledo JC. Hypertensive crisis: clinical-epidemiological profile. Hypertens Res. 2011;34(3):367–371. doi: 10.1038/hr.2010.245. [DOI] [PubMed] [Google Scholar]
- Ishimitsu T, Honda T, Ohno E, Furukata S, Sudo Y, Nakano N, Takahashi T, Ono H, Matsuoka H. Year-long antihypertensive therapy with candesartan completely prevents development of cardiovascular organ injuries in spontaneously hypertensive rats. Int Heart J. 2010;51(5):359–364. doi: 10.1536/ihj.51.359. [DOI] [PubMed] [Google Scholar]
- Hajdu MA, Baumbach GL. Mechanics of large and small cerebral arteries in chronic hypertension. Am J Physiol. 1994;266(3):H1027–H1033. doi: 10.1152/ajpheart.1994.266.3.H1027. [DOI] [PubMed] [Google Scholar]
- Behbahani J, Thandapilly SJ, Louis XL, Huang Y, Shao Z, Kopilas MA, Wojciechowski P, Netticadan T, Anderson HD. Resveratrol and small artery compliance and remodeling in the spontaneously hypertensive rat. Am J Hypertens. 2010;23(12):1273–1278. doi: 10.1038/ajh.2010.161. [DOI] [PubMed] [Google Scholar]
- Intengan HD, Schiffrin EL. Vascular remodeling in hypertension: roles of apoptosis, inflammation, and fibrosis. Hypertension. 2001;38(3):581–587. doi: 10.1161/hy09t1.096249. [DOI] [PubMed] [Google Scholar]
- Feihl F, Liaudet L, Waeber B. The macrocirculation and microcirculation of hypertension. Curr Hypertens Rep. 2009;11(3):182–189. doi: 10.1007/s11906-009-0033-6. [DOI] [PubMed] [Google Scholar]
- Sonoyama K, Greenstein A, Price A, Khavandi K, Heagerty T. Vascular remodeling: implications for small artery function and target organ damage. Ther Adv Cardiovasc Dis. 2007;1(2):129–137. doi: 10.1177/1753944707086358. [DOI] [PubMed] [Google Scholar]
- Ferrario CM, Flack JM. Pathologic consequences of increased angiotensin II activity. Cardiovasc Drugs Ther. 1996;10(5):511–518. doi: 10.1007/BF00050990. [DOI] [PubMed] [Google Scholar]
- Schiffrin EL. Remodeling of resistance arteries in essential hypertension and effects of antihypertensive treatment. Am J Hypertens. 2004;17(12):1192–1200. doi: 10.1016/j.amjhyper.2004.05.023. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Fifty years of framingham study contributions to understanding hypertension. J Hum Hypertens. 2000;14(2):83–90. doi: 10.1038/sj.jhh.1000949. [DOI] [PubMed] [Google Scholar]
- Bohle A, Muller GA, Wehrmann M, Mackensen-Haen S, Xiao JC. Pathogenesis of chronic renal failure in the primary glomerulopathies, renal vasculopathies, and chronic interstitial nephritides. Kidney Int Suppl. 1996;54:S2–S9. [PubMed] [Google Scholar]
- Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens. 2001;19(5):921–930. doi: 10.1097/00004872-200105000-00013. [DOI] [PubMed] [Google Scholar]
- Burlew BS, Weber KT. Cardiac fibrosis as a cause of diastolic dysfunction. Herz. 2002;27(2):92–98. doi: 10.1007/s00059-002-2354-y. [DOI] [PubMed] [Google Scholar]
- Brooks WW, Conrad CH, Robinson KG, Colucci WS, Bing OH. L-arginine fails to prevent ventricular remodeling and heart failure in the spontaneously hypertensive rat. Am J Hypertens. 2009;22(2):228–234. doi: 10.1038/ajh.2008.334. [DOI] [PubMed] [Google Scholar]
- Shen L, Han JZ, Li C, Yue SJ, Liu Y, Qin XQ, Liu HJ, Luo ZQ. Protective effect of ginsenoside Rg1 on glutamate-induced lung injury. Acta Pharmacol Sin. 2007;28(3):392–397. doi: 10.1111/j.1745-7254.2007.00511.x. [DOI] [PubMed] [Google Scholar]
- Najeeb UR, Mehmood MH, Alkharfy KM, Gilani AH. Prokinetic and laxative activities of lepidium sativum seed extract with species and tissue selective gut stimulatory actions. J Ethnopharmacol. 2011;134(3):878–883. doi: 10.1016/j.jep.2011.01.047. [DOI] [PubMed] [Google Scholar]
- Ghayur MN, Gilani AH. Species differences in the prokinetic effects of ginger. Int J Food Sci Nutr. 2006;57(1–2):65–73. doi: 10.1080/09637480600656074. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The representative chromatogram of high-performance liquid chromatography for Rg1. Agilent 1100 HPLC system was used and the detection wavelength was set at 280 nm. The mobile phase consisted of (A) acetonitrile and (B) 0.05% aqueous trifluoroacetic acid (V/V), using a gradient elution of 2%-10% A at 0–7 min, 10%–30% A at 7–20 min, 23%–27% A at 20–35 min, and 27%–60% A at 35–50 min .
Figure S2.13C NMR spectrum of Rg1 detected by Bruker AM-400 spectrometer.
Figure S3. Structure elucidation of Rg1. (A) Chemical structure of Rg1. (B)13C NMR (100 MHz) spectral data for Rg1.
Figure S4. Experimental protocol. Rg1 treatment was performed from week 9 to week 12.