Abstract
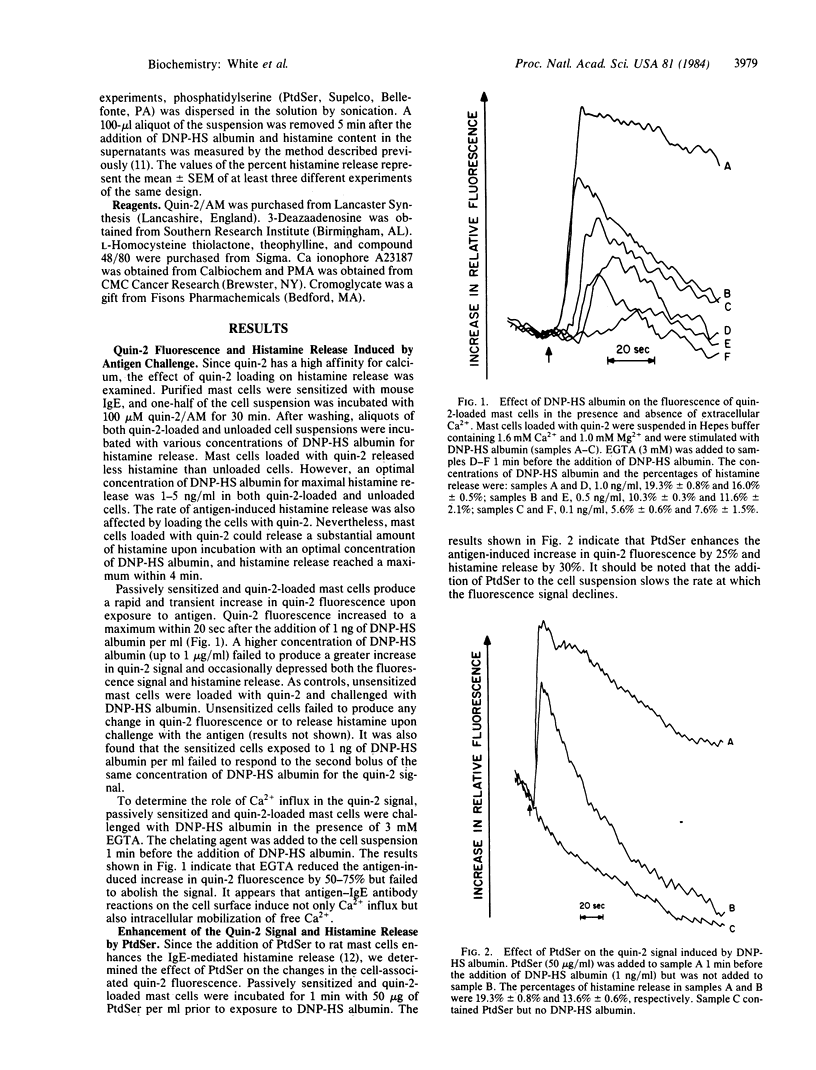
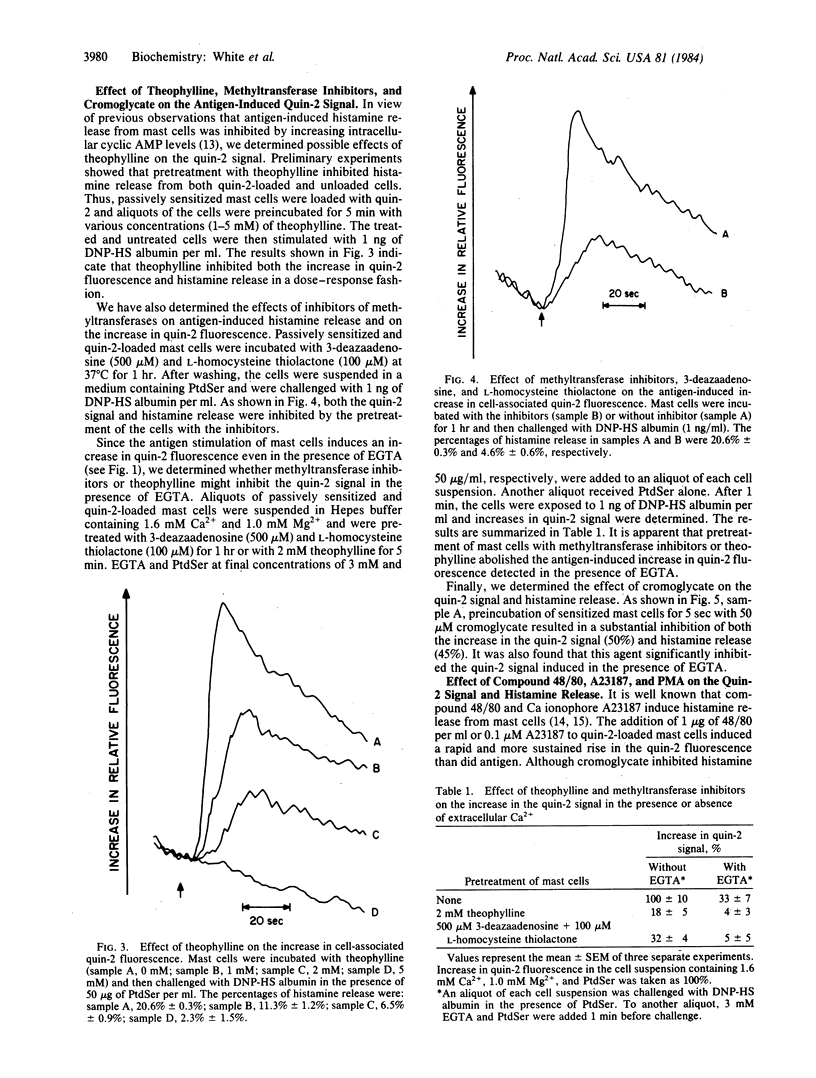
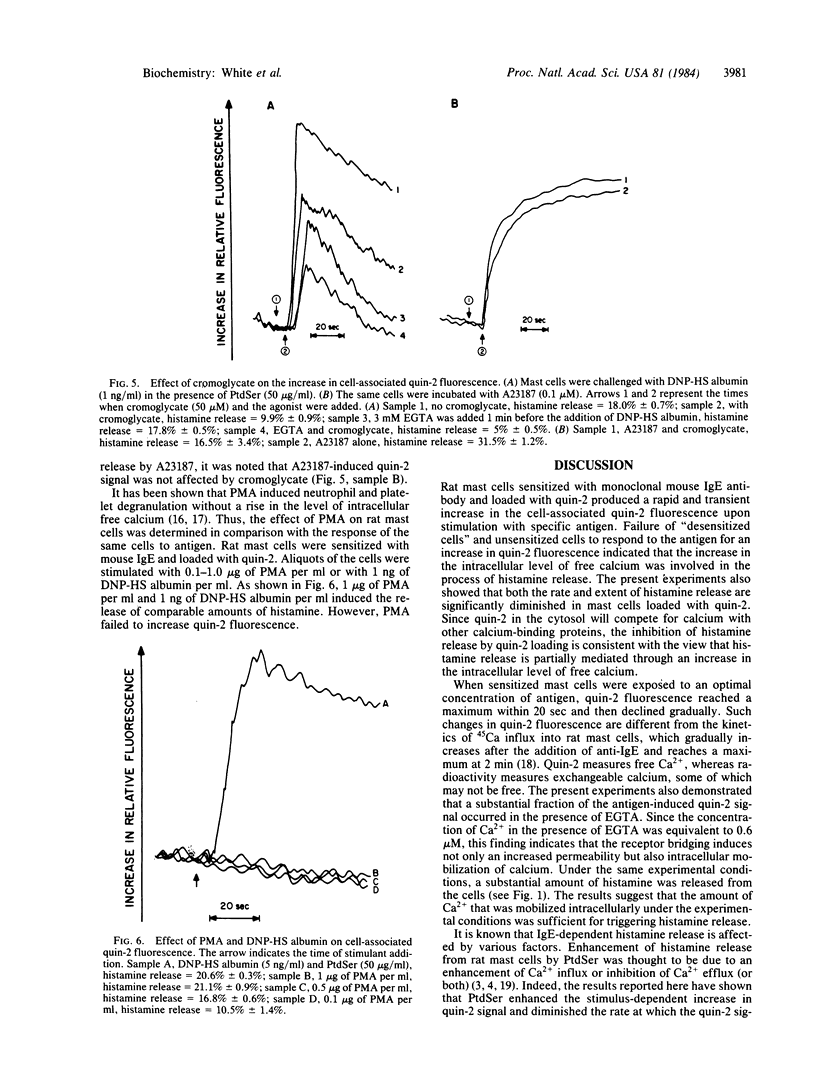
Rat mast cells, passively sensitized with monoclonal mouse IgE antibody, were stimulated with multi-valent antigen, and an increase in cytoplasmic Ca2+ was determined by using the fluorescent probe quin-2. The increase in quin-2 fluorescence reached maximum within 20 sec after the antigen challenge and then gradually declined. A substantial increase in quin-2 fluorescence was observed in the presence of EGTA, indicating that bridging of cell-bound IgE antibody molecules by antigen induced not only Ca2+ influx but also mobilization of intracellular Ca2+. Phosphatidylserine added to the medium enhanced both the antigen-induced histamine release and the increase in quin-2 fluorescence and slowed the rate at which the quin-2 signal returned to basal levels. Both the antigen-induced increase in quin-2 fluorescence and histamine release were inhibited by pretreatment of mast cells with inhibitors of methyltransferases, theophylline, or cromoglycate. It was also found that methyltransferase inhibitors and theophylline inhibited not only stimulus-dependent calcium influx but also release of bound calcium from intracellular stores. Other secretagogues, compound 48/80 (1 microgram/ml) and Ca ionophore A23187 (0.1 microM), induced a rapid increase in cytoplasmic Ca2+ in rat mast cells and subsequent histamine release. In contrast, the cocarcinogenic compound phorbol 12-myristate 13-acetate caused histamine release without increasing the quin-2 fluorescence.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Daëron M., Sterk A. R., Hirata F., Ishizaka T. Biochemical analysis of glucocorticoid-induced inhibition of IgE-mediated histamine release from mouse mast cells. J Immunol. 1982 Sep;129(3):1212–1218. [PubMed] [Google Scholar]
- Feinstein M. B., Egan J. J., Sha'afi R. I., White J. The cytoplasmic concentration of free calcium in platelets is controlled by stimulators of cyclic AMP production (PGD2, PGE1, forskolin). Biochem Biophys Res Commun. 1983 Jun 15;113(2):598–604. doi: 10.1016/0006-291x(83)91768-0. [DOI] [PubMed] [Google Scholar]
- Foreman J. C., Hallett M. B., Mongar J. L. The relationship between histamine secretion and 45calcium uptake by mast cells. J Physiol. 1977 Sep;271(1):193–214. doi: 10.1113/jphysiol.1977.sp011996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J. C., Mongar J. L., Gomperts B. D. Calcium ionophores and movement of calcium ions following the physiological stimulus to a secretory process. Nature. 1973 Oct 5;245(5423):249–251. doi: 10.1038/245249a0. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J., Crews F. T. Concanavalin A stimulates phospholipid methylation and phosphatidylserine decarboxylation in rat mast cells. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4813–4816. doi: 10.1073/pnas.76.10.4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Foreman J. C., Sterk A. R., Ishizaka K. Induction of calcium flux across the rat mast cell membrane by bridging IgE receptors. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5858–5862. doi: 10.1073/pnas.76.11.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Ishizaka K., Axelrod J. Stimulation of phospholipid methylation, Ca2+ influx, and histamine release by bridging of IgE receptors on rat mast cells. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1903–1906. doi: 10.1073/pnas.77.4.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Hirata F., Sterk A. R., Ishizaka K., Axelrod J. A. Bridging of IgE receptors activates phospholipid methylation and adenylate cyclase in mast cell plasma membranes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6812–6816. doi: 10.1073/pnas.78.11.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K. Biology of immunoglobulin E. Molecular basis of reaginic hypersensitivity. Prog Allergy. 1975;19:60–121. [PubMed] [Google Scholar]
- Liu F. T., Bohn J. W., Ferry E. L., Yamamoto H., Molinaro C. A., Sherman L. A., Klinman N. R., Katz D. H. Monoclonal dinitrophenyl-specific murine IgE antibody: preparation, isolation, and characterization. J Immunol. 1980 Jun;124(6):2728–2737. [PubMed] [Google Scholar]
- MONGAR J. L., SCHILD H. O. The effect of calcium and pH on the anaphylactic reaction. J Physiol. 1958 Feb 17;140(2):272–284. doi: 10.1113/jphysiol.1958.sp005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mongar J. L., Svec P. The effect of phospholipids on anaphylactic histamine release. Br J Pharmacol. 1972 Dec;46(4):741–752. doi: 10.1111/j.1476-5381.1972.tb06899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzan T., Arslan P., Tsien R. Y., Rink T. J. Anti-immunoglobulin, cytoplasmic free calcium, and capping in B lymphocytes. J Cell Biol. 1982 Aug;94(2):335–340. doi: 10.1083/jcb.94.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Röhlich P., Anderson P., Uvnäs B. Electron microscope observations on compounds 48-80-induced degranulation in rat mast cells. Evidence for sequential exocytosis of storage granules. J Cell Biol. 1971 Nov;51(21):465–483. doi: 10.1083/jcb.51.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHORE P. A., BURKHALTER A., COHN V. H., Jr A method for the fluorometric assay of histamine in tissues. J Pharmacol Exp Ther. 1959 Nov;127:182–186. [PubMed] [Google Scholar]
- Sha'afi R. I., White J. R., Molski T. F., Shefcyk J., Volpi M., Naccache P. H., Feinstein M. B. Phorbol 12-myristate 13-acetate activates rabbit neutrophils without an apparent rise in the level of intracellular free calcium. Biochem Biophys Res Commun. 1983 Jul 29;114(2):638–645. doi: 10.1016/0006-291x(83)90828-8. [DOI] [PubMed] [Google Scholar]
- Sullivan T. J., Parker K. L., Eisen S. A., Parker C. W. Modulation of cyclic AMP in purified rat mast cells. II. Studies on the relationship between intracellular cyclic AMP concentrations and histamine release. J Immunol. 1975 May;114(5):1480–1485. [PubMed] [Google Scholar]
- Tateson J. E., Trist D. G. Inhibition of adenosine-3',5'-cyclic monophosphate phosphodiesterase by potential antiallergic compounds. Life Sci. 1976 Jan 15;18(2):153–161. doi: 10.1016/0024-3205(76)90019-9. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander-Mallie R., Ishizaka T., Ishizaka K. Lymphocytes bearing Fc receptor for IgE. VIII. Affinity of mouse IgE for Fc epsilon R on Mouse B lymphocytes. J Immunol. 1982 May;128(5):2306–2312. [PubMed] [Google Scholar]
- White J. R., Naccache P. H., Molski T. F., Borgeat P., Sha'afi R. I. Direct demonstration of increased intracellular concentration of free calcium in rabbit and human neutrophils following stimulation by chemotactic factor. Biochem Biophys Res Commun. 1983 May 31;113(1):44–50. doi: 10.1016/0006-291x(83)90429-1. [DOI] [PubMed] [Google Scholar]



