Abstract
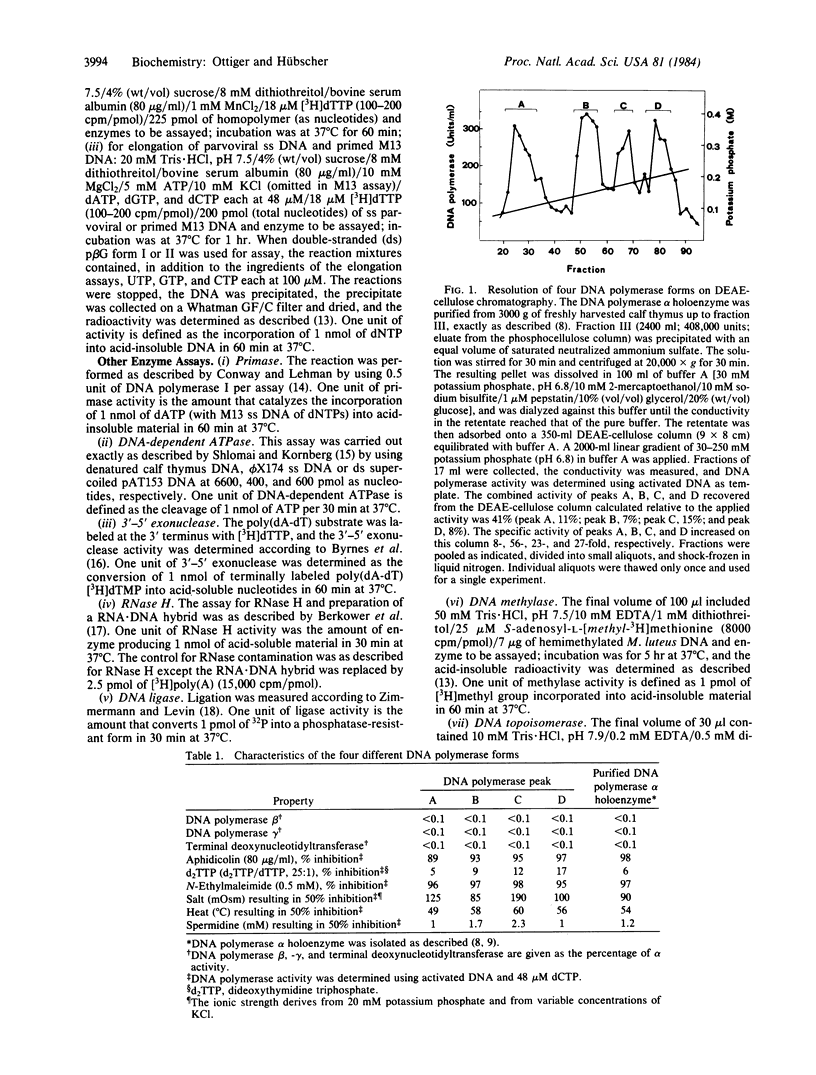
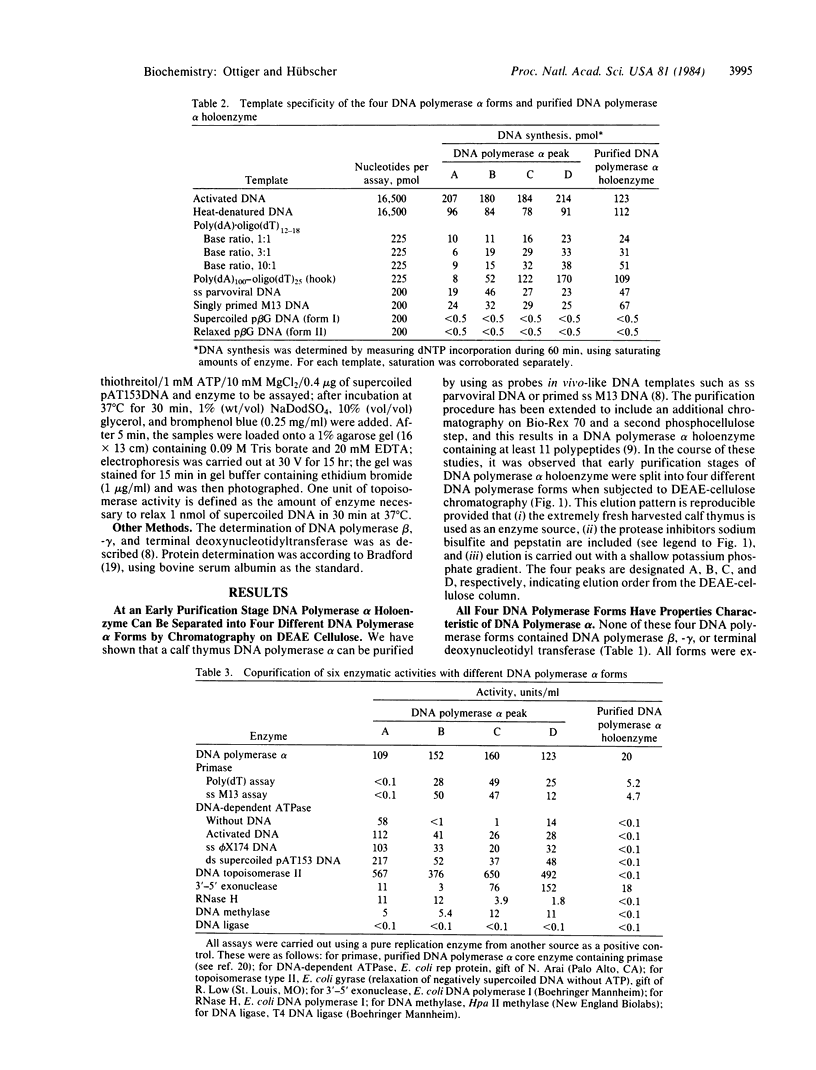
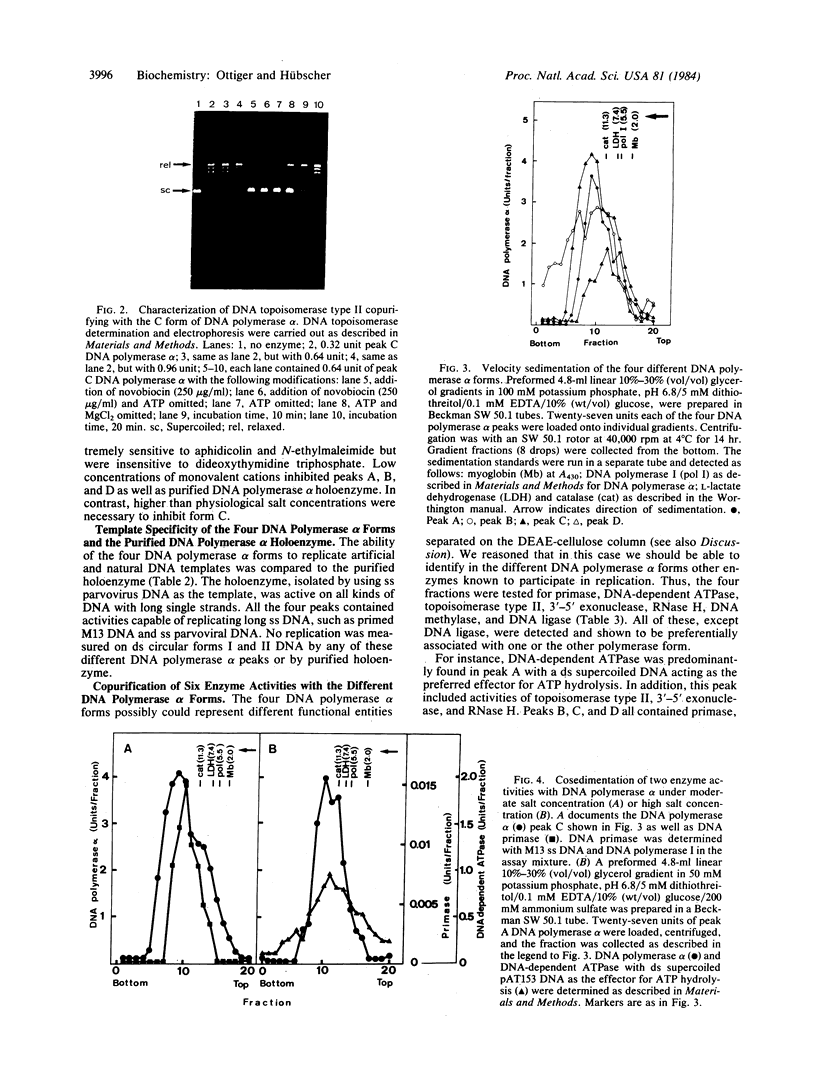
At an early purification stage, DNA polymerase alpha holoenzyme from calf thymus can be separated into four different forms by chromatography on DEAE-cellulose. All four enzyme forms (termed A, B, C, and D) are capable of replicating long single-stranded DNA templates, such as parvoviral DNA or primed M13 DNA. Peak A possesses, in addition to the DNA polymerase alpha, a double-stranded DNA-dependent ATPase, as well as DNA topoisomerase type II, 3'-5' exonuclease, and RNase H activity. Peaks B, C, and D all contain, together with DNA polymerase alpha, activities of primase and DNA topoisomerase type II. Furthermore, peak B is enriched in an RNase H, and peaks C and D are enriched in a 3'-5' exonuclease. DNA methylase (DNA methyltransferase) was preferentially identified in peaks C and D. Velocity sedimentation analyses of the four peaks gave evidence of unexpectedly large forms of DNA polymerase alpha (greater than 11.3 s), indicating that copurification of the above putative replication enzymes is not fortuitous. With moderate and high concentrations of salt, enzyme activities cosedimented with DNA polymerase alpha. Peak C is more resistant to inhibition by salt and spermidine than the other three enzyme forms. These results suggest the existence of a leading strand replicase (peak A) and several lagging strand replicase forms (peaks B, C, and D). Finally, the salt-resistant C form might represent a functional DNA polymerase alpha holoenzyme, possibly fitting in a higher-order structure, such as the replisome or even the chromatin.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert W., Grummt F., Hübscher U., Wilson S. H. Structural homology among calf thymus alpha-polymerase polypeptides. Nucleic Acids Res. 1982 Feb 11;10(3):935–946. doi: 10.1093/nar/10.3.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkower I., Leis J., Hurwitz J. Isolation and characterization of an endonuclease from Escherichia coli specific for ribonucleic acid in ribonucleic acid-deoxyribonucleic acid hybrid structures. J Biol Chem. 1973 Sep 10;248(17):5914–5921. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burgers P. M., Kornberg A. ATP activation of DNA polymerase III holoenzyme of Escherichia coli. I. ATP-dependent formation of an initiation complex with a primed template. J Biol Chem. 1982 Oct 10;257(19):11468–11473. [PubMed] [Google Scholar]
- Byrnes J. J., Downey K. M., Que B. G., Lee M. Y., Black V. L., So A. G. Selective inhibition of the 3' to 5' exonuclease activity associated with DNA polymerases: a mechanism of mutagenesis. Biochemistry. 1977 Aug 23;16(17):3740–3746. doi: 10.1021/bi00636a002. [DOI] [PubMed] [Google Scholar]
- Ciomei M., Spadari S., Pedrali-Noy G., Ciarrocchi G. Structural alterations of pathologically or physiologically modified DNA. Nucleic Acids Res. 1984 Feb 24;12(4):1977–1989. doi: 10.1093/nar/12.4.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaway R. C., Lehman I. R. A DNA primase activity associated with DNA polymerase alpha from Drosophila melanogaster embryos. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2523–2527. doi: 10.1073/pnas.79.8.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Chen J. T., Korn D. Enzymological characterization of KB cell DNA polymerase-alpha. Regulation of template binding by nucleic acid base composition. J Biol Chem. 1981 Jan 10;256(1):133–141. [PubMed] [Google Scholar]
- Fuller R. S., Kaguni J. M., Kornberg A. Enzymatic replication of the origin of the Escherichia coli chromosome. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7370–7374. doi: 10.1073/pnas.78.12.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse F., Krauss G. Purification of a 9S DNA polymerase alpha species from calf thymus. Biochemistry. 1981 Sep 15;20(19):5470–5475. doi: 10.1021/bi00522a019. [DOI] [PubMed] [Google Scholar]
- Hübscher U., Gerschwiler P., McMaster G. K. A mammalian DNA polymerase alpha holoenzyme functioning on defined in vivo-like templates. EMBO J. 1982;1(12):1513–1519. doi: 10.1002/j.1460-2075.1982.tb01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U., Kornberg A. The delta subunit of Escherichia coli DNA polymerase III holoenzyme is the dnaX gene product. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6284–6288. doi: 10.1073/pnas.76.12.6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübscher U. The mammalian primase is part of a high molecular weight DNA polymerase alpha polypeptide. EMBO J. 1983;2(1):133–136. doi: 10.1002/j.1460-2075.1983.tb01394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low R. L., Arai K., Kornberg A. Conservation of the primosome in successive stages of phi X174 DNA replication. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1436–1440. doi: 10.1073/pnas.78.3.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. A prepriming DNA replication enzyme of Escherichia coli. I. Purification of protein n': a sequence-specific, DNA-dependent ATPase. J Biol Chem. 1980 Jul 25;255(14):6789–6793. [PubMed] [Google Scholar]
- Zimmerman S. B., Levin C. J. A deoxyribonucleic acid ligase from nuclei of rat liver. Purification and properties. J Biol Chem. 1975 Jan 10;250(1):149–155. [PubMed] [Google Scholar]