Abstract
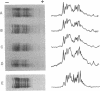
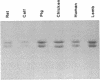
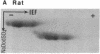
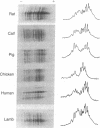
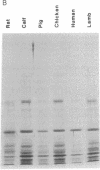
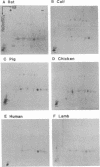
We have examined the extent of brain tubulin heterogeneity in six vertebrate species commonly used in tubulin research (rat, calf, pig, chicken, human, and lamb) using isoelectric focusing, two-dimensional electrophoresis, and peptide mapping procedures that provide higher resolution than previously available. The extent of heterogeneity is extremely similar in all of these organisms, as judged by number, range of isoelectric points, and distribution of the isotubulins. A minimum of 6 alpha and 12 beta tubulins was resolved from all sources. Even the pattern of spots on two-dimensional peptide maps is remarkably similar. These similarities suggest that the populations of tubulin in all of these brains should have similar overall physical properties. It is particularly interesting that chicken, which has only four or five beta-tubulin genes, contains approximately 12 beta tubulins. Thus, post-translational modification must generate at least some of the tubulin heterogeneity. Mammalian species, which contain 15-20 tubulin DNA sequences, do not show any more tubulin protein heterogeneity than does chicken. This suggests that expression of only a small number of the mammalian genes may be required to generate the observed tubulin heterogeneity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argaraña C. E., Arce C. A., Barra H. S., Caputto R. In vivo incorporation of [14C]tyrosine into the C-terminal position of the alpha subunit of tubulin. Arch Biochem Biophys. 1977 Apr 30;180(2):264–268. doi: 10.1016/0003-9861(77)90037-6. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Hinkle P. M. Thyroid and brain microtubules: a comparison. J Biol Chem. 1980 Apr 10;255(7):3186–3193. [PubMed] [Google Scholar]
- Bibring T., Baxandall J., Denslow S., Walker B. Heterogeneity of the alpha subunit of tubulin and the variability of tubulin within a single organism. J Cell Biol. 1976 May;69(2):301–312. doi: 10.1083/jcb.69.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond J. F., Farmer S. R. Regulation of tubulin and actin mRNA production in rat brain: expression of a new beta-tubulin mRNA with development. Mol Cell Biol. 1983 Aug;3(8):1333–1342. doi: 10.1128/mcb.3.8.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunke K. J., Collis P. S., Weeks D. P. Post-translational modification of tubulin dependent on organelle assembly. Nature. 1982 Jun 10;297(5866):516–518. doi: 10.1038/297516a0. [DOI] [PubMed] [Google Scholar]
- Bryan R. N., Bossinger J., Hayashi M. Tubulin and actin synthesis during brain development. Dev Biol. 1981 Jan 30;81(2):349–355. doi: 10.1016/0012-1606(81)90299-2. [DOI] [PubMed] [Google Scholar]
- Burland T. G., Gull K., Schedl T., Boston R. S., Dove W. F. Cell type-dependent expression of tubulins in Physarum. J Cell Biol. 1983 Dec;97(6):1852–1859. doi: 10.1083/jcb.97.6.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W. The tubulins: from DNA to RNA to protein and back again. Cell. 1983 Sep;34(2):330–332. doi: 10.1016/0092-8674(83)90366-5. [DOI] [PubMed] [Google Scholar]
- Dahl J. L., Weibel V. J. Changes in tubulin heterogeneity during postnatal development of rat brain. Biochem Biophys Res Commun. 1979 Feb 14;86(3):822–828. doi: 10.1016/0006-291x(79)91786-8. [DOI] [PubMed] [Google Scholar]
- Denoulet P., Edde B., Jeantet C., Gros F. Evolution of tubulin heterogeneity during mouse brain development. Biochimie. 1982 Mar;64(3):165–172. doi: 10.1016/s0300-9084(82)80466-5. [DOI] [PubMed] [Google Scholar]
- Denoulet P., Jeantet C., Gros F. Tubulin microheterogeneity during mouse liver development. Biochem Biophys Res Commun. 1982 Apr 14;105(3):806–813. doi: 10.1016/0006-291x(82)91041-5. [DOI] [PubMed] [Google Scholar]
- Doenges K. H., Weissinger M., Fritzsche R., Schroeter D. Assembly of nonneural microtubules in the absence of glycerol and microtubule-associated proteins. Biochemistry. 1979 May 1;18(9):1698–1702. doi: 10.1021/bi00576a010. [DOI] [PubMed] [Google Scholar]
- Edde B., Portier M. M., Sahuquillo C., Jeantet C., Gros F. Changes in some cytoskeletal proteins during neuroblastoma cell differentiation. Biochimie. 1982 Feb;64(2):141–151. doi: 10.1016/s0300-9084(82)80416-1. [DOI] [PubMed] [Google Scholar]
- Eddé B., Jeantet C., Gros F. One beta-tubulin subunit accumulates during neurite outgrowth in mouse neuroblastoma cells. Biochem Biophys Res Commun. 1981 Dec 15;103(3):1035–1043. doi: 10.1016/0006-291x(81)90913-x. [DOI] [PubMed] [Google Scholar]
- Eipper B. A. Rat brain microtubule protein: purification and determination of covalently bound phosphate and carbohydrate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2283–2287. doi: 10.1073/pnas.69.8.2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feit H., Shelanski M. L. Is tubulin a glycoprotein? Biochem Biophys Res Commun. 1975 Oct 6;66(3):920–927. doi: 10.1016/0006-291x(75)90728-7. [DOI] [PubMed] [Google Scholar]
- Feit H., Slusarek L., Shelanski M. L. Heterogeneity of tubulin subunits. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2028–2031. doi: 10.1073/pnas.68.9.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgue S. T., Dahl J. L. Rat brain tubulin: subunit heterogeneity and phosphorylation. J Neurochem. 1979 Mar;32(3):1015–1025. doi: 10.1111/j.1471-4159.1979.tb04588.x. [DOI] [PubMed] [Google Scholar]
- Gallo J. M., Anderton B. H. A subpopulation of trypanosome microtubules recognized by a monoclonal antibody to tubulin. EMBO J. 1983;2(4):479–483. doi: 10.1002/j.1460-2075.1983.tb01450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George H. J., Misra L., Field D. J., Lee J. C. Polymorphism of brain tubulin. Biochemistry. 1981 Apr 28;20(9):2402–2409. doi: 10.1021/bi00512a006. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., Behar L., Givol D., Littauer U. Z. The nucleotide sequence of rat alpha-tubulin: 3'-end characteristics, and evolutionary conservation. Nucleic Acids Res. 1981 Jun 25;9(12):2691–2697. doi: 10.1093/nar/9.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., Saya D., Littauer U. Z. Tubulin microheterogeneity in neuroblastoma and glioma cell lines differs from that of the brain. Brain Res. 1979 Jul 27;171(1):171–175. doi: 10.1016/0006-8993(79)90746-7. [DOI] [PubMed] [Google Scholar]
- Gozes I., de Baetselier A., Littauer U. Z. Translation in vitro of rat brain mRNA coding for a variety of tubulin forms. Eur J Biochem. 1980 Jan;103(1):13–20. doi: 10.1111/j.1432-1033.1980.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto F., Horigome T., Kanbayashi M., Yoshida K., Sugano H. An improved method for separation of low-molecular-weight polypeptides by electrophoresis in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1983 Feb 15;129(1):192–199. doi: 10.1016/0003-2697(83)90068-4. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. Developmental regulation of Drosophila alpha-tubulin genes. Cell. 1982 May;29(1):91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Raff R. A., Kaufman T. C., Raff E. C. Mutation in a structural gene for a beta-tubulin specific to testis in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3991–3995. doi: 10.1073/pnas.76.8.3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N., Flavin M. Preferential action of a brain detyrosinolating carboxypeptidase on polymerized tubulin. J Biol Chem. 1981 Jul 25;256(14):7678–7686. [PubMed] [Google Scholar]
- L'Hernault S. W., Rosenbaum J. L. Chlamydomonas alpha-tubulin is posttranslationally modified in the flagella during flagellar assembly. J Cell Biol. 1983 Jul;97(1):258–263. doi: 10.1083/jcb.97.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lemischka I. R., Farmer S., Racaniello V. R., Sharp P. A. Nucleotide sequence and evolution of a mammalian alpha-tubulin messenger RNA. J Mol Biol. 1981 Sep 5;151(1):101–120. doi: 10.1016/0022-2836(81)90223-0. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Little M. Identification of a second beta chain in pig brain tubulin. FEBS Lett. 1979 Dec 1;108(1):283–286. doi: 10.1016/0014-5793(79)81229-6. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Lu R. C., Elzinga M. Chromatographic resolution of the subunits of calf brain tubulin. Anal Biochem. 1977 Jan;77(1):243–250. doi: 10.1016/0003-2697(77)90310-4. [DOI] [PubMed] [Google Scholar]
- Margolis R. K., Margolis R. U., Shelanski M. L. The carbohydrate composition of brain microtubule protein. Biochem Biophys Res Commun. 1972 Apr 28;47(2):432–437. doi: 10.1016/0006-291x(72)90732-2. [DOI] [PubMed] [Google Scholar]
- Marotta C. A., Harris J. L., Gilbert J. M. Characterization of multiple forms of brain tubulin subunits. J Neurochem. 1978 Jun;30(6):1431–1440. doi: 10.1111/j.1471-4159.1978.tb10475.x. [DOI] [PubMed] [Google Scholar]
- McKeithan T. W., Lefebvre P. A., Silflow C. D., Rosenbaum J. L. Multiple forms of tubulin in Polytomella and Chlamydomonas: evidence for a precursor of flagellar alpha-tubulin. J Cell Biol. 1983 Apr;96(4):1056–1063. doi: 10.1083/jcb.96.4.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeithan T. W., Rosenbaum J. L. Multiple forms of tubulin in the cytoskeletal and flagellar microtubules of Polytomella. J Cell Biol. 1981 Nov;91(2 Pt 1):352–360. doi: 10.1083/jcb.91.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischke D., Pardue M. L. Organization and expression of alpha-tubulin genes in Drosophila melanogaster. One member of the alpha-tubulin multigene family is transcribed in both oogenesis and later embryonic development. J Mol Biol. 1982 Apr 15;156(3):449–466. doi: 10.1016/0022-2836(82)90260-1. [DOI] [PubMed] [Google Scholar]
- Moura Neto V., Mallat M., Jeantet C., Prochiantz A. Microheterogeneity of tubulin proteins in neuronal and glial cells from the mouse brain in culture. EMBO J. 1983;2(8):1243–1248. doi: 10.1002/j.1460-2075.1983.tb01576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Hiebsch R. R. Purification of microtubule protein from beef brain and comparison of the assembly requirements for neuronal microtubules isolated from beef and hog. Anal Biochem. 1979 Jul 1;96(1):225–235. doi: 10.1016/0003-2697(79)90577-3. [DOI] [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T. Brain and erythrocyte microtubules from chicken contain different beta-tubulin polypeptides. J Biol Chem. 1983 Jun 25;258(12):7870–7875. [PubMed] [Google Scholar]
- Nelles L. P., Bamburg J. R. Comparative peptide mapping and isoelectric focusing of isolated subunits from chick embryo brain tubulin. J Neurochem. 1979 Feb;32(2):477–489. doi: 10.1111/j.1471-4159.1979.tb00374.x. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff E. C., Fuller M. T., Kaufman T. C., Kemphues K. J., Rudolph J. E., Raff R. A. Regulation of tubulin gene expression during embryogenesis in Drosophila melanogaster. Cell. 1982 Jan;28(1):33–40. doi: 10.1016/0092-8674(82)90372-5. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Wilde C. D., Crowther C. E., Cripe T. P., Gwo-Shu Lee M., Cowan N. J. Evidence that a human beta-tubulin pseudogene is derived from its corresponding mRNA. Nature. 1982 May 6;297(5861):83–84. doi: 10.1038/297083a0. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lee J. C. Preparation of tubulin from brain. Methods Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- Wolff A., Denoulet P., Jeantet C. High level of tubulin microheterogeneity in the mouse brain. Neurosci Lett. 1982 Aug 31;31(3):323–328. doi: 10.1016/0304-3940(82)90041-6. [DOI] [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]
- von Hungen K., Chin R. C., Baxter C. F. Brain tubulin microheterogeneity in the mouse during development and aging. J Neurochem. 1981 Aug;37(2):511–514. doi: 10.1111/j.1471-4159.1981.tb00485.x. [DOI] [PubMed] [Google Scholar]