Abstract
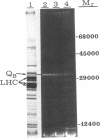
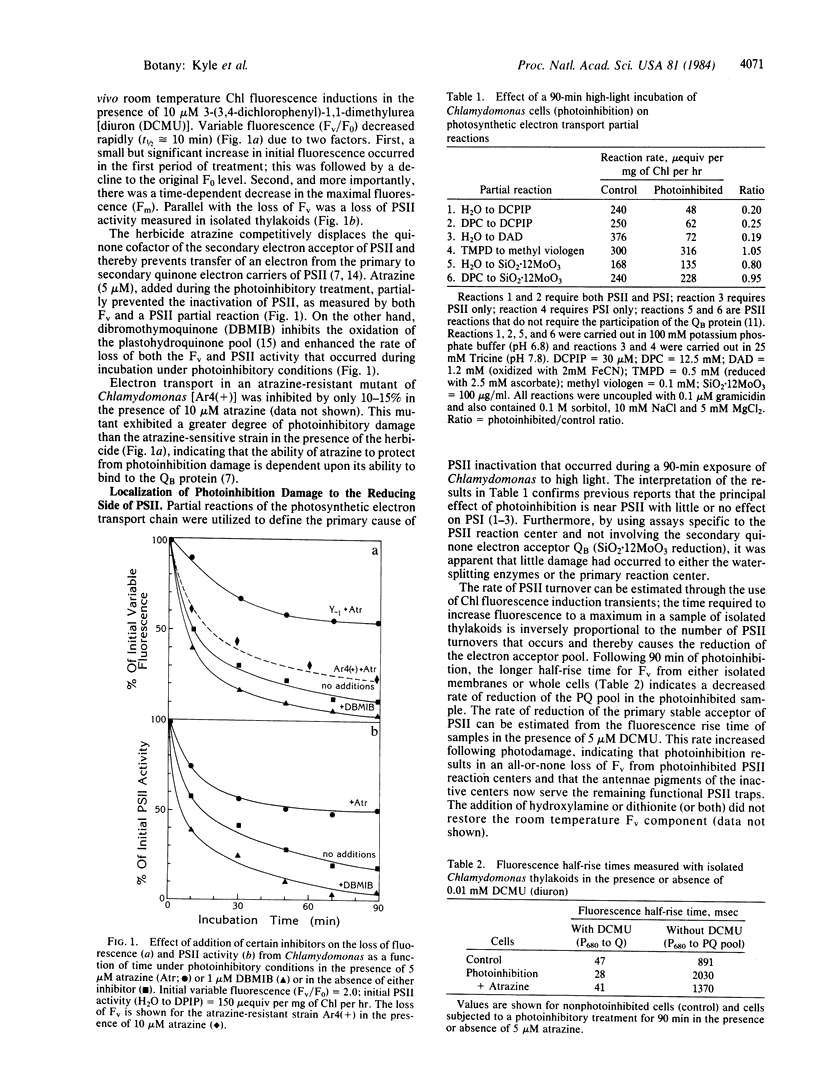
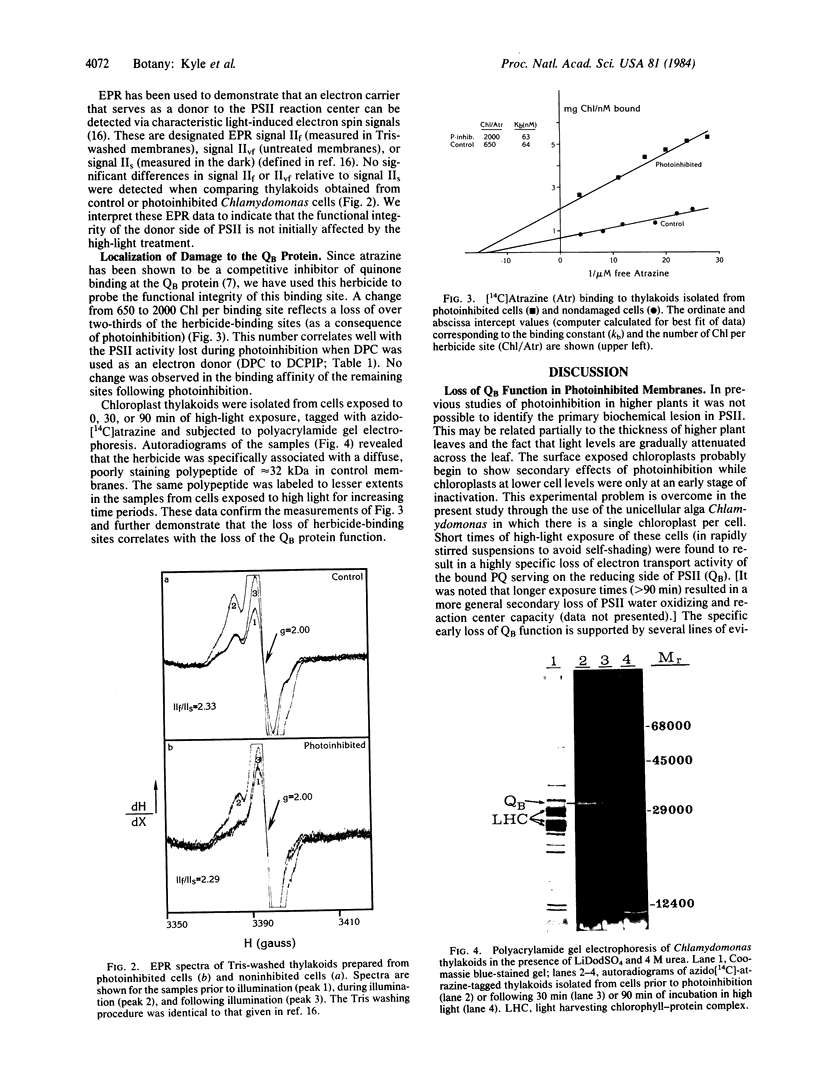
A loss of electron transport capacity in chloroplast membranes was induced by high-light intensities (photoinhibition). The primary site of inhibition was at the reducing side of photosystem II (PSII) with little damage to the oxidizing side or to the reaction center core of PSII. Addition of herbicides (atrazine or diuron) partially protected the membrane from photoinhibition; these compounds displace the bound plastoquinone (designated as QB), which functions as the secondary electron acceptor on the reducing side of PSII. Loss of function of the 32-kilodalton QB apoprotein was demonstrated by a loss of binding sites for [14C]atrazine. We suggest that quinone anions, which may interact with molecular oxygen to produce an oxygen radical, selectively damage the apoprotein of the secondary acceptor of PSII, thus rendering it inactive and thereby blocking photosynthetic electron flow under conditions of high photon flux densities.
Keywords: photoinhibition, Chlamydomonas, herbicide-binding protein, photosynthesis, triazine herbicide
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babcock G. T., Sauer K. A rapid, light-induced transient in electron paramagnetic resonance signal II activated upon inhibition of photosynthetic oxygen evolution. Biochim Biophys Acta. 1975 Feb 17;376(2):315–328. doi: 10.1016/0005-2728(75)90024-9. [DOI] [PubMed] [Google Scholar]
- Barr R., Crane F. L., Giaquinta R. T. Dichlorophenylurea-insensitive Reduction of Silicomolybdic Acid by Chloroplast Photosystem II. Plant Physiol. 1975 Mar;55(3):460–462. doi: 10.1104/pp.55.3.460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley C. Studies on the Mechanism of Photoinhibition in Higher Plants: I. EFFECTS OF HIGH LIGHT INTENSITY ON CHLOROPLAST ACTIVITIES IN CUCUMBER ADAPTED TO LOW LIGHT. Plant Physiol. 1981 Jun;67(6):1161–1165. doi: 10.1104/pp.67.6.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman-Falk H., Mattoo A. K., Marder J. B., Edelman M., Ellis R. J. General occurrence and structural similarity of the rapidly synthesized, 32,000-dalton protein of the chloroplast membrane. J Biol Chem. 1982 Apr 25;257(8):4583–4587. [PubMed] [Google Scholar]
- Horton P., Whitmarsh J., Cramer W. A. On the specific site of action of 3-)3,4-dichlorophenyl)-1,1-dimethylurea in chloroplasts: inhibiion of a dark acid-induced decrease in midpoint potential of cytochrome b-559. Arch Biochem Biophys. 1976 Oct;176(2):519–524. doi: 10.1016/0003-9861(76)90195-8. [DOI] [PubMed] [Google Scholar]
- Klimov V. V., Klevanik A. V., Shuvalov V. A., Kransnovsky A. A. Reduction of pheophytin in the primary light reaction of photosystem II. FEBS Lett. 1977 Oct 15;82(2):183–186. doi: 10.1016/0014-5793(77)80580-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ohad I., Siekevitz P., Palade G. E. Biogenesis of chloroplast membranes. II. Plastid differentiation during greening of a dark-grown algal mutant (Chlamydomonas reinhardi). J Cell Biol. 1967 Dec;35(3):553–584. doi: 10.1083/jcb.35.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinback K. E., McIntosh L., Bogorad L., Arntzen C. J. Identification of the triazine receptor protein as a chloroplast gene product. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7463–7467. doi: 10.1073/pnas.78.12.7463. [DOI] [PMC free article] [PubMed] [Google Scholar]