Abstract
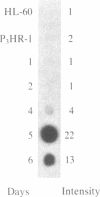
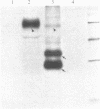
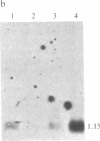
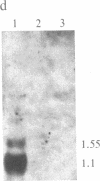
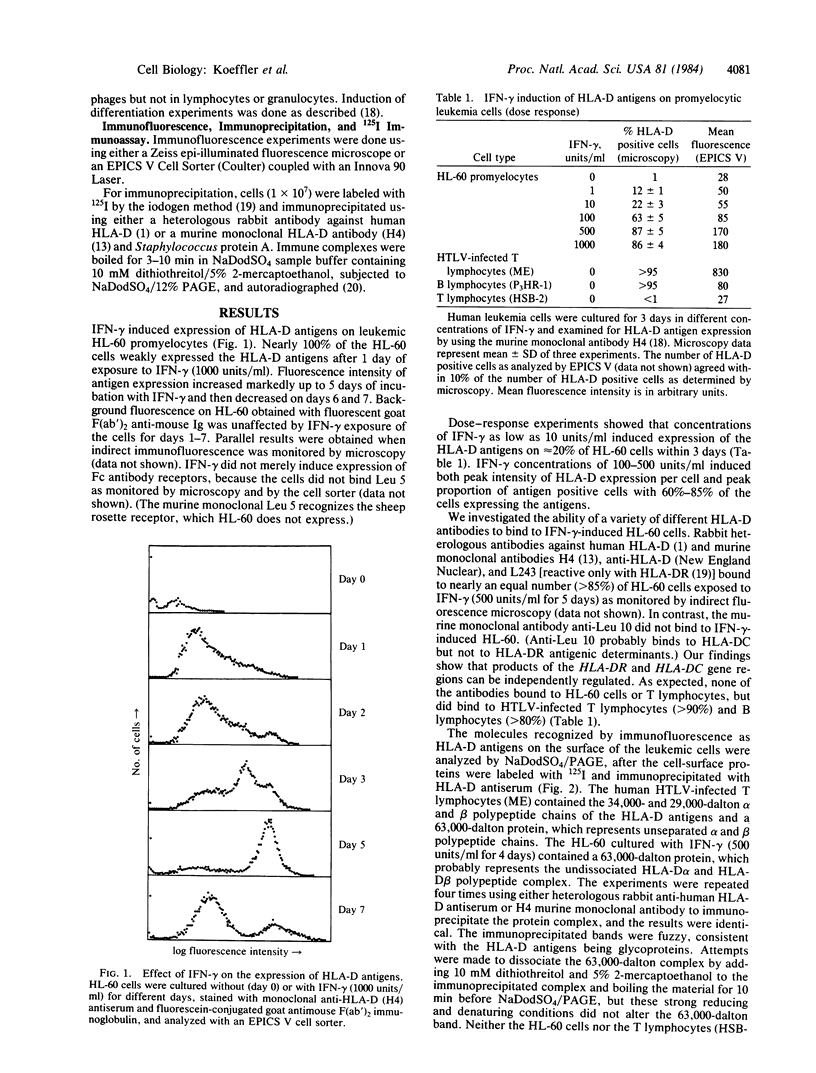
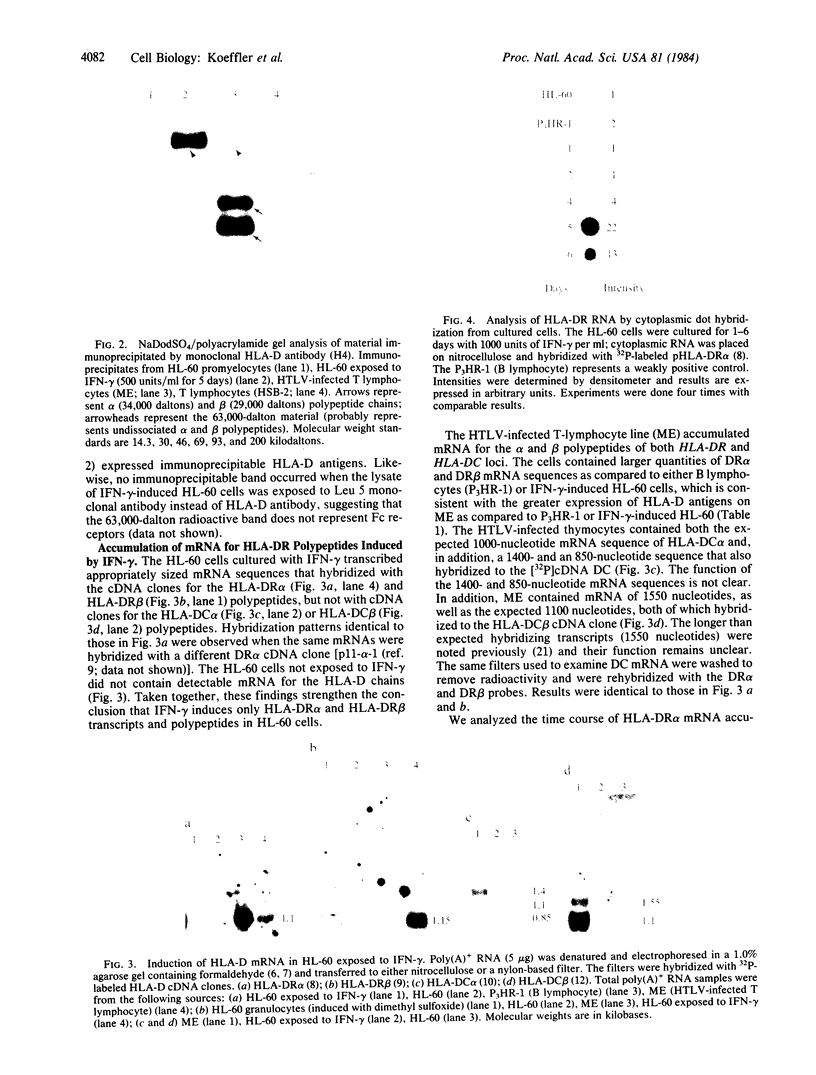
Gamma-Interferon (IFN-gamma) is a lymphokine produced by T lymphocytes. We find that recombinant human IFN-gamma induces expression of HLA-D antigens on human promyelocytic leukemia cells (HL-60) and enhances expression of HLA-D antigens on normal human monocytes and macrophages. Induction of both HLA-D antigen expression and HLA-D mRNA accumulation occurs within 1 day of exposure of HL-60 to IFN-gamma and is maximal by day 5. Maximal antigen expression occurs in the presence of 100-500 units of IFN-gamma per ml. IFN-gamma induces expression of DR but not DC antigens on HL-60, as confirmed by using four different murine monoclonal antibodies or one rabbit heterologous antibody. RNA blot data show that IFN-gamma-exposed HL-60 cells contain mRNA for DR alpha and DR beta polypeptides but not for DC alpha and DC beta polypeptides, which also suggests that HLA-DR and HLA-DC gene regions can be independently regulated. IFN-gamma induces 20% of HL-60 cells to differentiate to macrophage-like cells. However, IFN-gamma dose-response studies using both HL-60 cells and a nondifferentiation variant of HL-60 cells (HL-60 blast) clearly show that induction of transcription and expression of HLA-D gene products by IFN-gamma can be uncoupled from expression of other monocyte-macrophage characteristics. Further studies show that IFN-gamma enhances expression of HLA-D antigens on normal human monocytes and macrophages. Expression of the HLA-D antigens is necessary for the interaction of macrophages and T lymphocytes; IFN-gamma may play a fundamental role in this interaction.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Korman A. J., Roux-Dosseto M., Bono R., Strominger J. L. cDNA clone for the heavy chain of the human B cell alloantigen DC1: strong sequence homology to the HLA-DR heavy chain. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6337–6341. doi: 10.1073/pnas.79.20.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Billing R., Rafizadeh B., Drew I., Hartman G., Gale R., Terasaki P. Human B-lymphocyte antigens expressed by lymphocytic and myelocytic leukemia cells. I. Detection by rabbit antisera. J Exp Med. 1976 Jul 1;144(1):167–178. doi: 10.1084/jem.144.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Giphart M. J., Kaufman J. F., Fuks A., Albrechtsen D., Solheim B. G., Bruning J. W., Strominger J. L. HLA-D associated alloantisera react with molecules similar to Ia antigens. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3533–3536. doi: 10.1073/pnas.74.8.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin J., Munroe D., Major P., Kufe D. Induction of differentiation of human myeloid leukemia cells by inhibitors of DNA synthesis. Exp Hematol. 1982 Oct;10(9):774–781. [PubMed] [Google Scholar]
- Hocking W., Billing R., Foon K., Golde D. Human alveolar macrophages express Ia-like antigens. Blood. 1981 Nov;58(5):1040–1042. [PubMed] [Google Scholar]
- Kelley V. E., Fiers W., Strom T. B. Cloned human interferon-gamma, but not interferon-beta or -alpha, induces expression of HLA-DR determinants by fetal monocytes and myeloid leukemic cell lines. J Immunol. 1984 Jan;132(1):240–245. [PubMed] [Google Scholar]
- Koeffler H. P., Bar-Eli M., Territo M. C. Phorbol ester effect on differentiation of human myeloid leukemia cell lines blocked at different stages of maturation. Cancer Res. 1981 Mar;41(3):919–926. [PubMed] [Google Scholar]
- Koeffler H. P., Billing R., Levine A. M., Golde D. W. Ia antigen is a differentiation marker on human eosinophils. Blood. 1980 Jul;56(1):11–14. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Levy R. Two populations of Ia-like molecules on a human B cell line. J Immunol. 1980 Jul;125(1):293–299. [PubMed] [Google Scholar]
- Larhammar D., Gustafsson K., Claesson L., Bill P., Wiman K., Schenning L., Sundelin J., Widmark E., Peterson P. A., Rask L. Alpha chain of HLA-DR transplantation antigens is a member of the same protein superfamily as the immunoglobulins. Cell. 1982 Aug;30(1):153–161. doi: 10.1016/0092-8674(82)90021-6. [DOI] [PubMed] [Google Scholar]
- Larhammar D., Schenning L., Gustafsson K., Wiman K., Claesson L., Rask L., Peterson P. A. Complete amino acid sequence of an HLA-DR antigen-like beta chain as predicted from the nucleotide sequence: similarities with immunoglobulins and HLA-A, -B, and -C antigens. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3687–3691. doi: 10.1073/pnas.79.12.3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Trowsdale J., Bodmer W. F. cDNA clones coding for the heavy chain of human HLA-DR antigen. Proc Natl Acad Sci U S A. 1982 Jan;79(2):545–549. doi: 10.1073/pnas.79.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- Long E. O., Wake C. T., Strubin M., Gross N., Accolla R. S., Carrel S., Mach B. Isolation of distinct cDNA clones encoding HLA-DR beta chains by use of an expression assay. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7465–7469. doi: 10.1073/pnas.79.23.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Scher M. G., Beller D. I., Unanue E. R. Demonstration of a soluble mediator that induces exudates rich in Ia-positive macrophages. J Exp Med. 1980 Dec 1;152(6):1684–1698. doi: 10.1084/jem.152.6.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlossman S. F., Chess L., Humphreys R. E., Strominger J. L. Distribution of Ia-like molecules on the surface of normal and leukemic human cells. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1288–1292. doi: 10.1073/pnas.73.4.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Johnson H. M., Oppenheim J. J. Regulation of murine macrophage Ia antigen expression by a lymphokine with immune interferon activity. J Exp Med. 1982 Dec 1;156(6):1780–1793. doi: 10.1084/jem.156.6.1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Nogueira N., Witmer M. D., Tydings J. D., Mellman I. S. Lymphokine enhances the expression and synthesis of Ia antigens on cultured mouse peritoneal macrophages. J Exp Med. 1980 Nov 1;152(5):1248–1261. doi: 10.1084/jem.152.5.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake C. T., Long E. O., Strubin M., Gross N., Accolla R., Carrel S., Mach B. Isolation of cDNA clones encoding HLA-DR alpha chains. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6979–6983. doi: 10.1073/pnas.79.22.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]