Abstract
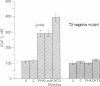
The human T-cell leukemia, Jurkat, and a T3-negative mutant of Jurkat (S.5) were used to study the role of T3 in human T-cell activation. Incubation of Jurkat with phytohemagglutinin (PHA) resulted in the production of interleukin 2, which was markedly increased by the addition of phorbol 12-myristate 13-acetate (PMA). Antibodies reactive with T3 could activate Jurkat only if added together with PMA. However, S.5 cells failed to produce interleukin 2 in response to PHA and produced 1/16th the interleukin 2 activity that Jurkat produced in response to PHA and PMA. Incubation of S.5 cells with the calcium ionophore A23187 and PMA resulted in the production of interleukin 2 activity comparable to that produced by Jurkat. Like antibodies reactive with T3, A23187 demonstrated an obligate requirement for PMA in order to activate Jurkat or S.5. These observations suggested that T3 might participate in T-cell activation through mechanisms that increase intracellular Ca2+. This was examined by using the Ca2+ sensitive fluor, quin-2, to measure levels of cytoplasmic free Ca2+ [( Ca2+]i). Addition of PHA, A23187, or monoclonal antibodies reactive with T3 to Jurkat cells resulted in substantial increases of [Ca2+]i. In contrast, only A23187 could induce an increase in [Ca2+]i in S.5 cells. Three other monoclonal antibodies reactive with other membrane antigens expressed on Jurkat or S.5 did not increase [Ca2+]i. These results suggest that T3 and/or associated molecules participate in T-cell activation through mechanisms that lead to increases in [Ca2+]i and that their expression is a relative requirement for T-cell activation by PHA.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borst J., Prendiville M. A., Terhorst C. The T3 complex on human thymus-derived lymphocytes contains two different subunits of 20 kDa. Eur J Immunol. 1983 Jul;13(7):576–580. doi: 10.1002/eji.1830130712. [DOI] [PubMed] [Google Scholar]
- Chang T. W., Kung P. C., Gingras S. P., Goldstein G. Does OKT3 monoclonal antibody react with an antigen-recognition structure on human T cells? Proc Natl Acad Sci U S A. 1981 Mar;78(3):1805–1808. doi: 10.1073/pnas.78.3.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang T. W., Testa D., Kung P. C., Perry L., Dreskin H. J., Goldstein G. Cellular origin and interactions involved in gamma-interferon production induced by OKt3 monoclonal antibody. J Immunol. 1982 Feb;128(2):585–589. [PubMed] [Google Scholar]
- Charest R., Blackmore P. F., Berthon B., Exton J. H. Changes in free cytosolic Ca2+ in hepatocytes following alpha 1-adrenergic stimulation. Studies on Quin-2-loaded hepatocytes. J Biol Chem. 1983 Jul 25;258(14):8769–8773. [PubMed] [Google Scholar]
- Gershengorn M. C., Thaw C. Calcium influx is not required for TRH to elevate free cytoplasmic calcium in GH3 cells. Endocrinology. 1983 Oct;113(4):1522–1524. doi: 10.1210/endo-113-4-1522. [DOI] [PubMed] [Google Scholar]
- Giles R. C., Hurley C. K., Capra J. D. Primary structural variation among serologically indistinguishable DS antigens: the MB3-bearing molecule in DR4 cells differs from the MB3-bearing molecule in DR5 cells. J Immunol. 1984 Jul;133(1):1–3. [PubMed] [Google Scholar]
- Gillis S., Watson J. Biochemical and biological characterization of lymphocyte regulatory molecules. V. Identification of an interleukin 2-producing human leukemia T cell line. J Exp Med. 1980 Dec 1;152(6):1709–1719. doi: 10.1084/jem.152.6.1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart P. A., Fisher R. I. Characterization of the lymphocyte surface receptors for Con A and PHA. J Immunol. 1975 Feb;114(2 Pt 1):710–714. [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Kanellopoulos J. M., Wigglesworth N. M., Owen M. J., Crumpton M. J. Biosynthesis and molecular nature of the T3 antigen of human T lymphocytes. EMBO J. 1983;2(10):1807–1814. doi: 10.1002/j.1460-2075.1983.tb01662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedel J. E., Kuhn L. J., Vandenbark G. R. Phorbol diester receptor copurifies with protein kinase C. Proc Natl Acad Sci U S A. 1983 Jan;80(1):36–40. doi: 10.1073/pnas.80.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Acuto O., Schlossman S. F. Comparison of T3-associated 49- and 43-kilodalton cell surface molecules on individual human T-cell clones: evidence for peptide variability in T-cell receptor structures. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4104–4108. doi: 10.1073/pnas.80.13.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Sanchez A., Hallam T. J. Diacylglycerol and phorbol ester stimulate secretion without raising cytoplasmic free calcium in human platelets. Nature. 1983 Sep 22;305(5932):317–319. doi: 10.1038/305317a0. [DOI] [PubMed] [Google Scholar]
- Tax W. J., Willems H. W., Reekers P. P., Capel P. J., Koene R. A. Polymorphism in mitogenic effect of IgG1 monoclonal antibodies against T3 antigen on human T cells. Nature. 1983 Aug 4;304(5925):445–447. doi: 10.1038/304445a0. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. T-cell mitogens cause early changes in cytoplasmic free Ca2+ and membrane potential in lymphocytes. Nature. 1982 Jan 7;295(5844):68–71. doi: 10.1038/295068a0. [DOI] [PubMed] [Google Scholar]
- Van Wauwe J. P., De Mey J. R., Goossens J. G. OKT3: a monoclonal anti-human T lymphocyte antibody with potent mitogenic properties. J Immunol. 1980 Jun;124(6):2708–2713. [PubMed] [Google Scholar]
- Venuta S., Mertelsmann R., Welte K., Feldman S. P., Wang C. Y., Moore M. A. Production and regulation of interleukin-2 in human lymphoblastic leukemias studied with T-cell monoclonal antibodies. Blood. 1983 Apr;61(4):781–789. [PubMed] [Google Scholar]
- van Agthoven A., Terhorst C., Reinherz E., Schlossman S. Characterization of T cell surface glycoproteins T 1 and T 3 present on all human peripheral T lymphocytes and functionally mature thymocytes. Eur J Immunol. 1981 Jan;11(1):18–21. doi: 10.1002/eji.1830110105. [DOI] [PubMed] [Google Scholar]