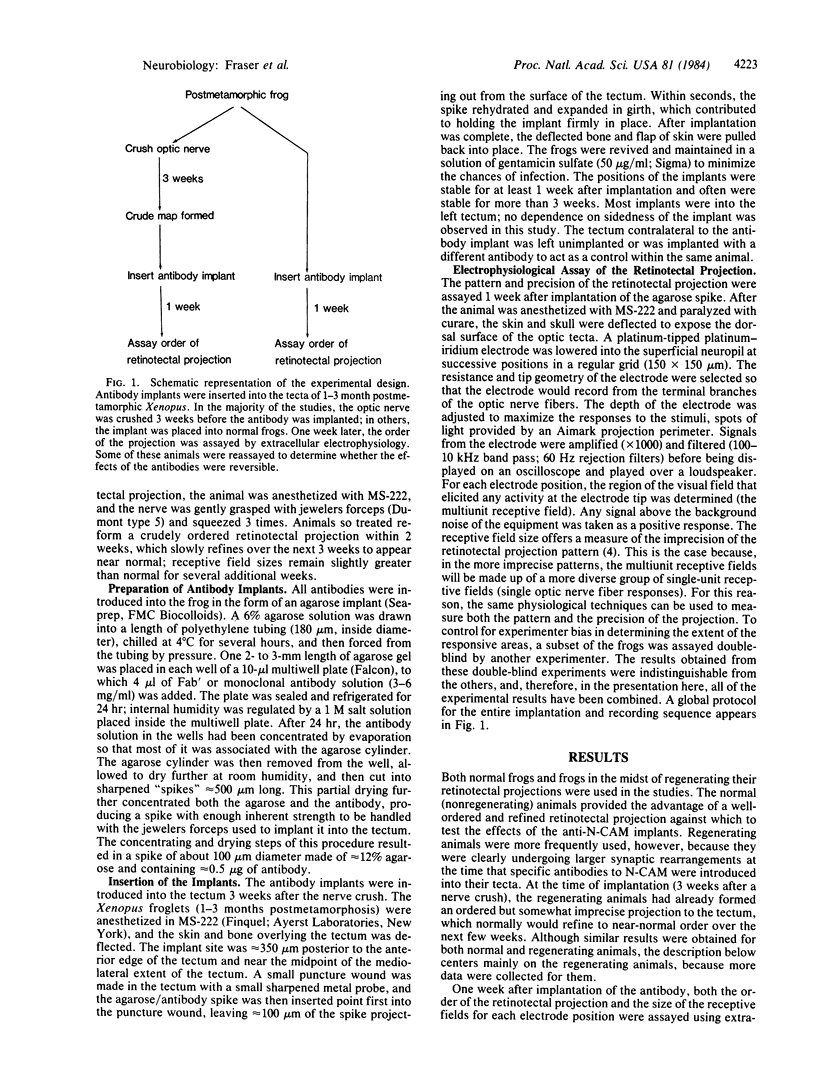
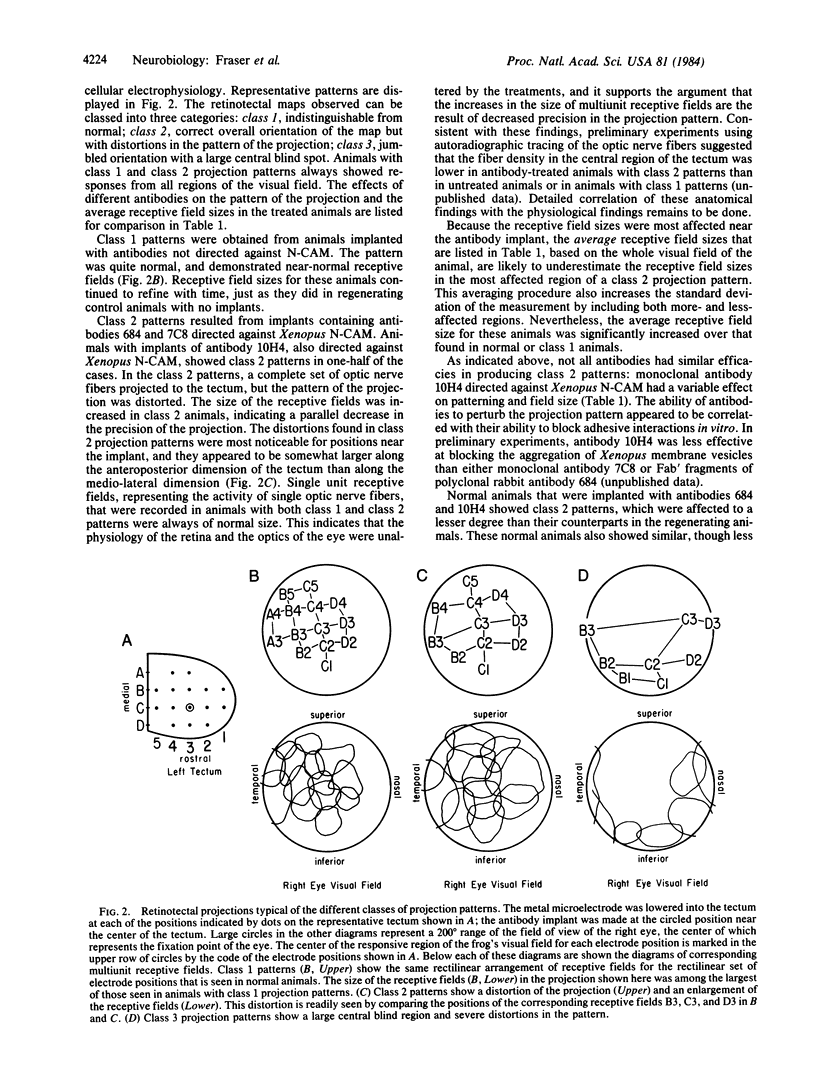
Abstract
The neural cell adhesion molecule (N-CAM) mediates neuron-neuron adhesion, is ubiquitous in the nervous system of developing and mature vertebrates, and undergoes major alterations in both amount and distribution during development. Perturbation of homophilic (N-CAM to N-CAM) binding by univalent fragments of specific anti-N-CAM antibodies has previously been found to alter neural tissue patterns in vitro. To show that significant alterations can also occur in vivo, antibodies to Xenopus N-CAM were embedded in agarose microcylinders and implanted in the tecta of juvenile Xenopus laevis frogs that were undergoing regeneration of their retinotectal projections; 1 week later, the effects of implantation on the projection pattern from the optic nerve were determined. Both polyclonal and monoclonal antibodies to N-CAM distorted the retinotectal projection pattern and greatly decreased the precision of the projection; these alterations recovered to near normal after an additional 3 weeks. Similar but smaller effects were obtained when normally developing froglets received tectal implants. In control animals, implants of immunoglobulins from preimmune serum and monoclonal antibodies not directed against N-CAM had little or no effect on the pattern. The results suggest that neuronal adhesion mediated by N-CAM is important in establishing and maintaining the precision and topography of neural patterns.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chuong C. M., McClain D. A., Streit P., Edelman G. M. Neural cell adhesion molecules in rodent brains isolated by monoclonal antibodies with cross-species reactivity. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4234–4238. doi: 10.1073/pnas.79.13.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Fraser S. E. Differential adhesion approach to the patterning of nerve connections. Dev Biol. 1980 Oct;79(2):453–464. doi: 10.1016/0012-1606(80)90130-x. [DOI] [PubMed] [Google Scholar]
- Fraser S. E. Fiber optic mapping of the Xenopus visual system: shift in the retinotectal projection during development. Dev Biol. 1983 Feb;95(2):505–511. doi: 10.1016/0012-1606(83)90053-2. [DOI] [PubMed] [Google Scholar]
- Fraser S. E., Hunt R. K. Retinotectal specificity: models and experiments in search of a mapping function. Annu Rev Neurosci. 1980;3:319–352. doi: 10.1146/annurev.ne.03.030180.001535. [DOI] [PubMed] [Google Scholar]
- Gallin W. J., Edelman G. M., Cunningham B. A. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci U S A. 1983 Feb;80(4):1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumet M., Edelman G. M. Heterotypic binding between neuronal membrane vesicles and glial cells is mediated by a specific cell adhesion molecule. J Cell Biol. 1984 May;98(5):1746–1756. doi: 10.1083/jcb.98.5.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris W. A. The effects of eliminating impulse activity on the development of the retinotectal projection in salamanders. J Comp Neurol. 1980 Nov 15;194(2):303–317. doi: 10.1002/cne.901940203. [DOI] [PubMed] [Google Scholar]
- Harris W. A. The transplantation of eyes to genetically eyeless salamanders: visual projections and somatosensory interactions. J Neurosci. 1982 Mar;2(3):339–353. doi: 10.1523/JNEUROSCI.02-03-00339.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman S., Edelman G. M. Kinetics of homophilic binding by embryonic and adult forms of the neural cell adhesion molecule. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5762–5766. doi: 10.1073/pnas.80.18.5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer R. L. Tetrodotoxin inhibits the formation of refined retinotopography in goldfish. Brain Res. 1983 Feb;282(3):293–298. doi: 10.1016/0165-3806(83)90068-8. [DOI] [PubMed] [Google Scholar]
- SPERRY R. W. CHEMOAFFINITY IN THE ORDERLY GROWTH OF NERVE FIBER PATTERNS AND CONNECTIONS. Proc Natl Acad Sci U S A. 1963 Oct;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. T., Edwards D. L. Activity sharpens the map during the regeneration of the retinotectal projection in goldfish. Brain Res. 1983 Jun 13;269(1):29–39. doi: 10.1016/0006-8993(83)90959-9. [DOI] [PubMed] [Google Scholar]
- Whitelaw V. A., Cowan J. D. Specificity and plasticity of retinotectal connections: a computational model. J Neurosci. 1981 Dec;1(12):1369–1387. doi: 10.1523/JNEUROSCI.01-12-01369.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]




