Abstract
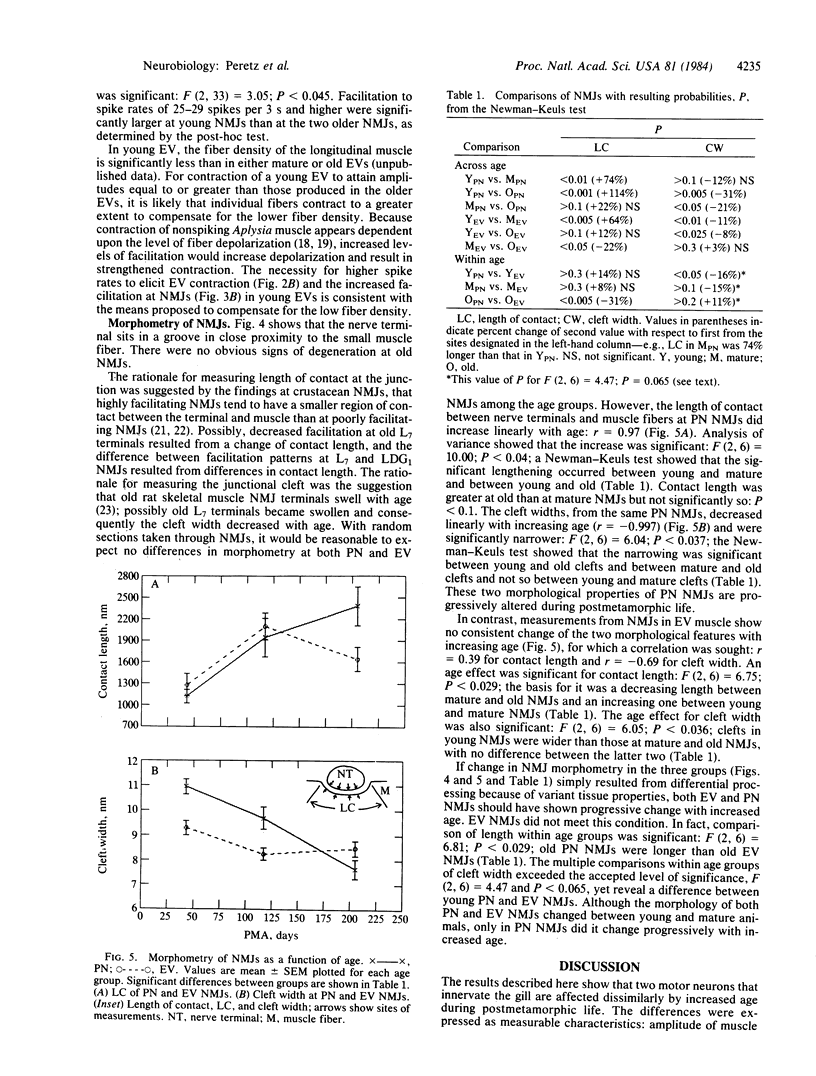
Because the viability of the gill withdrawal reflex is dependent on age in Aplysia, we examined physiologic and morphometric properties of two motor neurons, L7 and LDG1, involved in the reflex in three postmetamorphic age groups: young, mature, and old. L7's capability to elicit pinnule contraction, a major component of the reflex, was reduced markedly in old gills; facilitation at old L7 terminals, upon which contraction is dependent, was significantly reduced. The morphology of the pinnule neuromuscular junctions changed with increased age. In contrast, LDG1's capability to elicit efferent vessel contraction, a major component of respiratory movements, was not significantly altered by age; facilitation also was relatively unaffected; and morphologic changes at neuromuscular junctions were poorly correlated with age. Aging occurs differentially in two motor neurons innervating the gill. The dissimilarity in function and in frequency of activation of L7 and LDG1 may help explain the greater vulnerability of L7 to the effects of aging. The possibility that disuse is involved in the aging process is discussed.
Full text
PDF
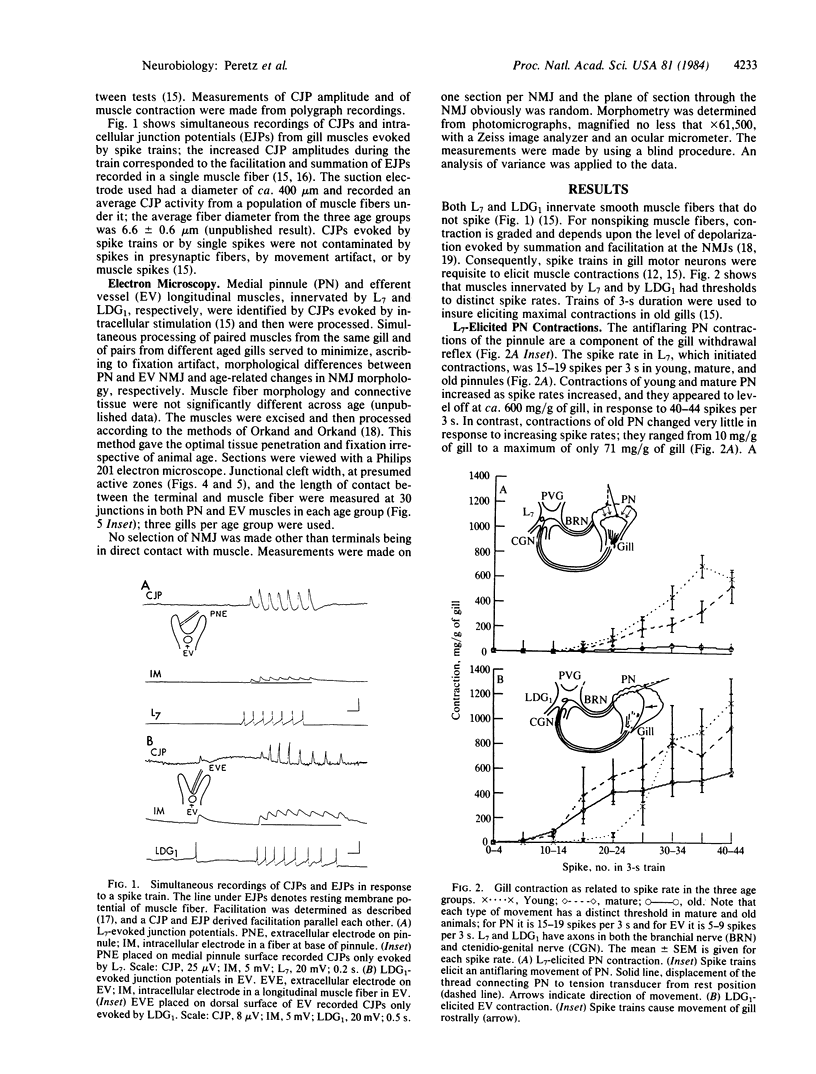
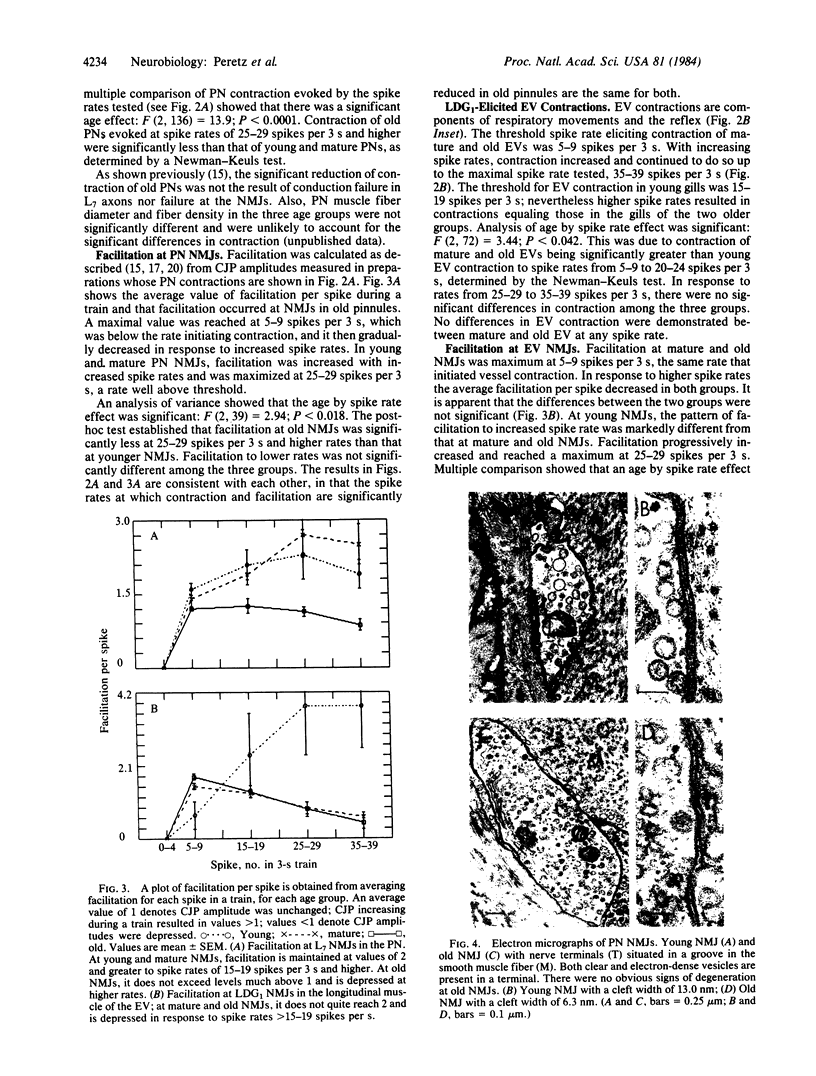

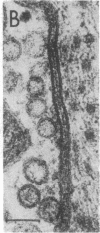
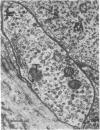
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwood H. L. Organization and synaptic physiology of crustacean neuromuscular systems. Prog Neurobiol. 1976;7(Pt 4):291–391. doi: 10.1016/0301-0082(76)90009-5. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Chen M. Morphological basis of long-term habituation and sensitization in Aplysia. Science. 1983 Apr 1;220(4592):91–93. doi: 10.1126/science.6828885. [DOI] [PubMed] [Google Scholar]
- Byrne J. H., Koester J. Respiratory pumping: neuronal control of a centrally commanded behavior in Aplysia. Brain Res. 1978 Mar 17;143(1):87–105. doi: 10.1016/0006-8993(78)90754-0. [DOI] [PubMed] [Google Scholar]
- Jacklet J. W., Rine J. Facilitation at neuromuscular junctions: contribution to habituation and dishabituation of the Aplysia gill withdrawal reflex. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1267–1271. doi: 10.1073/pnas.74.3.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriegstein A. R., Castellucci V., Kandel E. R. Metamorphosis of Aplysia californica in laboratory culture. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3654–3658. doi: 10.1073/pnas.71.9.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupfermann I., Carew T. J., Kandel E. R. Local, reflex, and central commands controlling gill and siphon movements in Aplysia. J Neurophysiol. 1974 Sep;37(5):996–1019. doi: 10.1152/jn.1974.37.5.996. [DOI] [PubMed] [Google Scholar]
- Lukowiak K. The development of central nervous system control of the gill withdrawal reflex evoked by siphon stimulation in Aplysia. Can J Physiol Pharmacol. 1979 Sep;57(9):987–997. doi: 10.1139/y79-148. [DOI] [PubMed] [Google Scholar]
- Magleby K. L. The effect of repetitive stimulation on facilitation of transmitter release at the frog neuromuscular junction. J Physiol. 1973 Oct;234(2):327–352. doi: 10.1113/jphysiol.1973.sp010348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallart A., Martin A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J Physiol. 1967 Dec;193(3):679–694. doi: 10.1113/jphysiol.1967.sp008388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkand P. M., Orkand R. K. Neuromuscular junctions in the buccal mass of Aplysia: fine structure and electrophysiology of excitatory transmission. J Neurobiol. 1975 Nov;6(6):531–548. doi: 10.1002/neu.480060602. [DOI] [PubMed] [Google Scholar]
- Peretz B. Central neuron initiation of periodic gill movements. Science. 1969 Nov 28;166(3909):1167–1172. doi: 10.1126/science.166.3909.1167. [DOI] [PubMed] [Google Scholar]
- Peretz B., Ringham G., Wilson R. Age-diminished motor neuronal function of central neuron L7 in Aplysia. J Neurobiol. 1982 Mar;13(2):141–151. doi: 10.1002/neu.480130206. [DOI] [PubMed] [Google Scholar]
- Rattan K. S., Peretz B. Age-dependent behavioral changes and physiological changes in identified neurons in Aplysia californica. J Neurobiol. 1981 Sep;12(5):469–478. doi: 10.1002/neu.480120506. [DOI] [PubMed] [Google Scholar]
- Weiss K. R., Cohen J., Kupfermann I. Potentiation of muscle contraction: a possible modulatory function of an identified serotonergic cell in Aplysia. Brain Res. 1975 Dec 5;99(2):381–386. doi: 10.1016/0006-8993(75)90041-4. [DOI] [PubMed] [Google Scholar]