Abstract
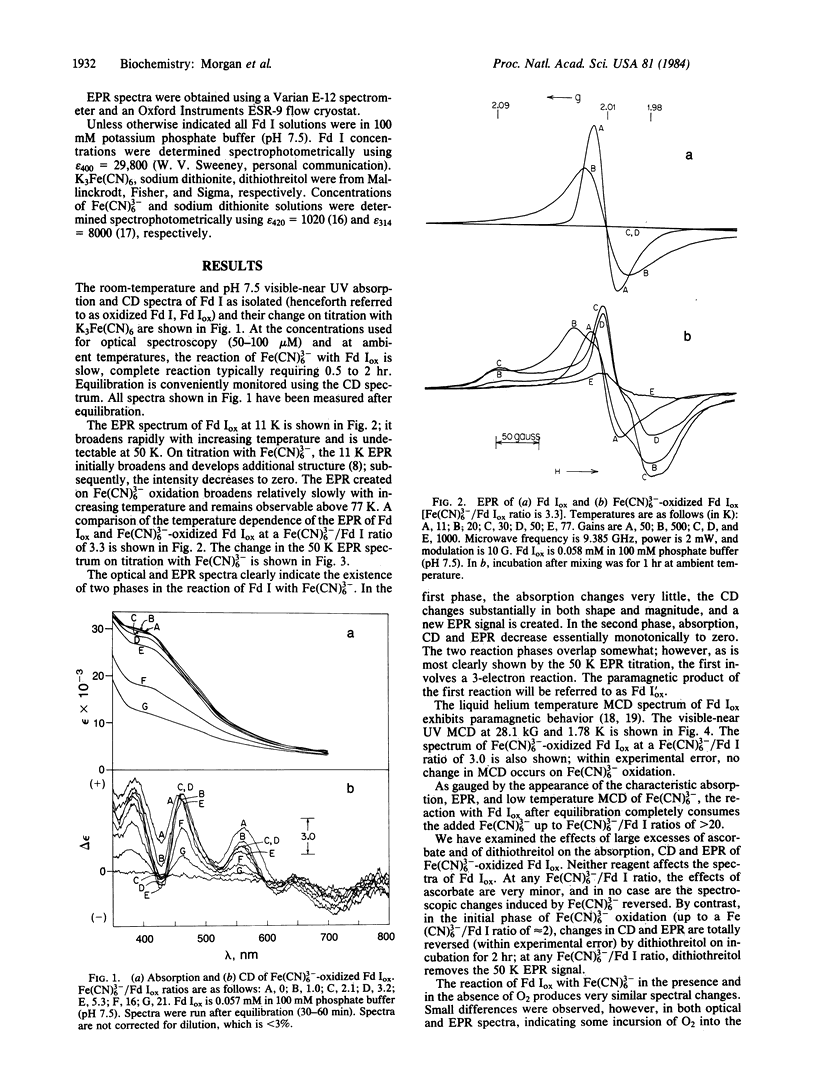
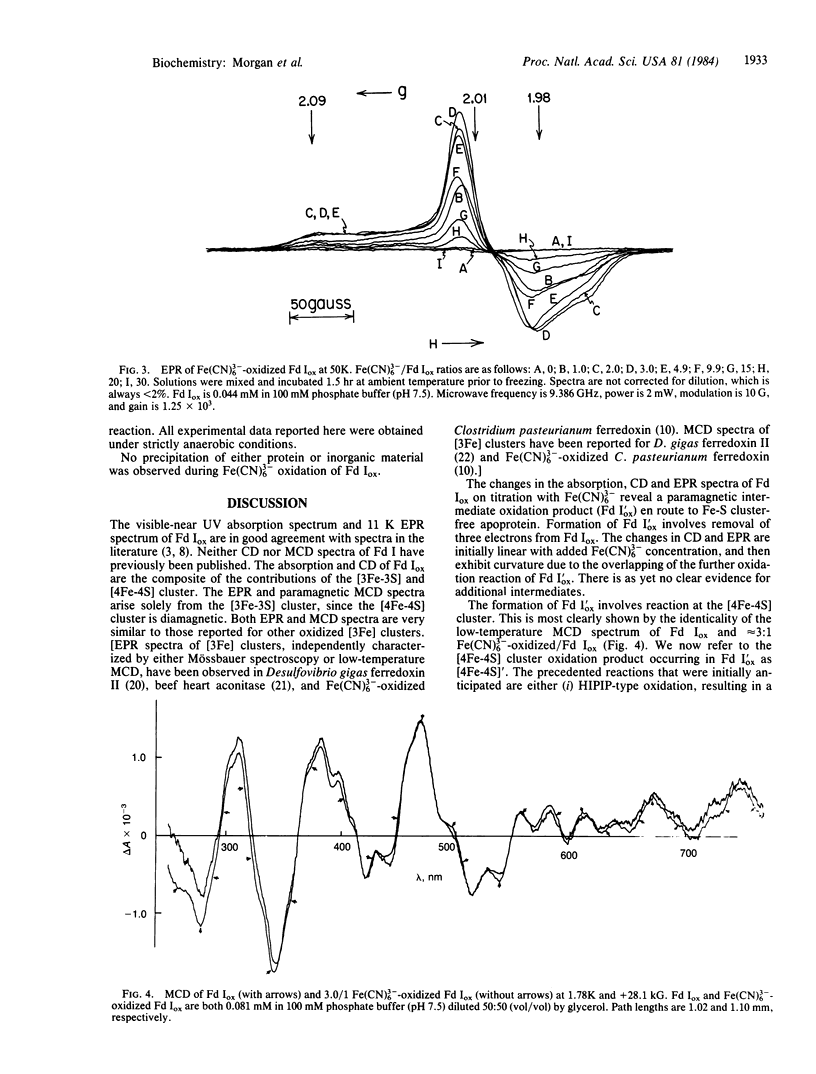
The Fe(CN)3-(6) oxidation of the crystallographically characterized [[3Fe-3S], [4Fe-4S]] ferredoxin I of Azotobacter vinelandii has been studied using absorption, circular dichroism, magnetic circular dichroism, and EPR spectroscopies. A paramagnetic intermediate is observed en route to Fe-S cluster-free apoprotein, possessing an anisotropic g approximately equal to 2 EPR signal, surviving to temperatures greater than 77 K. This species is shown to result from 3-electron oxidation of the [4Fe-4S] cluster, without modification of the [3Fe-3S] cluster. However, it does not give rise to observable paramagnetic magnetic circular dichroism in the visible-near UV spectral region and is therefore neither an oxidized HIPIP [4Fe-4S] cluster nor an oxidized [3Fe-3S] cluster. We identify the paramagnetic species as a cysteinyldisulfide radical formed on dissociation of an oxidized cysteinate and an oxidized sulfide ion from the [4Fe-4S] cluster. This conclusion is consistent with the observed reaction stoichiometry, the spectroscopic results obtained, known EPR spectra of disulfide radicals, and the reconstitution of the native [4Fe-4S] cluster by dithiothreitol alone. This reaction, earlier interpreted as a HIPIP-type oxidation, is a previously uncharacterized oxidation reaction of [4Fe-4S] clusters.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Siefker L. C., Jensen L. H. Structure of Peptococcus aerogenes ferredoxin. Refinement at 2 A resolution. J Biol Chem. 1976 Jun 25;251(12):3801–3806. doi: 10.2210/pdb1fdx/pdb. [DOI] [PubMed] [Google Scholar]
- Antanaitis B. C., Moss T. H. Magnetic studies of the four-iron high-potential, non-heme protein from Chromatium vinosum. Biochim Biophys Acta. 1975 Oct 20;405(2):262–279. doi: 10.1016/0005-2795(75)90093-8. [DOI] [PubMed] [Google Scholar]
- Beinert H., Emptage M. H., Dreyer J. L., Scott R. A., Hahn J. E., Hodgson K. O., Thomson A. J. Iron-sulfur stoichiometry and structure of iron-sulfur clusters in three-iron proteins: evidence for [3Fe-4S] clusters. Proc Natl Acad Sci U S A. 1983 Jan;80(2):393–396. doi: 10.1073/pnas.80.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess B. K., Jacobs D. B., Stiefel E. I. Large-scale purification of high activity Azotobacter vinelandII nitrogenase. Biochim Biophys Acta. 1980 Jul 10;614(1):196–209. doi: 10.1016/0005-2744(80)90180-1. [DOI] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O., Moura J. J., Xavier A. V., Bruschi M., Le Gall J., Deville A., Gayda J. P. Spectroscopic studies of the oxidation-reduction properties of three forms of ferredoxin from Desulphovibrio gigas. Biochim Biophys Acta. 1977 Feb 22;490(2):311–321. doi: 10.1016/0005-2795(77)90006-x. [DOI] [PubMed] [Google Scholar]
- Dixon M. The acceptor specificity of flavins and flavoproteins. I. Techniques for anaerobic spectrophotometry. Biochim Biophys Acta. 1971 Mar 2;226(2):241–258. doi: 10.1016/0005-2728(71)90092-2. [DOI] [PubMed] [Google Scholar]
- Dus K., De Klerk H., Sletten K., Bartsch R. G. Chemical characterization of high potential iron proteins from Chromatium and Rhodopseudomonas gelatinosa. Biochim Biophys Acta. 1967 Jun 27;140(2):291–311. doi: 10.1016/0005-2795(67)90470-9. [DOI] [PubMed] [Google Scholar]
- Emptage M. H., Kent T. A., Huynh B. H., Rawlings J., Orme-Johnson W. H., Münck E. On the nature of the iron-sulfur centers in a ferredoxin from Azotobacter vinelandii. Mössbauer studies and cluster displacement experiments. J Biol Chem. 1980 Mar 10;255(5):1793–1796. [PubMed] [Google Scholar]
- Ghosh D., Furey W., Jr, O'Donnell S., Stout C. D. Structure of a 7Fe ferredoxin from Azotobacter vinelandii. J Biol Chem. 1981 May 10;256(9):4185–4192. [PubMed] [Google Scholar]
- Ghosh D., O'Donnell S., Furey W., Jr, Robbins A. H., Stout C. D. Iron-sulfur clusters and protein structure of Azotobacter ferredoxin at 2.0 A resolution. J Mol Biol. 1982 Jun 15;158(1):73–109. doi: 10.1016/0022-2836(82)90451-x. [DOI] [PubMed] [Google Scholar]
- Johnson M. K., Thomson A. J., Robinson A. E., Rao K. K., Hall D. O. Low-temperature magnetic circular dichroism spectra and magnetisation curves of 4Fe clusters in iron-sulphur proteins from Chromatium and Clostridium pasteurianum. Biochim Biophys Acta. 1981 Feb 27;667(2):433–451. doi: 10.1016/0005-2795(81)90209-9. [DOI] [PubMed] [Google Scholar]
- Kent T. A., Dreyer J. L., Kennedy M. C., Huynh B. H., Emptage M. H., Beinert H., Münck E. Mössbauer studies of beef heart aconitase: evidence for facile interconversions of iron-sulfur clusters. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1096–1100. doi: 10.1073/pnas.79.4.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura J. J., Moura I., Kent T. A., Lipscomb J. D., Huynh B. H., LeGall J., Xavier A. V., Münck E. Interconversions of [3Fe-3S] and [4Fe-4S] clusters. Mössbauer and electron paramagnetic resonance studies of Desulfovibrio gigas ferredoxin II. J Biol Chem. 1982 Jun 10;257(11):6259–6267. [PubMed] [Google Scholar]
- Petering D., Fee J. A., Palmer G. The oxygen sensitivity of spinach ferredoxin and other iron-sulfur proteins. The formation of protein-bound sulfur-zero. J Biol Chem. 1971 Feb 10;246(3):643–653. [PubMed] [Google Scholar]
- Ruzicka F. J., Beinert H. The soluble "high potential" type iron-sulfur protein from mitochondria is aconitase. J Biol Chem. 1978 Apr 25;253(8):2514–2517. [PubMed] [Google Scholar]
- Shethna Y. I. Non-heme iron (iron-sulfur) proteins of Azotobacter vinelandii. Biochim Biophys Acta. 1970 Apr 7;205(1):58–62. doi: 10.1016/0005-2728(70)90061-7. [DOI] [PubMed] [Google Scholar]
- Stephens P. J., Thomson A. J., Dunn J. B., Keiderling T. A., Rawlings J., Rao K. K., Hall D. O. Circular dichroism and magnetic circular dichroism of iron-sulfur proteins. Biochemistry. 1978 Oct 31;17(22):4770–4778. doi: 10.1021/bi00615a026. [DOI] [PubMed] [Google Scholar]
- Stout C. D. Two crystal forms of Azotobacter ferredoxin. J Biol Chem. 1979 May 10;254(9):3598–3599. [PubMed] [Google Scholar]
- Strombaugh N. A., Burris R. H., Orme-Johnson W. H. Ferredoxins from Bacillus polymyxa. Low potential iron-sulfur proteins which appear to contain single four iron, four sulfur centers accepting a single electron on reduction. J Biol Chem. 1973 Nov 25;248(22):7951–7956. [PubMed] [Google Scholar]
- Sweeney W. V., Rabinowitz J. C. Proteins containing 4Fe-4S clusters: an overview. Annu Rev Biochem. 1980;49:139–161. doi: 10.1146/annurev.bi.49.070180.001035. [DOI] [PubMed] [Google Scholar]
- Sweeney W. V., Rabinowitz J. C., Yoch D. C. High and low reduction potential 4Fe-4S clusters in Azotobacter vinelandii (4Fe-4S) 2ferredoxin I. Influence of the polypeptide on the reduction potentials. J Biol Chem. 1975 Oct 10;250(19):7842–7847. [PubMed] [Google Scholar]
- Thomson A. J., Robinson A. E., Johnson M. K., Moura J. J., Moura I., Xavier A. V., Legall J. The three-iron cluster in a ferredoxin from Desulphovibrio gigas. A low-temperature magnetic circular dichroism study. Biochim Biophys Acta. 1981 Aug 28;670(1):93–100. doi: 10.1016/0005-2795(81)90053-2. [DOI] [PubMed] [Google Scholar]
- Yoch D. C., Arnon D. I. Two biologically active ferredoxins from the aerobic nitrogen-fixing bacteriu, Azotobacter vinelandii. J Biol Chem. 1972 Jul 25;247(14):4514–4520. [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Bacterial iron-sulfur proteins. Microbiol Rev. 1979 Sep;43(3):384–421. doi: 10.1128/mr.43.3.384-421.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch D. C., Carithers R. P. Potentiometric titration of the high- and low-potential 4Fe-4S* centers of Azotobacter vinelandii ferredoxin I. J Bacteriol. 1978 Nov;136(2):822–824. doi: 10.1128/jb.136.2.822-824.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


