Abstract
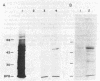
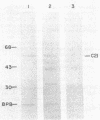
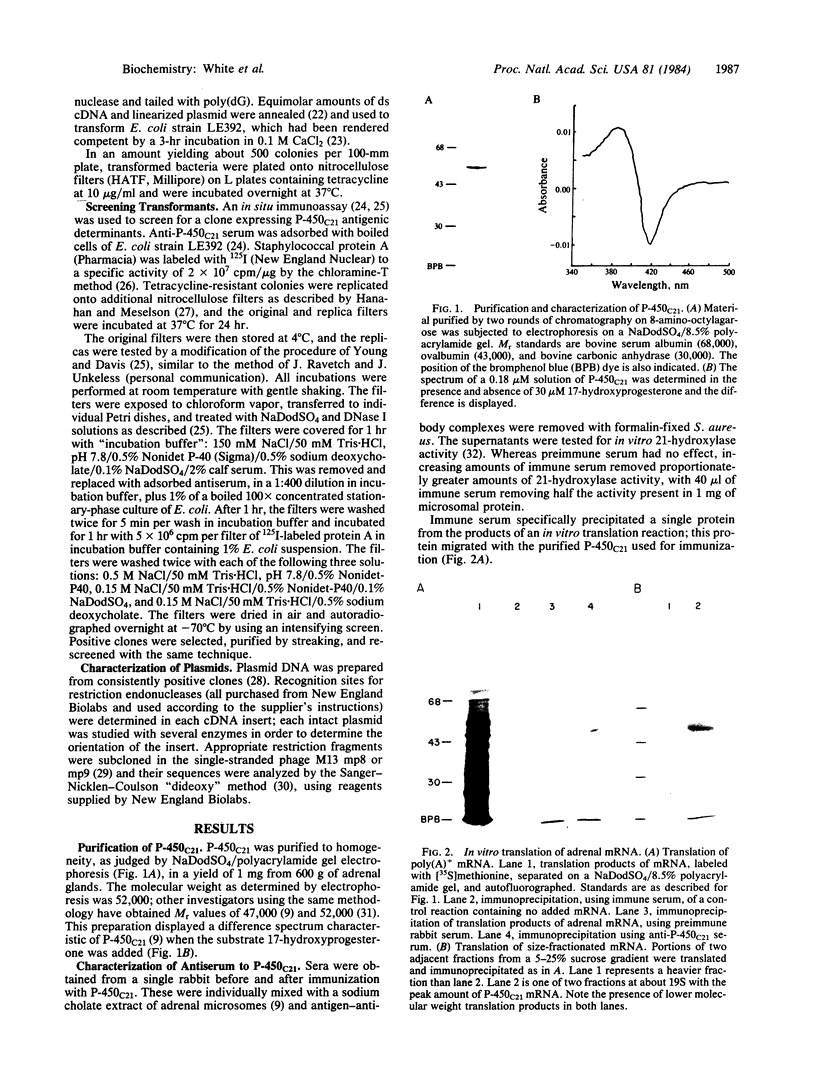
We isolated a cDNA clone encoding a bovine adrenal cytochrome P-450 specific for steroid 21-hydroxylation (P-450C21). Serum from rabbits immunized with purified P-450C21 precipitated a single protein from the products of an in vitro translation reaction using bovine adrenal mRNA. This protein migrated with P-450C21 on NaDodSO4/polyacrylamide gel electrophoresis. After sucrose gradient sedimentation, mRNA encoding P-450C21 was found in the 19S fraction. This fraction was reverse transcribed into double-stranded cDNA and inserted into the Pst I site of pBR322 by the dC X dG tailing procedure. Escherichia coli cells transformed with recombinant plasmids were screened with an in situ immunoassay using anti-P-450C21 serum and 125I-labeled staphylococcal protein A. Two colonies consistently bound anti-P-450C21 serum. They were identified as carrying the same plasmid by restriction mapping. This plasmid, pC21a, contains an insert of 520 base pairs. It hybridizes with mRNA encoding P-450C21. The peptide encoded by the insert in pC21a is highly homologous to two peptides isolated from porcine P-450C21 and shows limited homology to the P-450 induced by phenobarbital in rat liver. This clone may be useful in studying the molecular genetics of human congenital adrenal hyperplasia due to 21-hydroxylase deficiency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black S. D., Tarr G. E., Coon M. J. Structural features of isozyme 2 of liver microsomal cytochrome P-450. Identification of a highly conserved cysteine-containing peptide. J Biol Chem. 1982 Dec 25;257(24):14616–14619. [PubMed] [Google Scholar]
- Buell G. N., Wickens M. P., Payvar F., Schimke R. T. Synthesis of full length cDNAs from four partially purified oviduct mRNAs. J Biol Chem. 1978 Apr 10;253(7):2471–2482. [PubMed] [Google Scholar]
- Bumpus J. A., Dus K. M. Bovine adrenocortical microsomal hemeproteins P-45017 alpha and P-450C-21. Isolation, partial characterization, and comparison to P-450SCC. J Biol Chem. 1982 Nov 10;257(21):12696–12704. [PubMed] [Google Scholar]
- Chasalow F. I., Lieberman S. The activation of microsomal steroid 21-hydroxylase by cytosol from the cortex of bovine adrenals. J Biol Chem. 1979 May 25;254(10):3777–3781. [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Weiner R. I., Baxter J. D., Martial J. A. Structure of cloned DNA complementary to rat prolactin messenger RNA. J Biol Chem. 1980 Jul 10;255(13):6502–6510. [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Deng G., Wu R. An improved procedure for utilizing terminal transferase to add homopolymers to the 3' termini of DNA. Nucleic Acids Res. 1981 Aug 25;9(16):4173–4188. doi: 10.1093/nar/9.16.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupont B., Oberfield S. E., Smithwick E. M., Lee T. D., Levine L. S. Close genetic linkage between HLA and congenital adrenal hyperplasia (21-hydroxylase deficiency). Lancet. 1977 Dec 24;2(8052-8053):1309–1312. doi: 10.1016/s0140-6736(77)90362-2. [DOI] [PubMed] [Google Scholar]
- ESTABROOK R. W., COOPER D. Y., ROSENTHAL O. THE LIGHT REVERSIBLE CARBON MONOXIDE INHIBITION OF THE STEROID C21-HYDROXYLASE SYSTEM OF THE ADRENAL CORTEX. Biochem Z. 1963;338:741–755. [PubMed] [Google Scholar]
- Finkelstein M., Shaefer J. M. Inborn errors of steroid biosynthesis. Physiol Rev. 1979 Apr;59(2):353–406. doi: 10.1152/physrev.1979.59.2.353. [DOI] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Mizukami Y., Kawajiri K., Sogawa K., Muramatsu M. Primary structure of a cytochrome P-450: coding nucleotide sequence of phenobarbital-inducible cytochrome P-450 cDNA from rat liver. Proc Natl Acad Sci U S A. 1982 May;79(9):2793–2797. doi: 10.1073/pnas.79.9.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii-Kuriyama Y., Taniguchi T., Mizukami Y., Sakai M., Tashiro Y., Muramatsu M. Construction and identification of a hybrid plasmid containing DNA sequence complementary to phenobarbital-inducible cytochrome P-450 messenger RNA from rat liver. J Biochem. 1981 Jun;89(6):1869–1879. doi: 10.1093/oxfordjournals.jbchem.a133389. [DOI] [PubMed] [Google Scholar]
- Goodman H. M., MacDonald R. J. Cloning of hormone genes from a mixture of cDNA molecules. Methods Enzymol. 1979;68:75–90. doi: 10.1016/0076-6879(79)68007-2. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Helfman D. M., Feramisco J. R., Fiddes J. C., Thomas G. P., Hughes S. H. Identification of clones that encode chicken tropomyosin by direct immunological screening of a cDNA expression library. Proc Natl Acad Sci U S A. 1983 Jan;80(1):31–35. doi: 10.1073/pnas.80.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershfield V., Boyer H. W., Yanofsky C., Lovett M. A., Helinski D. R. Plasmid ColEl as a molecular vehicle for cloning and amplification of DNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3455–3459. doi: 10.1073/pnas.71.9.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Koizumi S., Kyoya S., Miyawaki T. M., Kidani H., Funabashi T. Cholesterol side-chain cleavage enzyme activity and cytochrome P-450 content in adrenal mitochondria of a patient with congenital lipoid adrenal hyperplasia (Prader disease). Clin Chim Acta. 1977 Jun 15;77(3):301–306. doi: 10.1016/0009-8981(77)90233-9. [DOI] [PubMed] [Google Scholar]
- Kominami S., Ochi H., Kobayashi Y., Takemori S. Studies on the steroid hydroxylation system in adrenal cortex microsomes. Purification and characterization of cytochrome P-450 specific for steroid C-21 hydroxylation. J Biol Chem. 1980 Apr 25;255(8):3386–3394. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Morville A. L., Thomas P., Levin W., Reik L., Ryan D. E., Raphael C., Adesnik M. The accumulation of distinct mRNAs for the immunochemically related cytochromes P-450c and P-450d in rat liver following 3-methylcholanthrene treatment. J Biol Chem. 1983 Mar 25;258(6):3901–3906. [PubMed] [Google Scholar]
- Negishi M., Swan D. C., Enquist L. W., Nebert D. W. Isolation and characterization of a cloned DNA sequence associated with the murine Ah locus and a 3-methylcholanthrene-induced form of cytochrome P-450. Proc Natl Acad Sci U S A. 1981 Feb;78(2):800–804. doi: 10.1073/pnas.78.2.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozols J., Heinemann F. S., Johnson E. F. Amino acid sequence of an analogous peptide from two forms of cytochrome P-450. J Biol Chem. 1981 Nov 25;256(22):11405–11408. [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payvar F., Schimke R. T. Methylmercury hydroxide enhancement of translation and transcription of ovalbumin and conalbumin mRNA's. J Biol Chem. 1979 Aug 25;254(16):7636–7642. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C., Newman R. A., Sutherland D. R., Asser U., Greaves M. F. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982 Sep 25;257(18):10766–10769. [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M. P., Buell G. N., Schimke R. T. Synthesis of double-stranded DNA complementary to lysozyme, ovomucoid, and ovalbumin mRNAs. Optimization for full length second strand synthesis by Escherichia coli DNA polymerase I. J Biol Chem. 1978 Apr 10;253(7):2483–2495. [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P. M., Nakajin S., Haniu M., Shinoda M., Hall P. F., Shively J. E. Steroid 21-hydroxylase (cytochrome P-450) from porcine adrenocortical microsomes: microsequence analysis of cysteine-containing peptides. Biochemistry. 1983 Jan 4;22(1):143–149. doi: 10.1021/bi00270a021. [DOI] [PubMed] [Google Scholar]
- Yuan P. M., Ryan D. E., Levin W., Shively J. E. Identification and localization of amino acid substitutions between two phenobarbital-inducible rat hepatic microsomal cytochromes P-450 by micro sequence analyses. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1169–1173. doi: 10.1073/pnas.80.5.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Martynoff G., Pays E., Vassart G. Synthesis of a full length DNA complementary to thyroglobulin 33 S messenger RNA. Biochem Biophys Res Commun. 1980 Apr 14;93(3):645–653. doi: 10.1016/0006-291x(80)91127-4. [DOI] [PubMed] [Google Scholar]