Abstract
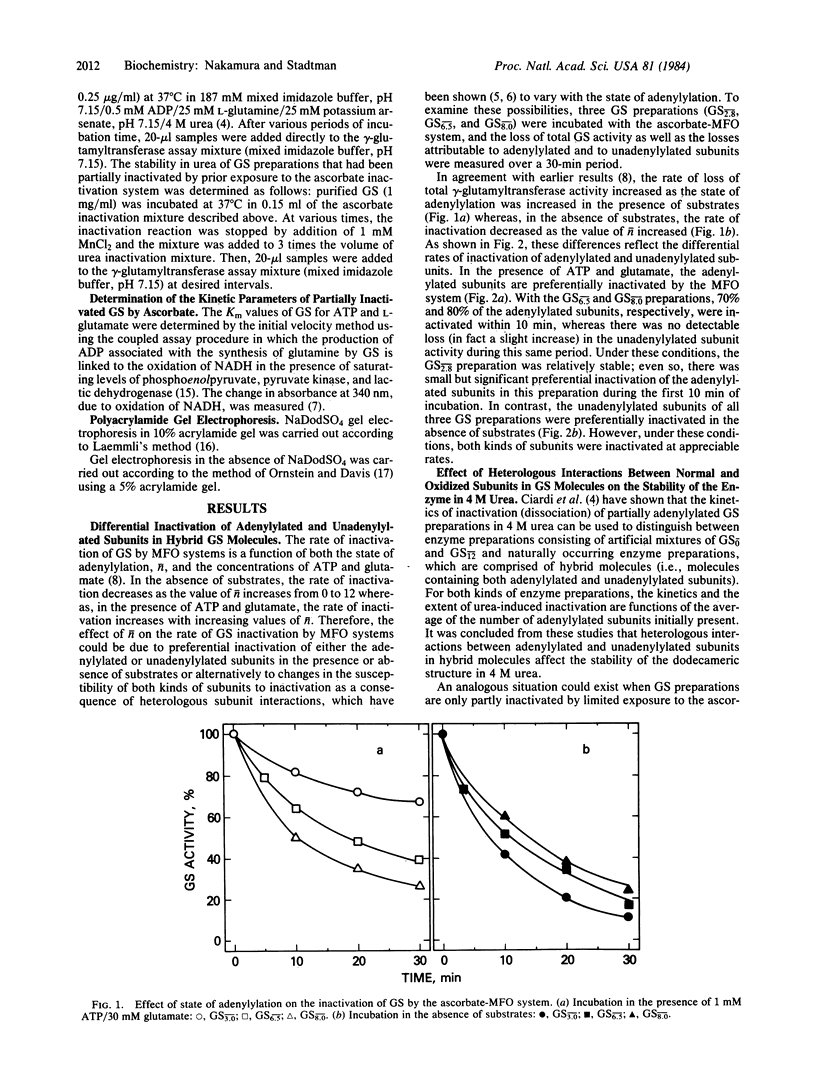
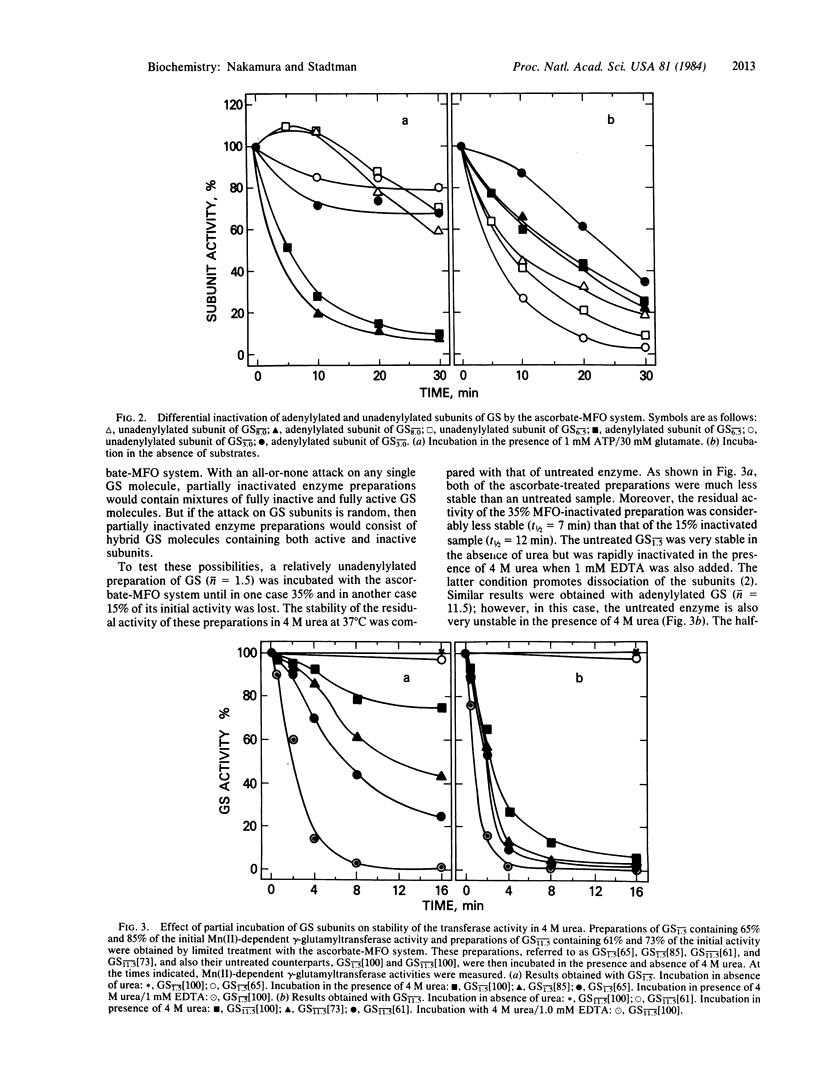
Escherichia coli glutamine synthetase (GS) was inactivated by a nonenzymic mixed-function oxidation system composed of ascorbate, O2, and Fe(III). Partial inactivation of GS by this system leads to the formation of hybrid GS molecules (dodecamers) composed of both active and inactive subunits. Subunit interactions in these hybrid molecules are weaker than in the native enzyme, as indicated by the kinetics of subunit dissociation in the presence of 4 M urea. Heterologous subunit interactions in these hybrid molecules do not affect the affinity of active subunits for glutamate. Incubation of partially adenylylated GS preparations (n = 6.7) with the ascorbate system in the absence of substrates leads to preferential oxidative inactivation of unadenylylated subunits, whereas incubation in the presence of ATP and glutamate leads to preferential inactivation of adenylylated subunits.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chock P. B., Stadtman E. R. Superiority of interconvertible enzyme cascades in metabolite regulation: analysis of multicyclic systems. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2766–2770. doi: 10.1073/pnas.74.7.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Cimino F., Stadtman E. R. Multiple forms of glutamine synthetase. Hybrid formation by association of adenylylated and unadenylylated subunits. Biochemistry. 1973 Oct 23;12(22):4321–4330. doi: 10.1021/bi00746a004. [DOI] [PubMed] [Google Scholar]
- Denton M. D., Ginsburg A. Some characteristics of the binding of substrates of glutamine synthetase from Escherichia coli. Biochemistry. 1970 Feb 3;9(3):617–632. doi: 10.1021/bi00805a024. [DOI] [PubMed] [Google Scholar]
- Fucci L., Oliver C. N., Coon M. J., Stadtman E. R. Inactivation of key metabolic enzymes by mixed-function oxidation reactions: possible implication in protein turnover and ageing. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1521–1525. doi: 10.1073/pnas.80.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdon H. S., Hubbard J. S., Stadtman E. R. Regulation of glutamine synthetase. XI. The nature and implications of a lag phase in the Escherichia coli glutamine synthetase reaction. Biochemistry. 1968 Jun;7(6):2136–2142. doi: 10.1021/bi00846a016. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. I. Inactivation is due to loss of one histidine residue. J Biol Chem. 1983 Oct 10;258(19):11823–11827. [PubMed] [Google Scholar]
- Levine R. L. Oxidative modification of glutamine synthetase. II. Characterization of the ascorbate model system. J Biol Chem. 1983 Oct 10;258(19):11828–11833. [PubMed] [Google Scholar]
- Miller R. E., Shelton E., Stadtman E. R. Zinc-induced paracrystalline aggregation of glutamine synthetase. Arch Biochem Biophys. 1974 Jul;163(1):155–171. doi: 10.1016/0003-9861(74)90465-2. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Rhee S. G., Park R., Chock P. B., Stadtman E. R. Allosteric regulation of monocyclic interconvertible enzyme cascade systems: use of Escherichia coli glutamine synthetase as an experimental model. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3138–3142. doi: 10.1073/pnas.75.7.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro B. M., Ginsburg A. Effects of specific divalent cations on some physical and chemical properties of glutamine synthetase from Escherichia coli. Taut and relaxed enzyme forms. Biochemistry. 1968 Jun;7(6):2153–2167. doi: 10.1021/bi00846a018. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Smyrniotis P. Z., Davis J. N., Wittenberger M. E. Enzymic procedures for determining the average state of adenylylation of Escherichia coli glutamine synthetase. Anal Biochem. 1979 May;95(1):275–285. doi: 10.1016/0003-2697(79)90217-3. [DOI] [PubMed] [Google Scholar]
- Woolfolk C. A., Shapiro B., Stadtman E. R. Regulation of glutamine synthetase. I. Purification and properties of glutamine synthetase from Escherichia coli. Arch Biochem Biophys. 1966 Sep 26;116(1):177–192. doi: 10.1016/0003-9861(66)90026-9. [DOI] [PubMed] [Google Scholar]




