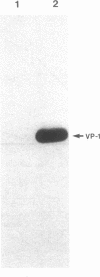
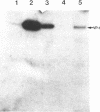
Abstract
The early simian virus 40 (SV40) gene product, large tumor (T) antigen, is responsible for the initiation of viral DNA replication and the autoregulation of early gene expression through direct protein-DNA interactions. We investigated the role of T antigen in late viral gene expression, independent of its function in amplifying templates through DNA replication. SV40 DNA was transfected into BSC-1 and COS-1 cells and cultured in the presence of inhibitors of DNA replication. Electrophoretic immunoblot analysis indicated that both the onset and the extent of SV40 late gene expression is increased in COS-1 cells, which constitutively express SV40 T antigen. Blot hybridization analysis of poly(A)-selected RNA demonstrated that the level of synthesis of the major late structural protein VP-1 in COS-1 cells was due to increased transcription. Similar results were obtained when plasmids that contain the SV40 late gene but lack both the origin for viral DNA replication and the early gene coding region were transfected onto COS-1 cells. Using lines of SV40-transformed monkey kidney cells that express altered T antigens, we found that enhanced expression of the late gene product is correlated with the ability of T antigen to bind SV40 DNA. These results indicate that large T antigen plays a role in the stimulation of late viral gene expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Khoury G. Effect of a tsA mutation of simian virus 40 late gene expression: variations between host cell lines. J Virol. 1980 Feb;33(2):920–925. doi: 10.1128/jvi.33.2.920-925.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Reed S. I., Stark G. R. Characterization of the autoregulation of simian virus 40 gene A. J Virol. 1977 Oct;24(1):22–27. doi: 10.1128/jvi.24.1.22-27.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Benoist C., Chambon P. In vivo sequence requirements of the SV40 early promotor region. Nature. 1981 Mar 26;290(5804):304–310. doi: 10.1038/290304a0. [DOI] [PubMed] [Google Scholar]
- Birkenmeier E. H., Chiu N., Radonovich M. F., May E., Salzman N. P. Regulation of simian virus 40 early and late gene transcription without viral DNA replication. J Virol. 1979 Mar;29(3):983–989. doi: 10.1128/jvi.29.3.983-989.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkenmeier E. H., May E., Salzman N. P. Characterization of simian virus 40 tsA58 transcriptional intermediates at restrictive temperatures: relationship between DNA replication and transcription. J Virol. 1977 Jun;22(3):702–710. doi: 10.1128/jvi.22.3.702-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Brady J. N., Radonovich M., Salzman N. P. Accurate transcription of simian virus 40 chromatin in a HeLa cell extract. J Virol. 1982 Dec;44(3):772–781. doi: 10.1128/jvi.44.3.772-781.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Radonovich M., Thoren M., Das G., Salzman N. P. Simian virus 40 major late promoter: an upstream DNA sequence required for efficient in vitro transcription. Mol Cell Biol. 1984 Jan;4(1):133–141. doi: 10.1128/mcb.4.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady J., Radonovich M., Vodkin M., Natarajan V., Thoren M., Das G., Janik J., Salzman N. P. Site-specific base substitution and deletion mutations that enhance or suppress transcription of the SV40 major late RNA. Cell. 1982 Dec;31(3 Pt 2):625–633. doi: 10.1016/0092-8674(82)90318-x. [DOI] [PubMed] [Google Scholar]
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Contreras R., Gheysen D., Knowland J., van de Voorde A., Fiers W. Evidence for the direct involvement of DNA replication origin in synthesis of late SV40 RNA. Nature. 1982 Dec 9;300(5892):500–505. doi: 10.1038/300500a0. [DOI] [PubMed] [Google Scholar]
- Cowan K., Tegtmeyer P., Anthony D. D. Relationship of replication and transcription of Simian Virus 40 DNA. Proc Natl Acad Sci U S A. 1973 Jul;70(7):1927–1930. doi: 10.1073/pnas.70.7.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLucia A. L., Lewton B. A., Tjian R., Tegtmeyer P. Topography of simian virus 40 A protein-DNA complexes: arrangement of pentanucleotide interaction sites at the origin of replication. J Virol. 1983 Apr;46(1):143–150. doi: 10.1128/jvi.46.1.143-150.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman L. T., Imperiale M. J., Nevins J. R. Activation of early adenovirus transcription by the herpesvirus immediate early gene: evidence for a common cellular control factor. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4952–4956. doi: 10.1073/pnas.79.16.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferdinand F. J., Brown M., Khoury G. Characterization of early simian virus 40 transcriptional complexes: late transcription in the absence of detectable DNA replication. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5443–5447. doi: 10.1073/pnas.74.12.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Berg P. Deletion mapping of DNA regions required for SV40 early region promoter function in vivo. J Mol Appl Genet. 1982;1(5):457–481. [PubMed] [Google Scholar]
- Ghosh P. K., Piatak M., Mertz J. E., Weissman S. M., Lebowitz P. Altered utilization of splice sites and 5' termini in late RNAs produced by leader region mutants of simian virus 40. J Virol. 1982 Nov;44(2):610–624. doi: 10.1128/jvi.44.2.610-624.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh P. K., Piatak M., Mertz J. E., Weissman S. M., Lebowitz P. Altered utilization of splice sites and 5' termini in late RNAs produced by leader region mutants of simian virus 40. J Virol. 1982 Nov;44(2):610–624. doi: 10.1128/jvi.44.2.610-624.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y., Davison J., Oren M., Winocour E. Properties of permissive monkey cells transformed by UV-irradiated simian virus 40. J Virol. 1977 May;22(2):256–266. doi: 10.1128/jvi.22.2.256-266.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Green M. R., Treisman R., Maniatis T. Transcriptional activation of cloned human beta-globin genes by viral immediate-early gene products. Cell. 1983 Nov;35(1):137–148. doi: 10.1016/0092-8674(83)90216-7. [DOI] [PubMed] [Google Scholar]
- Gruss P., Dhar R., Khoury G. Simian virus 40 tandem repeated sequences as an element of the early promoter. Proc Natl Acad Sci U S A. 1981 Feb;78(2):943–947. doi: 10.1073/pnas.78.2.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handa H., Sharp P. A. Expression of early and late simian virus 40 transcripts in the absence of protein synthesis. J Virol. 1980 Jun;34(3):592–597. doi: 10.1128/jvi.34.3.592-597.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen U., Sharp P. A. Sequences controlling in vitro transcription of SV40 promoters. EMBO J. 1983;2(12):2293–2303. doi: 10.1002/j.1460-2075.1983.tb01737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen U., Tenen D. G., Livingston D. M., Sharp P. A. T antigen repression of SV40 early transcription from two promoters. Cell. 1981 Dec;27(3 Pt 2):603–613. doi: 10.1016/0092-8674(81)90402-5. [DOI] [PubMed] [Google Scholar]
- Hartzell S. W., Byrne B. J., Subramanian K. N. Mapping of the late promoter of simian virus 40. Proc Natl Acad Sci U S A. 1984 Jan;81(1):23–27. doi: 10.1073/pnas.81.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N., Skolnik-David H., Aloni Y. Attenuation in the control of SV40 gene expression. Cell. 1982 May;29(1):183–193. doi: 10.1016/0092-8674(82)90102-7. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. An adenovirus type 5 early gene function regulates expression of other early viral genes. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3665–3669. doi: 10.1073/pnas.76.8.3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel M., Khoury G. Induction of cloned genes after transfer into eukaryotic cells. Gene Amplif Anal. 1983;3:233–260. [PubMed] [Google Scholar]
- Khoury G., May E. Regulation of early and late simian virus 40 transcription: overproduction of early viral RNA in the absence of a functional T-antigen. J Virol. 1977 Jul;23(1):167–176. doi: 10.1128/jvi.23.1.167-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange M., May E., May P. Ability of nonpermissive mouse cells to express a simian virus 40 late function(s). J Virol. 1981 Jun;38(3):940–951. doi: 10.1128/jvi.38.3.940-951.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowitz S. G. Simian virus 40 mutant with transposed T-antigen and VP1 genes. J Virol. 1982 Mar;41(3):1025–1037. doi: 10.1128/jvi.41.3.1025-1037.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen J., Consigli R. A. Immunological reactivity of antisera to sodium dodecyl sulfate-derived polypeptides of polyoma virions. J Virol. 1977 Mar;21(3):1113–1120. doi: 10.1128/jvi.21.3.1113-1120.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau P., Hen R., Wasylyk B., Everett R., Gaub M. P., Chambon P. The SV40 72 base repair repeat has a striking effect on gene expression both in SV40 and other chimeric recombinants. Nucleic Acids Res. 1981 Nov 25;9(22):6047–6068. doi: 10.1093/nar/9.22.6047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevins J. R. Induction of the synthesis of a 70,000 dalton mammalian heat shock protein by the adenovirus E1A gene product. Cell. 1982 Jul;29(3):913–919. doi: 10.1016/0092-8674(82)90453-6. [DOI] [PubMed] [Google Scholar]
- Nevins J. R. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981 Oct;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. A regulatory upstream promoter element in the Drosophila hsp 70 heat-shock gene. Cell. 1982 Sep;30(2):517–528. doi: 10.1016/0092-8674(82)90249-5. [DOI] [PubMed] [Google Scholar]
- Prives C., Covey L., Scheller A., Gluzman Y. DNA-binding properties of simian virus 40 T-antigen mutants defective in viral DNA replication. Mol Cell Biol. 1983 Nov;3(11):1958–1966. doi: 10.1128/mcb.3.11.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. I., Stark G. R., Alwine J. C. Autoregulation of simian virus 40 gene A by T antigen. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3083–3087. doi: 10.1073/pnas.73.9.3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaldy A., Feunteun J., Rosenberg B. H. Prereplicative events involving simian virus 40 DNA in permissive cells. J Virol. 1982 Jan;41(1):237–243. doi: 10.1128/jvi.41.1.237-243.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio D., Robbins A., Myers R., Tjian R. Regulation of simian virus 40 early transcription in vitro by a purified tumor antigen. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5706–5710. doi: 10.1073/pnas.77.10.5706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller A., Covey L., Barnet B., Prives C. A small subclass of SV40 T antigen binds to the viral origin of replication. Cell. 1982 Jun;29(2):375–383. doi: 10.1016/0092-8674(82)90154-4. [DOI] [PubMed] [Google Scholar]
- Schutzbank T., Robinson R., Oren M., Levine A. J. SV40 large tumor antigen can regulate some cellular transcripts in a positive fashion. Cell. 1982 Sep;30(2):481–490. doi: 10.1016/0092-8674(82)90245-8. [DOI] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Shortle D. R., Margolskee R. F., Nathans D. Mutational analysis of the simian virus 40 replicon: pseudorevertants of mutants with a defective replication origin. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6128–6131. doi: 10.1073/pnas.76.12.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Regulatory mutants of simian virus 40: constructed mutants with base substitutions at the origin of DNA replication. J Mol Biol. 1979 Jul 15;131(4):801–817. doi: 10.1016/0022-2836(79)90202-x. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenen D. G., Livingston D. M., Wang S. S., Martin R. G. Effect of a stem-loop structure within the SV40 replication origin upon SV40 T antigen binding to origin region sequences. Cell. 1983 Sep;34(2):629–639. doi: 10.1016/0092-8674(83)90395-1. [DOI] [PubMed] [Google Scholar]
- Thomas G. P., Mathews M. B. DNA replication and the early to late transition in adenovirus infection. Cell. 1980 Nov;22(2 Pt 2):523–533. doi: 10.1016/0092-8674(80)90362-1. [DOI] [PubMed] [Google Scholar]
- Tjian R. T antigen binding and the control of SV40 gene expression. Cell. 1981 Oct;26(1 Pt 1):1–2. doi: 10.1016/0092-8674(81)90026-x. [DOI] [PubMed] [Google Scholar]