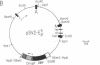
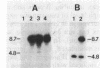
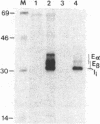
Abstract
Using the spheroplast fusion technique, we have introduced the cloned E beta b gene into two d haplotype cell lines, the B lymphoma line A20-2J and the macrophage tumor line P388D1. Analysis with a monoclonal antibody indicates that the product of the transfected E beta b gene associates with the endogenous E alpha chain to form an E alpha dE beta b complex. While expression of E alpha dE beta b is constitutive in A20-2J cells transfected with the E beta b gene, surface expression of E alpha dE beta b is detected in transfected macrophage cells only after treatment of cells with culture supernatants from concanavalin A (Con A)-stimulated T cells. Transfected B lymphoma cells and transfected Con A supernatant-treated macrophage cells have acquired the ability to present antigen to E alpha dE beta b-restricted T-cell hybridomas. The observed inducible expression of the transfected gene in the macrophage host indicates that sequences responsible for regulated expression of the E beta b gene may be associated with the transfected gene. In combination with directed mutagenesis, the system described here provides a means to study (i) E beta b sequences that are important in determining the restriction specificity of the E molecule and (ii) sequences associated with the E beta gene that may be important in the regulation of E beta chain expression.
Full text
PDF
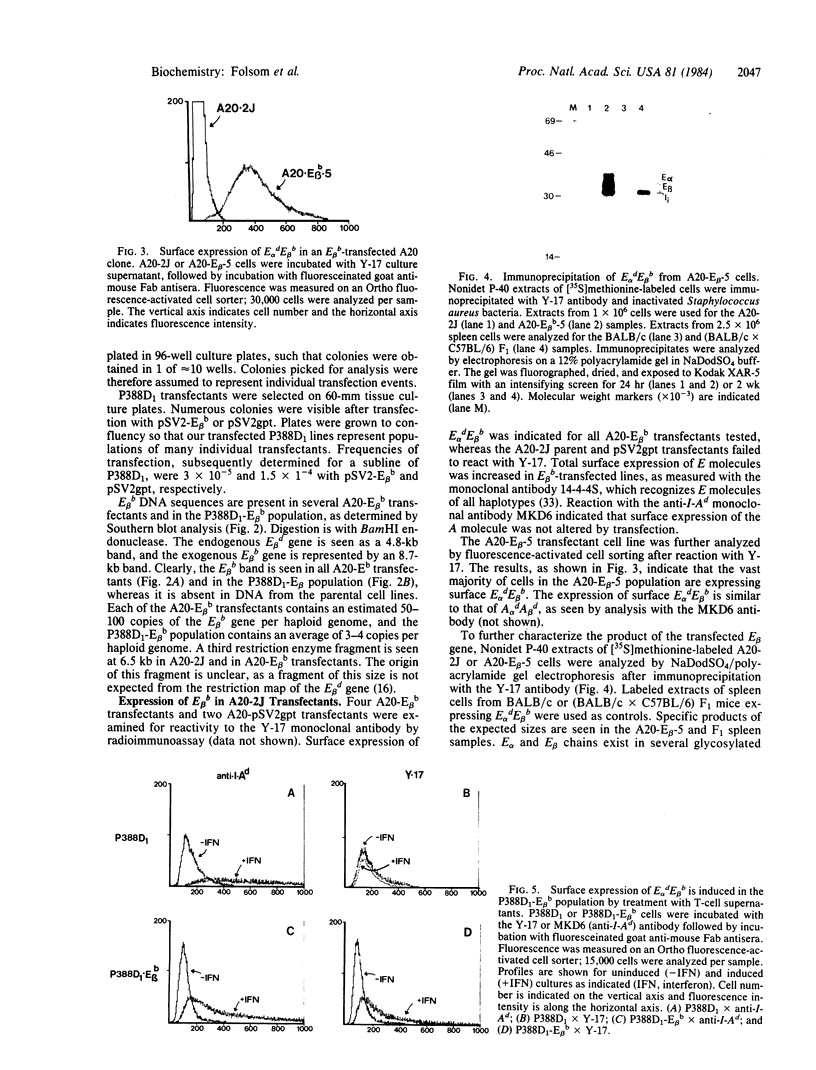
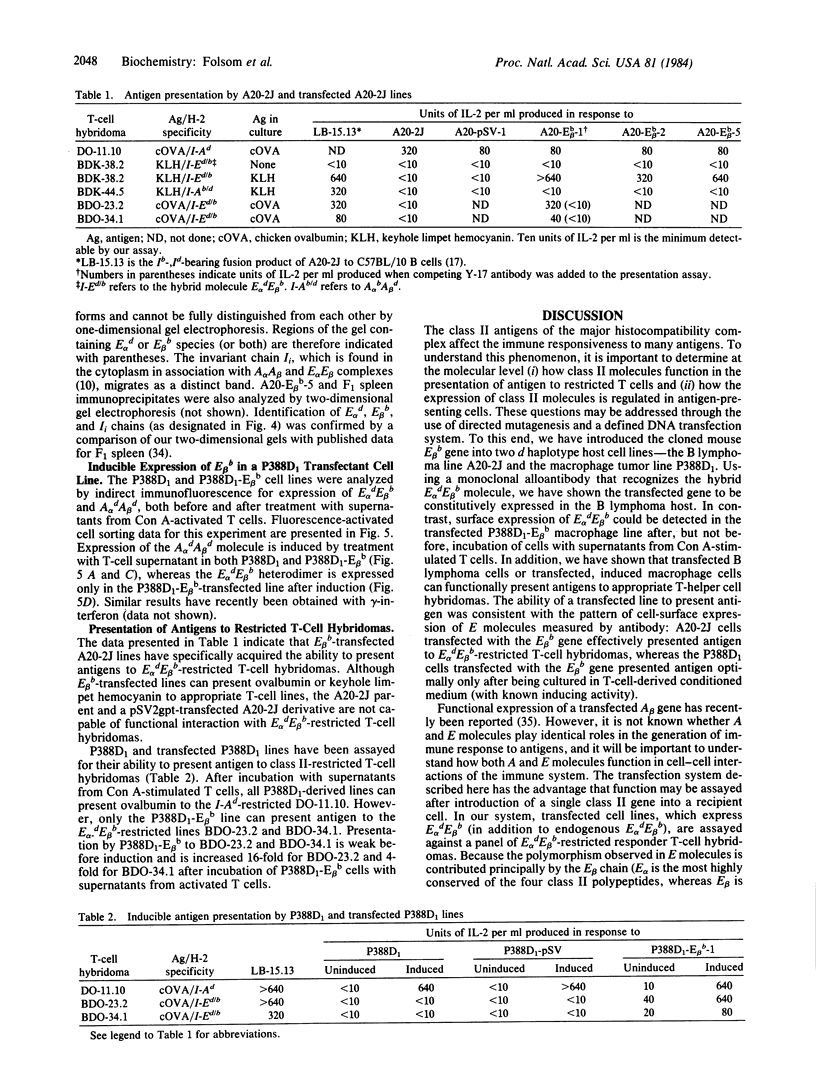

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benacerraf B., McDevitt H. O. Histocompatibility-linked immune response genes. Science. 1972 Jan 21;175(4019):273–279. doi: 10.1126/science.175.4019.273. [DOI] [PubMed] [Google Scholar]
- Benoist C. O., Mathis D. J., Kanter M. R., Williams V. E., 2nd, McDevitt H. O. The murine Ia alpha chains, E alpha and A alpha, show a surprising degree of sequence homology. Proc Natl Acad Sci U S A. 1983 Jan;80(2):534–538. doi: 10.1073/pnas.80.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham J. R., Chesnut R. W., Kappler J. W., Marrack P., Kubo R., Grey H. M. Antigen presentation to T cell hybridomas by a macrophage cell line: an inducible function. J Immunol. 1982 Mar;128(3):1491–1492. [PubMed] [Google Scholar]
- Chandler V. L., Maler B. A., Yamamoto K. R. DNA sequences bound specifically by glucocorticoid receptor in vitro render a heterologous promoter hormone responsive in vivo. Cell. 1983 Jun;33(2):489–499. doi: 10.1016/0092-8674(83)90430-0. [DOI] [PubMed] [Google Scholar]
- Choi E., McIntyre K., Germain R. N., Seidman J. G. Murine I-A beta chain polymorphism: nucleotide sequences of three allelic I-A beta genes. Science. 1983 Jul 15;221(4607):283–286. doi: 10.1126/science.6407114. [DOI] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Germain R. N., Norcross M. A., Margulies D. H. Functional expression of a transfected murine class II MHC gene. Nature. 1983 Nov 10;306(5939):190–194. doi: 10.1038/306190a0. [DOI] [PubMed] [Google Scholar]
- Gillies S. D., Morrison S. L., Oi V. T., Tonegawa S. A tissue-specific transcription enhancer element is located in the major intron of a rearranged immunoglobulin heavy chain gene. Cell. 1983 Jul;33(3):717–728. doi: 10.1016/0092-8674(83)90014-4. [DOI] [PubMed] [Google Scholar]
- Kappler J. W., Skidmore B., White J., Marrack P. Antigen-inducible, H-2-restricted, interleukin-2-producing T cell hybridomas. Lack of independent antigen and H-2 recognition. J Exp Med. 1981 May 1;153(5):1198–1214. doi: 10.1084/jem.153.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappler J., White J., Wegmann D., Mustain E., Marrack P. Antigen presentation by Ia+ B cell hybridomas to H-2-restricted T cell hybridomas. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3604–3607. doi: 10.1073/pnas.79.11.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D. P., Jones P. P. Induction of Ia and H-2 antigens on a macrophage cell line by immune interferon. J Immunol. 1983 Jul;131(1):315–318. [PubMed] [Google Scholar]
- Klein J., Juretic A., Baxevanis C. N., Nagy Z. A. The traditional and a new version of the mouse H-2 complex. Nature. 1981 Jun 11;291(5815):455–460. doi: 10.1038/291455a0. [DOI] [PubMed] [Google Scholar]
- Koren H. S., Handwerger B. S., Wunderlich J. R. Identification of macrophage-like characteristics in a cultured murine tumor line. J Immunol. 1975 Feb;114(2 Pt 2):894–897. [PubMed] [Google Scholar]
- Lerner E. A., Matis L. A., Janeway C. A., Jr, Jones P. P., Schwartz R. H., Murphy D. B. Monoclonal antibody against an Ir gene product? J Exp Med. 1980 Oct 1;152(4):1085–1101. doi: 10.1084/jem.152.4.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malissen M., Hunkapiller T., Hood L. Nucleotide sequence of a light chain gene of the mouse I-A subregion: A beta d. Science. 1983 Aug 19;221(4612):750–754. doi: 10.1126/science.6410508. [DOI] [PubMed] [Google Scholar]
- Mathis D. J., Benoist C. O., Williams V. E., 2nd, Kanter M. R., McDevitt H. O. The murine E alpha immune response gene. Cell. 1983 Mar;32(3):745–754. doi: 10.1016/0092-8674(83)90060-0. [DOI] [PubMed] [Google Scholar]
- McNicholas J. M., King D. P., Jones P. P. Biosynthesis and expression of Ia and H-2 antigens on a macrophage cell line are stimulated by products of activated spleen cells. J Immunol. 1983 Jan;130(1):449–456. [PubMed] [Google Scholar]
- McNicholas J. M., Murphy D. B., Matis L. A., Schwartz R. H., Lerner E. A., Janeway C. A., Jr, Jones P. P. Immune response gene function correlates with the expression of an Ia antigen. I. Preferential association of certain Ae and E alpha chains results in a quantitative deficiency in expression of an Ae:E alpha complex. J Exp Med. 1982 Feb 1;155(2):490–507. doi: 10.1084/jem.155.2.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Manser T., Pearson G. D., Daley M. J., Gefter M. L. Effect of IFN-gamma on the immune response in vivo and on gene expression in vitro. 1984 Jan 26-Feb 1Nature. 307(5949):381–382. doi: 10.1038/307381a0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Evans G. A., Shykind B., Margulies D. H., Seidman J. G. Hybrid H-2 histocompatibility gene products assign domains recognized by alloreactive T cells. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2040–2043. doi: 10.1073/pnas.80.7.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozato K., Mayer N., Sachs D. H. Hybridoma cell lines secreting monoclonal antibodies to mouse H-2 and Ia antigens. J Immunol. 1980 Feb;124(2):533–540. [PubMed] [Google Scholar]
- Rosa F., Hatat D., Abadie A., Wallach D., Revel M., Fellous M. Differential regulation of HLA-DR mRNAs and cell surface antigens by interferon. EMBO J. 1983;2(9):1585–1589. doi: 10.1002/j.1460-2075.1983.tb01628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Maki R. A., Clayton L. K., Tonegawa S. Complete primary structures of the E beta chain and gene of the mouse major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5520–5524. doi: 10.1073/pnas.80.18.5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandri-Goldin R. M., Goldin A. L., Levine M., Glorioso J. C. High-frequency transfer of cloned herpes simplex virus type 1 sequences to mammalian cells by protoplast fusion. Mol Cell Biol. 1981 Aug;1(8):743–752. doi: 10.1128/mcb.1.8.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder D. S., Beller D. I., Unanue E. R. Prostaglandins modulate macrophage Ia expression. Nature. 1982 Sep 9;299(5879):163–165. doi: 10.1038/299163a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Steeg P. S., Moore R. N., Oppenheim J. J. Regulation of murine macrophage Ia-antigen expression by products of activated spleen cells. J Exp Med. 1980 Dec 1;152(6):1734–1744. doi: 10.1084/jem.152.6.1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Minard K., Horvath S., McNicholas J., Srelinger J., Wake C., Long E., Mach B., Hood L. A molecular map of the immune response region from the major histocompatibility complex of the mouse. Nature. 1982 Nov 4;300(5887):35–42. doi: 10.1038/300035a0. [DOI] [PubMed] [Google Scholar]
- Walker E., Warner N. L., Chesnut R., Kappler J., Marrack P. Antigen-specific. I region-restricted interactions in vitro between tumor cell lines and T cell hybridomas. J Immunol. 1982 May;128(5):2164–2169. [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J., Haskins K. M., Marrack P., Kappler J. Use of I region-restricted, antigen-specific T cell hybridomas to produce idiotypically specific anti-receptor antibodies. J Immunol. 1983 Mar;130(3):1033–1037. [PubMed] [Google Scholar]
- Wong G. H., Clark-Lewis I., McKimm-Breschkin L., Harris A. W., Schrader J. W. Interferon-gamma induces enhanced expression of Ia and H-2 antigens on B lymphoid, macrophage, and myeloid cell lines. J Immunol. 1983 Aug;131(2):788–793. [PubMed] [Google Scholar]
- Zlotnik A., Roberts W. K., Vasil A., Blumenthal E., Larosa F., Leibson H. J., Endres R. O., Graham S. D., Jr, White J., Hill J. Coordinate production by a T cell hybridoma of gamma interferon and three other lymphokine activities: multiple activities of a single lymphokine? J Immunol. 1983 Aug;131(2):794–800. [PubMed] [Google Scholar]