Abstract
Objectives
The present study examines gender differences in changes in functional status after age 50 and how such differences vary across different age groups.
Methods
Data came from the Health and Retirement Study, involving up to six repeated observations of a national sample of Americans older than 50 years of age between 1995 and 2006. We employed hierarchical linear models with time-varying covariates in depicting temporal variations in functional status between men and women.
Results
As a quadratic function, the worsening of functional status was more accelerated in terms of the intercept and rate of change among women and those in older age groups. In addition, gender differences in the level of functional impairment were more substantial in older persons than in younger individuals, although differences in the rate of change between men and women remained constant across age groups.
Discussion
A life course perspective can lead to new insights regarding gender variations in health within the context of intrapersonal and interpersonal differences. Smaller gender differences in the level of functional impairment in the younger groups may reflect improvement of women’s socioeconomic status, greater rate of increase in chronic diseases among men, and less debilitating effects of diseases.
Keywords: Functional status, gender, hierarchical linear models
Extensive research has focused on gender differences in health. Verbrugge (1989) suggested that women show higher morbidity, whereas men suffer from greater mortality. Subsequent research revealed that gender differences in morbidity are more complicated in that their directions and magnitudes vary according to the particular condition and the stage of the life cycle (Arber & Cooper, 1999; Macintyre, Hunt, & Sweeting, 1996). However, there is very limited understanding of how men and women differ in the way health changes in middle and later life.
In cross-sectional studies of health states, intrapersonal change cannot be distinguished from interpersonal differences. Studies of health transitions between two points in time (e.g., Anderson, James, Miller, Worley, & Longino, 1998; Mor, Wilcox, Rakowski, & Hiris, 1994) have suggested that women are more likely to experience deterioration in functional status than men. However, these studies provide little information on the underlying growth curve or trajectory, which consists of multiple health transitions over time. Given the dynamic nature of health, transitions between two points in time often do not provide an accurate reflection of the true picture as they provide no basis for distinguishing among alternative growth curves or trajectories (Rogosa, 1988). A more complete understanding dictates an analysis of how men and women differ in the level of health as well as the speed of health change.
Numerous investigators have viewed aging and health from a life course perspective (Elder, 1985; Riley, 1987; Ryder, 1965). According to this perspective, health changes are transitions in later life, and, thus, the probability of these transitions, their timing, durations, and trajectories are important research concerns. In addition, researchers predict that increasing heterogeneity is a result of the accumulation of the effects of risk factors over the life course.
Current research regarding gender differences in health may be informed by a life course perspective. First, this requires an examination of the shape of intrapersonal changes (i.e., the level of health and how fast it changes) over an extended period of time. Empirically one would analyze the intercept and rate of change associated with a health growth curve or trajectory. Emphasizing the notion of gendered health careers, Moen and Chermack (2005) called for research to delineate gender differences in the nature and range of health pathways over the life course. A key research question in this regard is how men and women differ in the level of functional status and its rate of change over time.
Second, health trajectories in later life may differ significantly across age groups because of variations in birth cohorts and life stages. Birth cohorts may vary in a number of ways, including (a) composition (e.g., size, gender and racial mix, educational attainment), (b) history (e.g., changes in family and work, savings and pension accumulation, and war experience) of cohorts arriving at older age at different times, (c) transformations of social institutions (e.g., old-age support and health care), (d) technological development (e.g., information technology, biotechnology), and (e) changing relationships with other birth cohorts, e.g., intergenerational support (Uhlenberg & Miner, 1996). Researchers have attributed the recent decline of disability prevalence rates among older Americans to better income, education, and health care (Crimmins, Saito, & Reynolds, 1997; Manton, Corder, & Stallard, 1993; Schoeni, Freedman, & Wallace, 2001). In contrast, studies of health transitions have suggested that older age is associated with a greater probability of functional decline and decreased odds of stability and improvement over time (Anderson et al., 1998; Crimmins & Saito, 1993; Mor et al., 1994).
The present study aims to contribute to current knowledge of aging and health in four respects. We first offer quantitative estimates of the trajectory of functional health by using longitudinal data derived from a national sample of Americans older than 50 years of age for a period of up to 11 years (1995–2006). Second, we examine how the level and speed of change associated with functional status differ between men and women. Third, we evaluate how changes in functional status differ across age groups. Finally, we explore whether the gender gap in functional status varies across age groups.
To address our research questions, we propose the following four hypotheses.
Hypothesis 1: After age 50, functional status declines over time
Although it is widely recognized that functional health declines in middle and older life, most estimates are based on cross-sectional data confounding intrapersonal and interindividual differences. Quantitative depictions of the trajectory of functional decline after 50 have rarely been derived from national data over an extended period of time. Although some investigators have observed recovery from disability (Anderson et al., 1998; Hardy, Dubin, Holford, & Gill, 2005), such findings have been largely based upon health transitions over a relatively short interval of time (ranging from 1 month to 4 years). In the longer term (10 years or longer), functional status tends to decline (Beckett et al., 1996; Kahng, Dunkle, & Jackson, 2004; Liang et al., 2003).
Hypothesis 2: Relative to men, women experience more functional impairment at the baseline as well as a greater rate of increase in functional impairment
Current findings regarding gender differences in changes in functional status are mixed. There is some evidence that men and women are similar in the incidence of disability or rate of functional decline (Guralnik & Kaplan, 1989; Kahng et al., 2004; Liang et al., 2003). In contrast, some findings have revealed that women experience greater odds of functional impairment (Anderson et al., 1998; Leveille, Penninx, Melzer, Izmirlian, & Guralnik, 2000) or a greater rate of functional decline than men (Beckett et al., 1996). Still others have indicated that men suffer from more accelerated functional decline than women (Maddox & Clark, 1992; Mendes de Leon, Barnes, Bienias, Skarupski, & Evans, 2005). Finally, the vast majority of longitudinal studies treated gender as a control variable, and differences between men and women in health changes were not the primary focus of analysis (e.g., Kahng et al., 2004; Liang et al., 2003; Mendes de Leon et al., 2005). Further research is required to clarify gender differences in the trajectory of functional health. In the present study, we hypothesize that women not only experience higher functional impairment on average but also tend to decline functionally at a greater rate.
Hypothesis 3: Older individuals have not only a higher level of functional impairment at the baseline but also a greater rate of increase in functional impairment than younger people
Age differences in the trajectories of functional impairment observed over time reflect a combination of cohort and age effects. According to Gruenberg (1977), even with increasing life expectancy, there is little change in the ages of onset of morbidity and disability. Hence, there is little or no difference in disability between older cohorts and younger cohorts. In contrast, Fries (1983) proposed the notion of compression of morbidity, in that the onsets of morbidity and disability are delayed significantly in younger cohorts relative to older cohorts. Finally, even though declines in mortality may increase the prevalence of chronic diseases, the rates of progression for these diseases and thus disability may fall (Manton, 1982). Thus, there would be greater functional impairment among members of older cohorts compared to younger cohorts at the same ages.
Costa (2003) recently documented the decline in functional limitations (i.e., difficulty walking, difficulty bending, paralysis, blindness in at least one eye, and deafness in at least one ear) by comparing their prevalence rates among men aged 50 to 74 for the Union Army, National Health and Nutrition Examination Survey (1988–1994), and National Health Interview Survey (1988–1994). On average, functional limitations declined by about 40% from the early 1900s to the 1990s, with 24% of the decline attributable to reduction in the debilitating effect of chronic conditions and 37% to reduced rates of chronic conditions.
Fogel (2005) reinforced this observation by suggesting that age-specific prevalence rates of chronic diseases have declined substantially, and there has been a significant delay in the onset of chronic diseases during the course of the 20th century. These findings have led to a theory of technophysio evolution that points to a synergy between technological and physiological improvement leading to a form of human evolution that is biological but not genetic, rapid, culturally transmitted, and not necessarily stable (Fogel, 2005). Thus, there is evidence that health in a given population improves with the year of birth, with younger cohorts enjoying better health.
In addition, within a given birth cohort, age plays an important role. Research on health transitions strongly suggests that age is associated positively with the risk of functional decline and negatively with functional improvement (Anderson et al., 1998; Crimmins & Saito, 1993; Mor et al., 1994). Extrapolating from these findings, it is reasonable to hypothesize that health trajectories observed over different stages of life course could assume different patterns (e.g., health changes between ages 50 and 60 could differ from those between ages 75 and 85). In particular, among middle-age and older individuals, age is significantly associated with not only a higher level of functional impairment but also a greater rate of increase in impairment over time.
Hypothesis 4: Gender differences in changes in functional status increase with age
A key factor underlying the gender gap in disability is that women have more comorbidity and chronic health problems than men (Newman & Brach, 2001; Verbrugge, Lepkowski, & Imanaka, 1989). At the same time, there is evidence that the impact of chronic diseases on disability has been reduced over the years (Freedman & Martin, 2001). Indeed, Manton and colleagues (1993, Table 5) observed that women aged 65 and older experienced greater reduction than men in rates of activities of daily living (ADLs) and instrumental ADLs (IADLs) between 1982 and 1989. Moreover, Maddox and Clark (1992) showed that in the young-old (aged 58–63), functional trajectories converge over a 10-year period. Extrapolating from this body of research, we hypothesize that gender differences in the intercept and rate of change in functional status are smaller in younger ages than in older ages.
We evaluated these hypotheses within the context of the following conceptual framework. In particular, gender, age, ethnicity, and socioeconomic status (SES) are key dimensions of social stratification, with SES a function of the other three. Furthermore, gender and age differences in functional status are partially mediated by social networks and prior health status. A number of investigators (Crimmins & Seeman, 2000; House, Lantz, & Herd, 2005) have articulated this framework.
A brief rationale for including self-rated health and depressive symptoms as covariates is in order. In particular, Idler and Kasl (1995) found that self-rated health was a significant predictor of functional status. Self-rated health differs from a biomedical definition of health in that individuals evaluate health by using more inclusive criteria including not only diseases and physical functioning but also social comparison, role activities, and even emotional and spiritual well-being (Idler, Hudson, & Leventhal, 1999). Thus, in addition to morbidity, subjective health may affect physical disability. In contrast, Penninx, Leveille, Ferrucci, van Eijk, and Guralnik (1999) suggested that depression may affect functional status via unhealthy behaviors (e.g., smoking and excessive drinking and eating) and failure to obtain adequate health care and social support. In addition, depression may be an early sign of medical conditions that lead to physical disability.
METHODS
Design and Data
Data for the present study came from the Health and Retirement Study (HRS), which began in 1992 by surveying a national sample of more than 12,600 persons of the 1931–1941 birth cohort. In 1993, the Asset and Health Dynamics Among the Oldest Old (AHEAD) study was launched with a national sample of individuals aged 70 and older (i.e., born in 1923 or earlier). Thereafter follow-ups have been made of the HRS and AHEAD respondents approximately every 2 years. In 1998, the HRS and AHEAD studies were merged (i.e., the questionnaires were integrated and data collection was conducted concurrently), and two new subsamples were added: Children of the Depression (CODA)—persons born 1924 through 1930, and War Babies (WB)—persons born 1942 through 1947. As of 2006, these four components of the HRS yielded a total of 26,988 respondents, representing all individuals older than 50 years of age in the United States. Extensive documentation of the HRS is available at its Web site (http://hrsonline.isr.umich.edu).
In the present study, baseline data were obtained from respondents in 1995 for AHEAD, 1996 for HRS, and 1998 for CODA and WB. Follow-up data were gathered in 1998 (for AHEAD and HRS cohorts), 2000, 2002, 2004, and 2006. Hence, up to five or six repeated observations were obtained for each admission cohort over a period of 8 to 11 years. We included in our analysis only individuals who responded to two consecutive interviews at least once during the 1995–2006 period (because of our use of lagged time-varying covariates in the analysis; see the Measures section for more details). We excluded HRS data collected in 1992 and 1994 and AHEAD data collected in 1993 because of incomparable measures of functional status.
Response rates for the baseline waves as defined in the present study ranged from 70% (for WB in 1998) to 74% (for AHEAD in 1995). Follow-up response rates (i.e., for sample members who were already in the study) ranged from 84% (HRS in 2000) to 92% (CODA in 2000). As of 2004, the cumulative mortality rates were 16%, 55%, 18%, and 4% for the HRS, AHEAD, CODA, and WB, respectively. Exit interviews for deceased respondents have been conducted in every wave since 1995. They aim to ascertain the status and activities of the respondent from the last interview until death. Mortality rates were further linked to the National Death Index for validation. When a respondent was unable to be interviewed because of physical or cognitive limitations, a proxy interview was conducted. Occasionally, this was done when the individual was unwilling to take the time to be interviewed but consented to having someone else be interviewed as the proxy. Rates of proxy interviews ranged from 4.9% to 18.4% depending on the wave of data collection and birth cohort, with higher rates for members of older cohorts.
In all, 22,185 individuals completed the baseline interviews (i.e., 1995 for AHEAD, 1996 for HRS, and 1998 for CODA and WB). From this initial sample, we excluded 2,657 individuals with zero analytical weights (respondents were assigned zero weights if they were age-ineligible spouses, living outside the United States, or institutionalized.) In order to assess the effects of ethnicity, we also deleted 734 respondents who identified themselves as other than Black, White, or Hispanic American. Because our model specifications required lagged time-varying covariates, we further limited our analysis to individuals who responded to two consecutive waves of interview at least once. We deleted 308 individuals because they never responded to consecutive interviews between 1995 and 2006. This resulted in a final analytic sample of 18,486 individuals with a total of 71,124 observations of consecutive interview pairs (median number of interview pairs completed = 4). About half of these individuals (47%) responded to all six waves of the HRS (1995–2006), providing five pairs of consecutive interviews, and 79% completed at least three pairs of consecutive interviews.
Measures
We assessed functional status by using a count of difficulties with six ADLs (i.e., dressing, walking, bathing or showering, eating, getting into or out of bed, and using the toilet) and five IADLs (i.e., preparing hot meals, grocery shopping, making phone calls, taking medications, and managing own money and expenses). Each item was scored 0 as having no difficulty at all, and 1 as having at least some difficulty. The sum of these ADL and IADL items ranged from 0 to 11, and a higher score represented greater functional impairment. We based the decision to combine ADLs and IADLs on our separate analyses of these two indices that yielded very similar trajectories. Furthermore, using item response theory methods, Spector and Fleishman (1998) showed that ADL and IADL items could be combined to measure functional status with enhanced range and sensitivity.
We included several measures of social stratification. We measured age differences by the year of birth subtracted from 1995 or age in 1995, with a higher value representing an older age at baseline (range = 48–103; those aged 51 in 1998 who entered as a WB would be 48 in 1995). We created binary variables of gender and ethnicity (i.e., non-Hispanic White, non-Hispanic Black, and Hispanic). We indexed education by the number of years of schooling (starting at 0 and capped at 17 years). In addition, we took baseline functional status into account.
The model also adjusted for several time-varying covariates such as marital status and health conditions, which were measured at each wave of the survey. We used marital status as an indicator for social networks and constructed it as a binary variable (1 if the individual was married or living with a partner, and 0 otherwise). Diseases were a count of the number of health conditions (i.e., heart disease, cancer, stroke, diabetes, hypertension, lung disease, and arthritis) reported (range = 0–7). Self-rated health was a single-item rating of the respondent’s present health (1 = excellent, 2 = very good, 3 = good, 4 = fair, and 5 = poor). Depressive symptoms were represented by a count of nine dichotomous items drawn from the Center for Epidemiologic Studies–Depression scale (Radloff, 1977). These included (a) felt depressed, (b) everything was an effort, (c) restless sleep, (d) felt happy, (e) felt lonely, (f) enjoyed life, (g) felt sad, (h) couldn’t get going, and (i) had a lot of energy. We recoded items so that a higher score reflected more reported depressive symptoms. Finally, we coded as a binary variable whether, in each wave of the survey, the observation was obtained through a proxy interview.
To ensure that a clear time sequence was defined between the time-varying covariates and the outcome measure, our model involved the lagged measure (i.e., observation from the last interview) and the change term (i.e., change between the previous observation and the current observation) for each of the time-varying covariates. Table 1 presents the descriptive statistics of all of the measures.
Table 1.
Descriptive Statistics for Measures at Levels 1 and 2
| Unweighted | Weighted | |||
|---|---|---|---|---|
| Measure | M | SD | M | SD |
| Level 1 (n = 71,124) | ||||
| Functional status | 0.68 | 1.75 | 0.64 | 1.67 |
| Time since baseline (years) | 5.64 | 2.74 | 5.65 | 2.70 |
| Proxy status (lagged) | 0.07 | 0.25 | 0.06 | 0.24 |
| Marital status (lagged) | 0.69 | 0.46 | 0.68 | 0.47 |
| Self-rated health (lagged) | 2.79 | 1.12 | 2.72 | 1.12 |
| Diseases (lagged) | 1.74 | 1.26 | 1.66 | 1.26 |
| Depressive symptoms (lagged) | 1.89 | 2.09 | 1.86 | 2.07 |
| Δ Proxy status | 0.01 | 0.21 | 0.01 | 0.20 |
| Δ Marital status | −0.03 | 0.21 | −0.03 | 0.20 |
| Δ Self-rated health | 0.10 | 0.91 | 0.10 | 0.90 |
| Δ Diseases | 0.18 | 0.60 | 0.18 | 0.59 |
| Δ Depressive symptoms | 0.12 | 2.02 | 0.11 | 2.01 |
| Level 2 (n = 18,486) | ||||
| Died (between baseline and 2006) | 0.26 | 0.44 | 0.23 | 0.42 |
| Ever attrited (between baseline and 2006) | 0.09 | 0.29 | 0.09 | 0.29 |
| Age (in 1995) | 64.28 | 10.23 | 62.75 | 10.64 |
| Female | 0.57 | 0.50 | 0.55 | 0.50 |
| Non-Hispanic Black | 0.14 | 0.35 | 0.09 | 0.29 |
| Hispanic | 0.07 | 0.26 | 0.06 | 0.24 |
| Education | 11.94 | 3.37 | 12.26 | 3.22 |
| Baseline functional status | 0.42 | 1.36 | 0.39 | 1.30 |
Notes: At Level 1, attributes are those associated with repeated observations within individuals. Level 2 measures are those associated with individuals at baseline (i.e., 1995 for Health and Retirement Study, 1996 for Asset and Health Dynamics Among the Oldest Old, and 1998 for Children of the Depression and War Babies). When weighted, case weights at the time of survey for the Level 1 units and case weights in 1998 for the Level 2 units were applied.
Data Analysis
We used hierarchical linear modeling to describe how functional status changes over time (Raudenbush & Bryk, 2002) as follows (Level 1 or repeated observation model):
| (1) |
where YiT is the functional status for individual i at time T (e.g., 1998); π0i is the intercept of functional status for individual i; Time refers to the timing of assessment from the baseline; π1i is the rate of change (slope) in functional status for individual i over time; XkiT are the time-varying covariates such as lagged marital status, lagged health status, and their corresponding change terms associated with individual i at time T; πki represents the effect of Xk on individual i’s functional status; εiT represents random error in functional status for individual i at time T; and π1i represents intrapersonal changes or aging since the baseline.
For the sample as a whole, we considered both linear and nonlinear changes in health. We centered time at its mean in order to minimize the possibility of multicollinearity when we evaluated nonlinear functions of changes with time. Hence, the intercept represented the level of functional impairment at the mean time of follow-up.
Furthermore, we specified time-varying covariates because some explanatory variables (e.g., marital status and prior health status) may have changed over time. This is particularly true if an extended period of observation is involved. The inclusion of time-varying covariates has been rare in the study of health changes in old age.
To examine age and gender differences in the changes of functional status, we included these as predictors in the Level 2 (or person-level) equation in the multilevel analysis:
| (2) |
Here, Xqi is the qth time constant covariate (e.g., age in 1995 and gender) associated with individual i, and βpq represents the effect of Xq on the pth growth parameter (πp). rpi is a random effect with a mean of 0.
The HRS involves a national sample of households augmented by oversamples of African Americans, Hispanics, and Floridians. A major challenge confronted by an analyst of longitudinal data derived from the HRS is whether to weight the data. We chose not to weight the data based on the following reasons. First, although there is a consensus for weighting data in generating descriptive statistics for a given target population, there is no such agreement in multivariate analyses (Gelman, 2006; Groves, 1989, pp. 279–290). Second, many of the attributes (e.g., ethnicity, age, marital status) upon which unequal selection probabilities were based were explicitly controlled in the multivariate analyses. When sampling weights are solely a function of independent variables included in the model, unweighted ordinary least squares estimates are preferred because they are unbiased, are consistent, and have smaller standard errors than weighted estimates (Winship & Radbill, 1994). Third, we undertook multivariable analyses by using unweighted data as well as weighted data (by applying case weights at the time of survey for the Level 1 units and case weights in 1998 for the Level 2 units) and obtained very similar results.
Because of the massive sample of the HRS database (18,486 respondents with more than 71,000 observations), there was a concern about the overabundance of significant results due to the large sample size. To offset this, we considered only estimates with a p value less than .01 as statistically significant.
Missing Items, Mortality, Attrition, and Proxy Interview
To minimize the loss of participants due to item missing, we undertook multiple imputation. In particular, we imputed three complete data sets with the NORM software developed by Schafer (1997) and ran analyses on each of these three data sets. We derived parameter estimates and their standard errors by averaging across three imputations and by adjusting for their variance.
To assess the selection bias due to mortality and attrition, we included binary variables in the Level 2 equation to differentiate those with complete data during the period of study from those who died or dropped out of the study. However, we identified in the Level 1 equation those from whom a proxy interview was obtained because the proxy status could vary across interviews.
RESULTS
Trajectory of Change in Functional Status
We charted the trajectory of functional status over time by using linear and nonlinear functions and found strong evidence for a quadratic model defined by three parameters, including the intercept, linear slope, and quadratic slope. Consistent with Hypothesis 1, functional impairment at the mean time of follow-up was 0.903 out of a total of 11 points (M1 in Table 2). In addition, functional impairment increased over time at an increasing rate (with a linear slope of .155, p < .001, and a quadratic slope of .014, p < .001). The parameters associated with this quadratic function (i.e., intercept, linear and quadratic slopes) remained significant even when we adjusted all sociodemographic and health conditions (M4 in Table 2). For the sample as a whole, functional impairment increased from 0.44 ADL/IADLs at the baseline to 1.88 over 11 years (see Table 3).
Table 2.
Intrapersonal and Interpersonal Differences in Functional Status: Results From Hierarchical Linear Models (Unweighted Data)
| Covariate | M0 | M1 | M2 | M3 | M4 |
|---|---|---|---|---|---|
| Fixed effect | |||||
| Time variant variables | |||||
| Proxy (lagged) | 1.503*** | 1.506*** | 1.499*** | 1.023*** | |
| Married (lagged) | −0.003 | ||||
| Self-rated health (lagged) | 0.183*** | ||||
| Diseases (lagged) | 0.032*** | ||||
| Depressive symptoms (lagged) | 0.119*** | ||||
| Δ Proxy | 1.149*** | 1.144*** | 1.140*** | 0.932*** | |
| Δ Marital status | 0.042 | ||||
| Δ Self-rated health | 0.155*** | ||||
| Δ Diseases | 0.056*** | ||||
| Δ Depressive symptoms | 0.101*** | ||||
| For intercept, π0 | |||||
| Intercept | 0.864*** | 0.903*** | 0.906*** | 0.904*** | 0.877*** |
| Death | 1.602*** | 1.304*** | 1.309*** | 0.765*** | |
| Ever attrited | −0.116** | −0.123** | −0.126** | −0.089** | |
| Age (in 1995) | 0.028*** | 0.028*** | 0.018*** | ||
| Female | 0.361*** | 0.366*** | 0.183*** | ||
| Non-Hispanic Black | 0.467*** | 0.469*** | 0.117** | ||
| Hispanic | 0.406*** | 0.406*** | 0.026 | ||
| Age (in 1995) × Female | 0.012*** | 0.007** | |||
| Education | 0.003 | ||||
| Baseline functional status | 0.601*** | ||||
| For linear time slope, π1 | |||||
| Intercept | 0.096*** | 0.155*** | 0.149*** | 0.149*** | 0.134*** |
| Death | 0.353*** | 0.282*** | 0.283*** | 0.266*** | |
| Ever attrited | 0.005 | 0.003 | 0.003 | 0.003 | |
| Age (in 1995) | 0.006*** | 0.006*** | 0.005*** | ||
| Female | 0.017*** | 0.017*** | 0.019*** | ||
| Non-Hispanic Black | 0.025** | 0.025*** | 0.025*** | ||
| Hispanic | 0.008 | 0.008 | −0.005 | ||
| Age (in 1995) × Female | 0.001 | 0.001 | |||
| Education | –0.005*** | ||||
| Baseline functional status | −0.009** | ||||
| For quadratic rime slope, π2 | |||||
| Intercept | 0.000 | 0.014*** | 0.012*** | 0.012*** | 0.010*** |
| Death | 0.039*** | 0.033*** | 0.033*** | 0.031*** | |
| Ever attrited | 0.005 | 0.004 | 0.004 | 0.004 | |
| Age (in 1995) | 0.001*** | 0.001*** | 0.001*** | ||
| Female | −0.002* | −0.002 | −0.001 | ||
| Non-Hispanic Black | 0.001 | 0.001 | 0.001 | ||
| Hispanic | 0.003 | 0.003 | 0.001 | ||
| Age (in 1995) × Female | 0.000 | 0.000 | |||
| Education | −0.000 | ||||
| Baseline functional status | −0.002 | ||||
| Random effect | Variance | Variance | Variance | Variance | Variance |
| Intercept 1 | 3.117*** | 2.359*** | 2.229*** | 2.226*** | 1.222*** |
| Linear time slope | 0.042*** | 0.032*** | 0.028*** | 0.028*** | 0.026*** |
| Quadratic time slope | 0.001*** | 0.001*** | 0.001*** | 0.001*** | 0.001*** |
| Level 1, R | 0.806 | 0.781 | 0.783 | 0.783 | 0.767 |
| Akaike information criterion fit | 245,774.764 | 239,166.667 | 237,654.103 | 237,665.644 | |
| index | 224,592.653 | ||||
Notes: Reliability estimates are based on 14,542 of 18,486 units that had sufficient data for computation. Fixed effects and variance components are based on all the data. M = model.
p <.05;
p < .01;
p < .001.
Table 3.
Predicted Values of Functional Status (ADL/IADL) by Gender, Age in 1995, and Gender by Age in 1995
| Time Since Baseline |
Aggregate | Gender | Age in 1995 | Gender by Age in 1995 | |||||
|---|---|---|---|---|---|---|---|---|---|
| Male | Female | 55 | 75 | Male 55 | Male 75 | Female 55 | Female 75 | ||
| 0 | 0.44 | 0.40 | 0.47 | 0.39 | 0.50 | 0.38 | 0.41 | 0.39 | 0.57 |
| 1 | 0.47 | 0.42 | 0.51 | 0.42 | 0.53 | 0.40 | 0.43 | 0.43 | 0.60 |
| 2 | 0.52 | 0.46 | 0.57 | 0.46 | 0.59 | 0.43 | 0.48 | 0.48 | 0.67 |
| 3 | 0.59 | 0.52 | 0.65 | 0.52 | 0.68 | 0.48 | 0.56 | 0.55 | 0.77 |
| 4 | 0.68 | 0.60 | 0.75 | 0.58 | 0.80 | 0.53 | 0.67 | 0.62 | 0.90 |
| 5 | 0.80 | 0.70 | 0.87 | 0.66 | 0.96 | 0.60 | 0.82 | 0.70 | 1.06 |
| 6 | 0.93 | 0.82 | 1.01 | 0.74 | 1.14 | 0.67 | 0.99 | 0.80 | 1.25 |
| 7 | 1.08 | 0.96 | 1.17 | 0.84 | 1.35 | 0.76 | 1.19 | 0.90 | 1.48 |
| 8 | 1.25 | 1.12 | 1.35 | 0.95 | 1.60 | 0.85 | 1.43 | 1.02 | 1.73 |
| 9 | 1.44 | 1.30 | 1.55 | 1.06 | 1.87 | 0.96 | 1.69 | 1.14 | 2.01 |
| 10 | 1.65 | 1.50 | 1.77 | 1.19 | 2.18 | 1.08 | 1.99 | 1.28 | 2.33 |
| 11 | 1.88 | 1.72 | 2.01 | 1.33 | 2.52 | 1.21 | 2.31 | 1.43 | 2.67 |
Notes: All values are derived on the basis of Model 4 (M4) in Table 2 with time-varying covariates and mortality, attrition, and proxy interview adjusted. Time since the baseline was defined in years. The baseline was 1995 for Asset and Health Dynamics Among the Oldest Old, 1996 for Health and Retirement Study, and 1998 for War Babies and Children of the Depression. Although data were collected only in 1995, 1996, 1998, 2000, 2002, 2004, and 2006, estimates in other years were extrapolated from M4. ADL = activities of daily living; IADL = instrumental ADL.
Gender Differences in Functional Health Trajectory
Our findings lent support to Hypothesis 2 in that women experienced not only a higher level of impairment at mean time of follow-up (b = 0.361, p < .001) but also a greater linear rate of increase in disability (b = 0.017, p < .001; M2 in Table 2). The quadratic slope, however, did not differ by gender, indicating that functional disability increased with equal acceleration for men and women. These results remained robust even when we controlled marital status, prior health, and recent health changes, although some of the gender gap in the intercept could have been explained by differences in prior health and recent health changes (M4 in Table 2). Specifically, for men, the number of ADL/IADLs increased from 0.40 at the baseline to 1.72 over the 11 years, whereas it rose from 0.47 to 2.01 for women during the same period (see Table 3).
Age Differences in Functional Health Trajectory
Hypothesis 3 was supported in that older individuals had greater functional impairment at the mean time of follow-up (b = 0.028, p < .001) and a greater rate of worsening of functional health (b = 0.006, p < .001; M2 in Table 2). Moreover, the rate of change in functional disability accelerated more among older adults than younger adults (quadratic slope = 0.001, p < .001). Although sociodemographic and health covariates accounted for some age differences in the parameters of functional health trajectory, age differences remained significant with all covariates controlled for (M4 in Table 2). Thus, for someone aged 55 in 1995, the number of ADL/IADLs increased from 0.39 to 1.33 in 11 years, whereas for a person 75 years of age in 1995, it grew from 0.50 to 2.52 (see Table 3).
Gender by Age Interaction
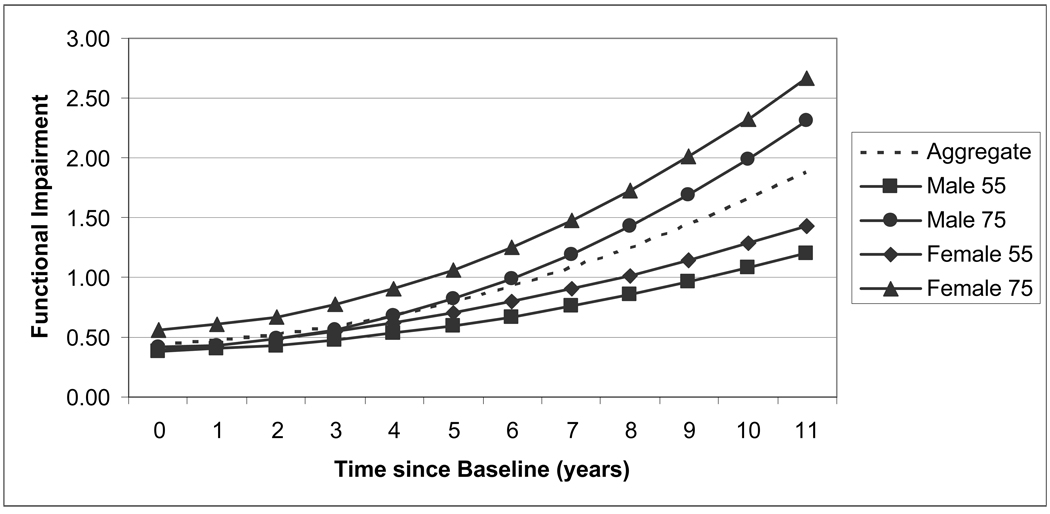
According to Hypothesis 4, gender differences in functional impairment in terms of the intercept and linear and quadratic slopes were greater in older age groups than younger age groups. Our results revealed that there was a statistically significant gender by age interaction effect on the intercept (b = 0.012, p < .001), but there was no such difference in the linear or quadratic slopes (M3 in Table 2). As shown in Table 3, the gender gap was consistently greater in the older age group than the younger age group. In particular, among men aged 55 in 1995, functional impairment increased from 0.38 to 1.21, whereas it increased from 0.39 to 1.43 for women over the 11-year period (see Table 3 and Figure 1). The gender gap increased from 0.01 to 0.22 over this period. In contrast, among those aged 75 in 1995, gender differences in changes in functional impairment were substantially greater. Functional impairment was 0.41 for men and 0.57 for women at the baseline and increased to 2.31 for men and 2.67 for women in 11 years. In short, the gender gap in functional status increased from 0.16 to 0.36 over 11 years.
Figure 1.
Functional impairment by gender and age in 1995. Time since baseline is the number of years since the baseline, which was 1995 for Asset and Health Dynamics Among the Oldest Old, 1996 for the Health and Retirement Study, and 1998 for War Babies and Children of the Depression.
Although the gender differences in both age groups increased over time, they grew by the same amount. That is, gender differences in ADL/IADLs increased equally by 0.20 over the 11-year period for those aged 55 and 75. Thus, the observed gender by age differences in the trajectory of functional impairment were essentially due to variations in the intercept instead of the rates of change, which remained robust even when we adjusted all sociodemographic and health covariates (b = 0.007, p < .01, M4 in Table 2).
Effects of Other Covariates
Even with SES, marital status, and health conditions adjusted, the main and interaction gender and age differences remained statistically significant, although they attenuated somewhat (see M3 and M4 in Table 2). This suggested that the observed gender and age differences in changes in functional status were only partially accounted for by education and health status.
Change in functional health was correlated with baseline sociodemographic and health attributes. African Americans had not only a higher level of functional impairment (b = 0.467, p < .001) but also a greater rate of worsening functional status (b = 0.025, p < .001) than White Americans (M2 in Table 2). Although Hispanics also had a higher level of functional impairment (b = 0.406, p < .001), they did not differ from White Americans in the rate of change (M2 in Table 2). Higher education was associated with a slower rise in functional impairment over time (b = −0.005, p < .001) but did not influence functional impairment at the baseline (M4 in Table 2).
Furthermore, functional impairment at the baseline was associated with greater functional impairment at the mean time of follow-up (b = 0.601, p < .001) but a slower rate of subsequent functional decrement (b = −0.009, p < .01; M4 in Table 2). Finally, persons with poor prior health conditions and/or recent decline in health (in terms of self-rated health, diseases, and depressive symptoms) experienced additional worsening in their functional status.
Mortality, Attrition, and Proxy Interview
Mortality was significantly associated with the intercept and slope of change in functional health. In particular, those who died during the period of follow-up had an elevated level of functional impairment (b = 1.602, p < .001) and a greater rate of worsening functional health (b = 0.353, p < .001) that became more accelerated over time (b = 0.039, p < .001; M1 in Table 2). Mortality remained significantly associated with changes in functional status even with the adjustment of sociodemographic characteristics and health (M4 in Table 2). In contrast, attrition during the period of follow-up did not seem to matter except that these persons had less functional impairment at the mean time of follow-up (b = −0.116, p < .01). Finally, having a proxy interview in the previous wave or newly having a proxy interview in the current wave was significantly associated with elevated functional impairment (b = 1.503, p < .001; and b = 1.149, p < .001, respectively; M1 in Table 2). Parameter estimates associated with the trajectory of functional decline would have been biased had we not controlled for mortality and proxy interview.
DISCUSSION
This study provides evidence that functional impairment accelerates with time among Americans older than 50 years of age. It lends support to the hypothesis that women not only have a higher level of functional impairment than men but also experience a faster decline in functional status after age 50. In addition, the increase in functional impairment is more accelerated in older age groups than younger age groups. Finally, gender differences in functional decline are more substantial in older age groups than younger age groups. These differences are a result of gender by age variations in the level of functional impairment instead of how fast functional impairment changes. To the best of our knowledge, no other study has focused on the gender by age interaction effects on the trajectory of functional status.
Methodologically, the present study differs from prior studies in that it is based upon multiwave longitudinal data derived from a national sample of Americans older than 50 years of age over an extended period of time. Moreover, by incorporating time-varying covariates and their changes during two adjacent waves, we analyzed how functional status evolves over time in a more dynamic fashion. Previous research was largely cross-sectional (e.g., Denton, Prus, & Walters, 2004; Verbrugge, 1989) or focused on health transitions between two points in time (e.g., Anderson et al., 1998; Strawbridge, Camacho, Cohen, & Kaplan, 1993). Even when these studies used multiwave longitudinal data, they often drew the data from limited locations or narrower age ranges (e.g., Beckett et al., 1996; Maddox & Clark, 1992; Mendes de Leon et al., 2005).
The present study complements prior studies based on cross-sectional data and health transitions by offering quantitative estimates of the parameters of the growth curve for functional health across gender and age groups. Whereas prior studies document that women have worse functional status than men and are more likely to experience functional decline than men, we are able to depict gender differences in terms of the level and the speed of change over a more extended period of time. At the same time, we are able to show that the trajectory of functional status is significantly more accelerated in older age groups than younger age groups. Although this result is consistent with the perspective of compression of morbidity, one needs to exercise caution because age and cohort effects are confounded in a time-based analysis such as ours.
Why is the gender gap in changes in functional status smaller in younger age groups than in older age groups? At least three mechanisms may account for this observation. First, improvement of women’s SES—such as education, occupation, income, and wealth—may lead to a significant reduction in functional impairment (Guralnik, Land, Blazer, Fillenbaum, & Branch, 1993; House et al., 2005). Indeed, younger cohorts of women fare better than older cohorts in terms of educational attainment, employment status, and division of household labor, and the large gender gaps that once existed have significantly diminished or disappeared altogether (Bae, Choy, Geddes, Sable, & Snyder, 2000). Nevertheless, the gender gap is far from erased for even the youngest cohorts in the HRS.
Second, the prevalence of chronic diseases has been increasing, and the rate of this increase may differ between men and women. Based on three repeated observations of a national sample of Americans aged 65 and older during the period 1986–1994, Kahng and associates (2004) observed that the rate of increase in chronic diseases was greater among men than women. Analyzing the prevalence of diseases between 1984 and 1994 among Americans 70 years of age or older, Crimmins and Saito (2000) found a significantly greater increase in most of the diseases among men than women.
Third, the effects of chronic diseases on functional status have become less debilitating (Freedman & Martin, 2001). If the diminishing effects are greater among women than men, then the gender gap may become smaller in younger age groups. Indeed, Crimmins and Saito (2000) reported that the severity of disability among women with most diseases (e.g., heart disease, stroke, and arthritis) has reduced, whereas there has been no reduction among men. Moreover, on the basis of data from the National Long Term Care Surveys, Manton and colleagues (1993, Table 5) observed that women aged 65 and older experienced greater reduction than men in rates of ADLs and IADLs between 1982 and 1989.
According to our findings, SES and prior health status partially mediate gender and age variations in the changes of functional status. This underscores the view that differences in SES and prior health status are not merely confounders of gender differences in functional status but an essential part of the causal pathway through which gender influences physical functioning. Gender differences in functional status reflect not only biological differences between men and women but also differences in privilege and power entailed in gender identities (Williamson & Boehmer, 1997). This calls for the examination of the total, direct, indirect, and interaction effects due to gender on health changes in later life.
Although the present study does not focus on ethnic differences in functional health, it offers some interesting observations in this regard. For instance, older Black Americans suffer a higher level of functional impairment as well as a greater rate of decline. In contrast, Hispanics experience a similar rate of functional decline as White Americans. Despite this, Hispanics have a significantly more elevated level of functional impairment. More important, ethnic differences in functional health appear to be explained by differences in SES and prior health. The extent to which gender differences interact with ethnic variations remains to be explored.
Substantively we are not quite sure why a higher level of baseline functional impairment is associated with a lower rate of functional decline. Nevertheless, we may suggest some clues for future inquiries. First, there could be a selection effect. Older adults who have a functional deficit and live in the community may get worse functionally, but the rate of the decline cannot be very rapid. Those with rapid functional decline would be institutionalized or would die quickly and thus would no longer be in the community. Although we have mortality as a control variable in our model, it is conceivable that this may not have eliminated all of the selection bias. Second, there might be significant heterogeneity in how functional status changes that was not explored in the present study. Depending on the underlying causes, as well as individual and environmental factors, disability may begin abruptly, progress slowly, remain stable, and even diminish over time. The average survival time after disability onset is highly variable, and it is not clear which factors determine length of survival (Ferrucci et al., 1996). Hence, among individuals who are significantly impaired, there is a possibility of a reduced rate of functional decline or even some modest improvement, whereas for those with no functional deficit at the baseline, the only possible change would be to remain functionally intact or to get worse at an accelerated pace. Further research on this is certainly required.
The present study can be improved in several respects. First, we based our analysis on time-based models, i.e., intrapersonal changes over the period of observation; (Alwin, Hofer, & McCammon, 2006). In such a specification, change is modeled as a function of time since the baseline, whereas in an age-based analysis, age rather than time since baseline is used in estimating the growth parameters. Even with multiple birth cohorts involved, a time-based analysis does not differentiate age effect from cohort effect.
We decided not to pursue an age-based analysis in this study because the HRS data are currently not suitable for the correct identification of cohort effects on changes in functional health. Such an analysis would require members of all cohorts to have been observed at the same ages over an extended period of time (i.e., 40 or more years). Most longitudinal studies, including the HRS, yield only data collected from members of different birth cohorts at different ages over a period of less than 20 years (Miyazaki & Raudenbush, 2000; Willson, Shuey, & Elder, 2007; Yang, 2007). Because different birth cohorts were observed at different ages, cohort and age effects were highly confounded. Although this is well known in a cross-sectional design, it is less obvious that such confounding exists in a longitudinal design with limited duration as well. Attempts to identify cohort effects by using age-based models with such data require extrapolations of intrapersonal changes to unobserved age ranges. This may lead to serious bias.
Second, because respondents of the HRS were sampled in middle and later life, differential mortality had already altered the representativeness of the original birth cohorts before they were eligible for inclusion (George, 2005). This is often referred to as left truncation, and it may lead to selection bias. The left-truncated cases sampled at the beginning of the observation period tend to overrepresent low-risk cases in a given cohort, or those who have a greater probability of survival. Furthermore, if the dependent variable of interest (e.g., functional status) is related to the risk, repeated observations over time derive from a biased population. In a sense, the selection process in a longitudinal study is actually a survival process. Currently there is very limited research addressing left truncation in longitudinal data analysis. This is because to control for selection bias due to left truncation, one would require information concerning the survival process from birth (or a very early age) up to the point that the respondents were recruited for the baseline observation. Such data are rarely available (for an exception, see Willson et al., 2007).
Left truncation is a salient issue when a dependent variable is highly correlated with the risk of dying and when survival is increasingly selective. Within the context of the present study, left truncation is likely to lead to a higher proportion of healthy respondents being included in the sample. This might underestimate the rate of increase in functional impairment over time. However, survival may be more selective among men than women. If this were the case, more women with poor health than men might be included in the HRS panel. This could lead to overstated gender differences in health. Nevertheless, it is unclear how large such biases are. More research on ways of adjusting for the biases due to left truncation is clearly warranted.
Third, assessing functional status once every 2 or 3 years could miss a significant portion of health dynamics. More frequent observations (i.e., weekly, monthly, or every 3–6 months) can yield much fine-grained data on changes in health and functioning (Hardy et al., 2005; Verbrugge, Reoma, & Gruber-Baldini, 1994). Such information is of great clinical and management value in improving the quality and efficiency of health care for the elderly. To synthesize data and knowledge of long-term as well as short-term health changes, the recently developed Bayesian hierarchical changepoint and mixture models could be quite useful (Skates, Pauler, & Jacobs, 2001).
Fourth, functional status is but one of the multiple dimensions of health and well-being. The disabling process consists of pathology, impairment, functional limitation, and disability. In contrast, key components of well-being include performance of social roles, physical status, emotional status, social interaction, intellectual functioning, economic status, and self-rated health (Pope & Tarlov, 1991). Conceivably, researchers can chart health trajectories in terms of all of these dimensions, and, more important, they need to examine how these trajectories vary across gender and age groups. The structural linkages among various dimensions of health and well-being have been of long-standing interest to researchers (e.g., Liang, 1986). Nevertheless, they have not been cast in a dynamic framework.
Finally, significant gaps remain in researchers’ knowledge concerning gender differences in health. A promising strategy would be to incorporate biomedical factors with psychosocial variables in future research. Genetic factors can explain up to one third of the variations in human life expectancy. Moreover, overall functioning, muscle strength, and gait speed have shown substantial heritability, suggesting genetic variations in the timing of the development of physical impairments (Melzer, Hurst, & Frayling, 2007). Neither biological nor social research alone can explain the complexity of gender differences in health. Only an integration of these perspectives can lead to the interdisciplinary dialogue and investigations required to close the gaps in the current knowledge (Rieker & Bird, 2005).
ACKNOWLEDGMENTS
This research was supported by Grants R01-AG154124 and R01-AG028116 (Jersey Liang, principal investigator) from the National Institute on Aging. The Japanese Ministry of Health, Labor and Welfare Longevity Foundation;the Tokyo Metropolitan Institute of Gerontology; and the Michigan Claude D. Pepper Older Americans Independence Center (P60-AG08808) provided additional support. We thank Steve Heeringa, Neil Alexander, and Caroline Blaum for their insightful comments and useful advice on data weighting and interpretation of findings.
J. Liang planned the study, supervised the data analysis, interpreted the findings, and drafted the manuscript. J. M. Bennett and A. R. Quiñones shared the responsibility of data management and statistical analysis and contributed to writing and revising the paper. B. A. Shaw helped in planning the study, analyzing the data, and writing and revising the manuscript. W. Ye provided statistical consultation and assisted in writing and revising the manuscript. X. Xu played a major role in implementing the dynamic modeling with time-varying covariates and in revising the manuscript. M. B. Ofstedal contributed to the compilation and management of longitudinal data derived from the Health and Retirement Study and assisted in writing and revising the manuscript.
REFERENCES
- Alwin DF, Hofer SM, McCammon RJ. Modeling the effects of time: Integrating demographic and developmental perspectives. In: Binstock RH, George LK, editors. Handbook of aging and the social sciences. San Diego, CA: Elsevier; 2006. pp. 20–38. [Google Scholar]
- Anderson RT, James MK, Miller ME, Worley AS, Longino CF. The timing of change: Patterns in transitions in functional status among elderly persons. Journal of Gerontology: Social Sciences. 1998;53B:S17–S27. doi: 10.1093/geronb/53b.1.s17. [DOI] [PubMed] [Google Scholar]
- Arber S, Cooper H. Gender differences in health in later life: The new paradox? Social Science & Medicine. 1999;48:61–76. doi: 10.1016/s0277-9536(98)00289-5. [DOI] [PubMed] [Google Scholar]
- Bae Y, Choy S, Geddes C, Sable J, Snyder T. Washington, DC: U.S. Government Printing Office; Trends in educational equity of girls and women. 2000 (National Center for Educational Statistics Report No. NCES200-30)
- Beckett LA, Brock DB, Lemke JH, Mendes de Leon CF, Guralnik JM, Fillenbaum GG, et al. Analysis of change in self-reported physical function among older persons in four population studies. American Journal of Epidemiology. 1996;143:766–778. doi: 10.1093/oxfordjournals.aje.a008814. [DOI] [PubMed] [Google Scholar]
- Costa DL. Changing chronic disease rates and long-term declines in functional limitation among older men. Demography. 2003;39(1):119–137. doi: 10.1353/dem.2002.0003. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Saito Y. Getting better and getting worse: Transitions in functional status among older Americans. Journal of Aging and Health. 1993;5(1):3–36. [Google Scholar]
- Crimmins EM, Saito Y. Change in the prevalence of diseases among older Americans: 1984–1994. [Retrieved May 4, 2007];Demographic Research. 2000 3 Article 9. from www.demographic-research.org/Volumes/Vol3/9/
- Crimmins EM, Saito Y, Reynolds SL. Further evidence on recent tends in the prevalence and incidence of disability among older Americans from two sources: The LSOA and the NHIS. Journal of Gerontology: Social Sciences. 1997;52B:S59–S71. doi: 10.1093/geronb/52b.2.s59. [DOI] [PubMed] [Google Scholar]
- Crimmins EM, Seeman T. Integrating biology into demographic research on health and aging (with a focus on the MacArthur Study of successful aging) In: Finch C, Vaupel J, Kinsella K, editors. Cells and surveys: Should biological measures be included in social science research? Washington, DC: National Academy Press; 2001. pp. 9–41. [PubMed] [Google Scholar]
- Denton M, Prus S, Walters V. Gender differences in health: A Canadian study of the psychosocial, structural and behavioral determinants of health. Social Science & Medicine. 2004;58:2585–2600. doi: 10.1016/j.socscimed.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Elder GH. Perspectives on the life course. In: Elder GH, editor. Life course dynamics. Ithaca, NY: Cornell University Press; 1985. pp. 23–49. [Google Scholar]
- Ferrucci L, Guralnik JM, Simonsick E, Salive ME, Corti C, Lanlois J. Progressive versus catastrophic disability: A longitudinal view of the disablement process. Journal of Gerontology: Medical Sciences. 1996;51A:M123–M130. doi: 10.1093/gerona/51a.3.m123. [DOI] [PubMed] [Google Scholar]
- Fogel RW. Changes in the disparities in chronic diseases during the course of the 20th century. Perspectives in Biology and Medicine. 2005;48(1):S150–S165. [PubMed] [Google Scholar]
- Freedman VA, Martin L. Contribution of chronic conditions to aggregate changes in old-age functioning. American Journal of Public Health. 2001;90:1755–1760. doi: 10.2105/ajph.90.11.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries JF. The compression of morbidity. Milbank Memorial Fund Quarterly. 1983;61:397–419. [PubMed] [Google Scholar]
- Gelman A. [Retrieved March 29, 2007];Struggle with survey weighting and regression modeling. 2006 from www.stat.columbia.edu/~gelman/
- George LK. Socioeconomic status and health across the life course: Progress and prospects. Journals of Gerontology: Psychological Sciences and Social Sciences. 2005;60B(Special Issue II):135–139. doi: 10.1093/geronb/60.special_issue_2.s135. [DOI] [PubMed] [Google Scholar]
- Groves RM. Survey errors and survey costs. New York: Wiley; 1989. [Google Scholar]
- Gruenberg EM. The failure of success. Milbank Memorial Fund Quarterly. 1977;55:3–24. [PubMed] [Google Scholar]
- Guralnik JM, Kaplan GA. Predictors of healthy aging: Prospective evidence from the Alameda County Study. American Journal of Public Health. 1989;79:703–708. doi: 10.2105/ajph.79.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guralnik JM, Land KC, Blazer D, Fillenbaum GG, Branch LG. Educational status and active expectancy among older blacks and whites. New England Journal of Medicine. 1993;329:110–111. doi: 10.1056/NEJM199307083290208. [DOI] [PubMed] [Google Scholar]
- Hardy SE, Dubin JA, Holford TR, Gill TM. Transitions between states of disability and independence among older persons. American Journal of Epidemiology. 2005;161:575–584. doi: 10.1093/aje/kwi083. [DOI] [PubMed] [Google Scholar]
- House JS, Lantz PM, Herd P Americans’ Changing Lives Study. Continuity and change in the social stratification of aging and health over the life course: Evidence from a nationally representative longitudinal study from 1986 to 2001/2002. Journals of Gerontology: Psychological Sciences and Social Sciences. 2005;60B(Special Issue II):15–26. doi: 10.1093/geronb/60.special_issue_2.s15. [DOI] [PubMed] [Google Scholar]
- Idler EL, Hudson SV, Leventhal H. The meaning of self-ratings of health. Research on Aging. 1999;21:458–476. [Google Scholar]
- Idler EL, Kasl SV. Self-ratings of health: Do they also predict change in functional ability? Journal of Gerontology: Social Sciences. 1995;50B:S344–S353. doi: 10.1093/geronb/50b.6.s344. [DOI] [PubMed] [Google Scholar]
- Kahng SK, Dunkle RE, Jackson JS. The relationship between the trajectory of body mass index and health trajectory among older adults: Multilevel modeling analyses. Research on Aging. 2004;26:31–61. [Google Scholar]
- Leveille SG, Penninx BWJH, Melzer D, Izmirlian G, Guralnik JM. Sex differences in the prevalence of mobility disability in old age: The dynamics of incidence, recovery, and mortality. Journal of Gerontology: Social Sciences. 2000;55B:S41–S50. doi: 10.1093/geronb/55.1.s41. [DOI] [PubMed] [Google Scholar]
- Liang J. The structure of self-reported physical health among aged adults. Journal of Gerontology. 1986;41:248–260. doi: 10.1093/geronj/41.2.248. [DOI] [PubMed] [Google Scholar]
- Liang J, Shaw BA, Krause N, Bennett JM, Blaum C, Kobayashi E, et al. Changes in functional status among older adults in Japan: Successful and usual aging. Psychology and Aging. 2003;18:684–695. doi: 10.1037/0882-7974.18.4.684. [DOI] [PubMed] [Google Scholar]
- Macintyre S, Hunt K, Sweeting H. Gender differences in health: Are things really as simple as they seem? Social Science & Medicine. 1996;42:617–624. doi: 10.1016/0277-9536(95)00335-5. [DOI] [PubMed] [Google Scholar]
- Maddox GL, Clark DO. Trajectories of functional impairment in later life. Journal of Health and Social Behavior. 1992;33(2):114–125. [PubMed] [Google Scholar]
- Manton KG. Changing concepts of morbidity and mortality in the elderly population. Milbank Memorial Fund Quarterly. 1982;60:183–244. [PubMed] [Google Scholar]
- Manton KG, Corder L, Stallard E. Estimate of change in chronic disability and institutional incidence and prevalence rates in the U.S. elderly population from the 1982, 1984, and 1989 National Long Term Care Survey. Journal of Gerontology: Social Sciences. 1993;48:S153–S166. doi: 10.1093/geronj/48.4.s153. [DOI] [PubMed] [Google Scholar]
- Melzer D, Hurst AJ, Frayling T. Genetic variation and human aging: Progress and prospects. Journal of Gerontology: Medical Sciences. 2007;62A:301–307. doi: 10.1093/gerona/62.3.301. [DOI] [PubMed] [Google Scholar]
- Mendes de Leon CF, Barnes LL, Bienias JL, Skarupski KA, Evans DA. Racial disparities in disability: Recent evidence from self-reported and performance-based disability measures in a population-based study of older adults. Journal of Gerontology: Social Sciences. 2005;60B:S263–S271. doi: 10.1093/geronb/60.5.s263. [DOI] [PubMed] [Google Scholar]
- Miyazaki Y, Raudenbush SW. Tests for linkage of multiple cohorts in an accelerated longitudinal design. Psychological Methods. 2000;5:44–63. doi: 10.1037/1082-989x.5.1.44. [DOI] [PubMed] [Google Scholar]
- Moen P, Chermack K. Gender disparities in health: Strategic selection, careers, and cycles of control. Journals of Gerontology: Psychological Sciences and Social Sciences. 2005;60B(Special Issue II):99–108. doi: 10.1093/geronb/60.special_issue_2.s99. [DOI] [PubMed] [Google Scholar]
- Mor V, Wilcox V, Rakowski W, Hiris J. Functional transitions among the elderly: Patterns, predictors, and related hospital use. American Journal of Public Health. 1994;84:1274–1280. doi: 10.2105/ajph.84.8.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman AB, Brach JS. Gender gap in longevity and disability in older persons. Epidemiologic Review. 2001;23:343–350. doi: 10.1093/oxfordjournals.epirev.a000810. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Leveille S, Ferrucci L, van Eijk JT, Guralnik JM. Exploring the effect of depression on physical disability: Longitudinal evidence from the Established Populations for Epidemiologic Studies of the Elderly. American Journal of Public Health. 1999;89:1346–1352. doi: 10.2105/ajph.89.9.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope AM, Tarlov AR. Disability in America: Toward a national agenda for prevention. Washington, DC: National Academy Press; 1991. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models. 2nd ed. Thousand Oaks, CA: Sage; 2002. [Google Scholar]
- Rieker PP, Bird CE. Rethinking gender differences in health: Why we need to integrate social and biological perspectives. Journals of Gerontology: Psychological Sciences and Social Sciences. 2005;60B(Special Issue II):40–47. doi: 10.1093/geronb/60.special_issue_2.s40. [DOI] [PubMed] [Google Scholar]
- Riley MW. On the significance of age in sociology. American Sociological Review. 1987;52:1–14. [Google Scholar]
- Rogosa DR. Myths about longitudinal research. In: Warner Schaie K, Campbell RT, Meredith WM, Rawlings SC, editors. Methodological issues in aging research. New York: Springer Publishing; 1988. pp. 171–209. [Google Scholar]
- Ryder N. The cohort as a concept in the study of social change. American Sociological Review. 1965;30:843–861. [PubMed] [Google Scholar]
- Schafer JL. Analysis of incomplete multivariate data. London: Chapman & Hall; 1997. [Google Scholar]
- Schoeni R, Freedman VA, Wallace R. Persistent, consistent, widespread, and robust? Another look at recent trends in old-age disability. Journal of Gerontology: Social Sciences. 2001;56B:S206–S218. doi: 10.1093/geronb/56.4.s206. [DOI] [PubMed] [Google Scholar]
- Skates SJ, Pauler DK, Jacobs IJ. Screening based on the risk of cancer calculation from Bayesian hierarchical changepoint and mixture models of longitudinal markers. Journal of the American Statistical Association. 2001;96:429–439. [Google Scholar]
- Spector WD, Fleishman JA. Combining activities of daily living with instrumental activities of daily living to measure functional disability. Journal of Gerontology: Social Sciences. 1998;53B:S46–S57. doi: 10.1093/geronb/53b.1.s46. [DOI] [PubMed] [Google Scholar]
- Strawbridge WJ, Camacho TC, Cohen RD, Kaplan GA. Gender differences in factors associated with change in physical functioning in old age: A 6-year longitudinal study. The Gerontologist. 1993;33:603–609. doi: 10.1093/geront/33.5.603. [DOI] [PubMed] [Google Scholar]
- Uhlenberg P, Miner S. Life course and aging: A cohort perspective. In: Binstock RH, George LK, editors. Handbook of aging and the social sciences. 4th ed. San Diego, CA: Academic Press; 1996. pp. 208–228. [Google Scholar]
- Verbrugge LM. The twain meet: Empirical explanations of sex differences in health and mortality. Journal of Health and Social Behavior. 1989;30(3):282–304. [PubMed] [Google Scholar]
- Verbrugge LM, Lepkowski JM, Imanaka Y. Comorbidity and its impact on disability. Milbank Quarterly. 1989;67:450–484. [PubMed] [Google Scholar]
- Verbrugge LM, Reoma JM, Gruber-Baldini AL. Short-term dynamics of disability and well-being. Journal of Health and Social Behavior. 1994;35(2):97–117. [PubMed] [Google Scholar]
- Williamson JB, Boehmer U. Female life expectancy, gender stratification, health status, and level of economic development: A cross-national study of less developed nations. Social Science & Medicine. 1997;45:305–317. doi: 10.1016/s0277-9536(96)00346-2. [DOI] [PubMed] [Google Scholar]
- Willson AE, Shuey KM, Elder GH., Jr Cumulative advantage processes as mechanisms of inequality in life-course health. American Journal of Sociology. 2007;112:1886–1924. [Google Scholar]
- Winship C, Radbill L. Sampling weights and regression analysis. Sociological Methods & Research. 1994;23(2):230–257. [Google Scholar]
- Yang Y. Is old age depressing? Growth trajectories and cohort variations in late life depression. Journal of Health and Social Behavior. 2007;48(1):16–32. doi: 10.1177/002214650704800102. [DOI] [PubMed] [Google Scholar]