Abstract
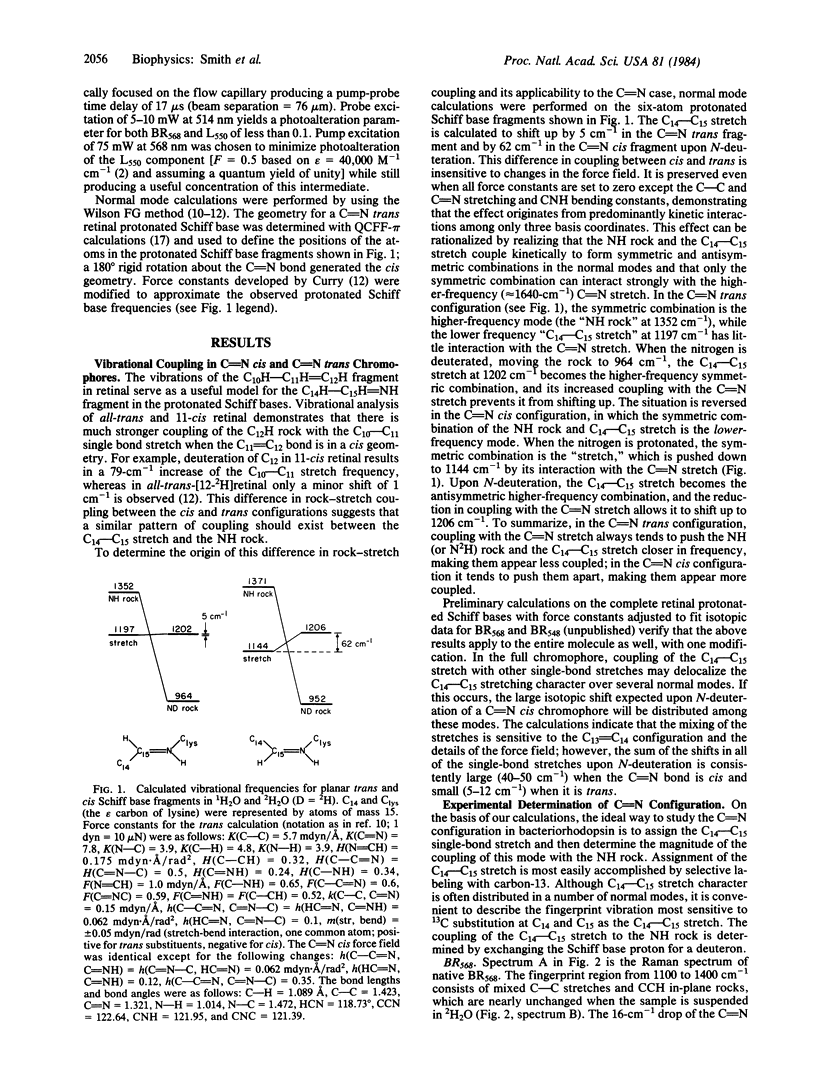
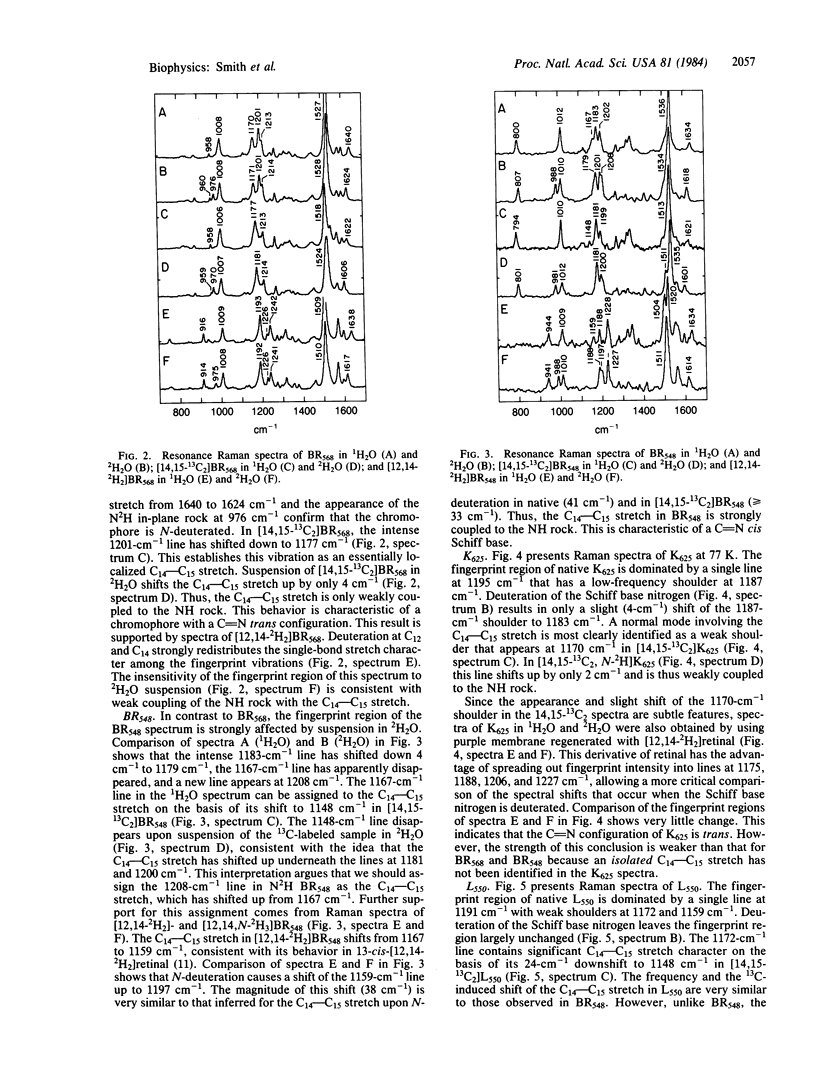
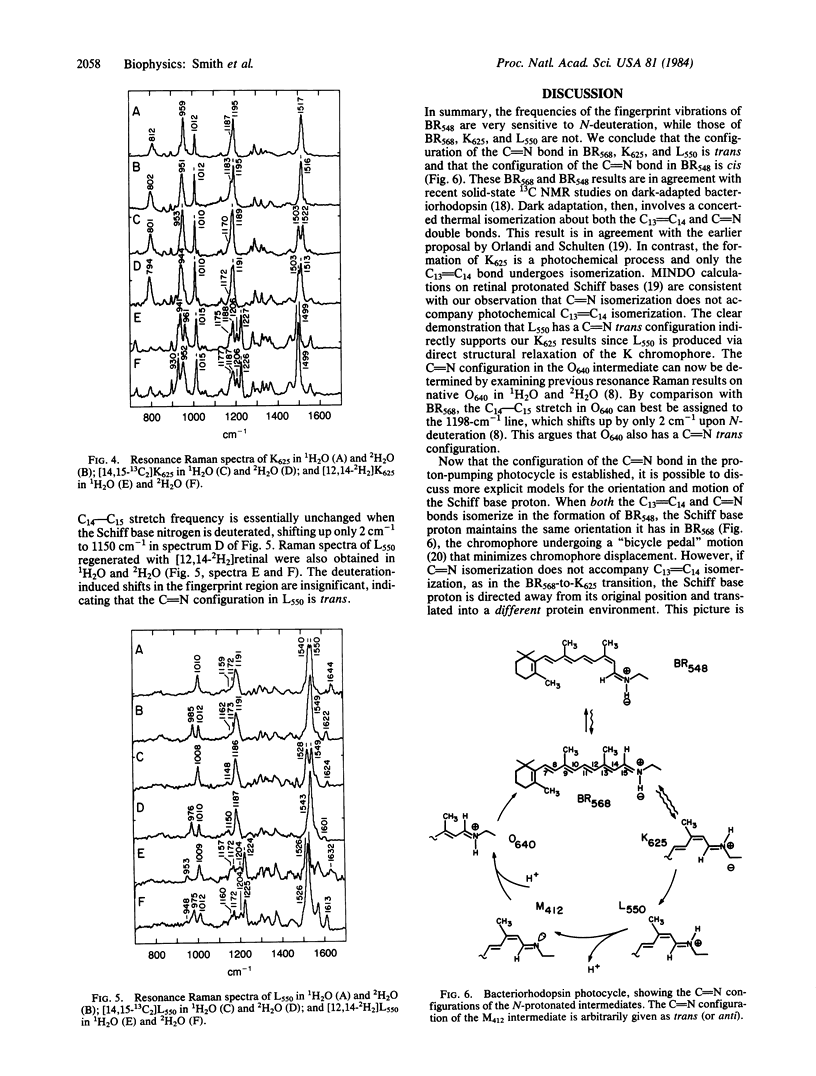
Resonance Raman spectra of the BR568, BR548, K625, and L550 intermediates of the bacteriorhodopsin photocycle have been obtained in 1H2O and 2H2O by using native purple membrane as well as purple membrane regenerated with 14,15-13C2 and 12,14-2H2 isotopic derivatives of retinal. These derivatives were selected to determine the contribution of the C14—C15 stretch to the normal modes in the 1100- to 1400-cm-1 fingerprint region and to characterize the coupling of the C14—C15 stretch with the NH rock. Normal mode calculations demonstrate that when the retinal Schiff base is in the C[unk]N cis configuration the C14—C15 stretch and the NH rock are strongly coupled, resulting in a large (≈50-cm-1) upshift of the C14—C15 stretch upon deuteration of the Schiff base nitrogen. In the C[unk]N trans geometry these vibrations are weakly coupled and only a slight (<5-cm-1) upshift of the C14—C15 stretch is predicted upon N-deuteration. In BR568, the insensitivity of the 1201-cm-1 C14—C15 stretch to N-deuteration demonstrates that its retinal C[unk]N configuration is trans. The C14—C15 stretch in BR548, however, shifts up from 1167 cm-1 in 1H2O to 1208 cm-1 in 2H2O, indicating that BR548 contains a C[unk]N cis chromophore. Thus, the conversion of BR568 to BR548 (dark adaptation) involves isomerization about the C[unk]N bond in addition to isomerization about the C13[unk]C14 bond. The insensitivity of the native, [14,15-13C2]-, and [12,14-2H2]K625 and L550 spectra to N-deuteration argues that these intermediates have a C[unk]N trans configuration. Thus, the primary photochemical step in bacteriorhodopsin (BR568 → K625) involves isomerization about the C13[unk]C14 bond alone. The significance of these results for the mechanism of proton-pumping by bacteriorhodopsin is discussed.
Keywords: resonance Raman spectroscopy, retinal isotopic derivatives, proton-pump mechanism, cis-trans isomerization, charge separation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braiman M., Mathies R. Resonance Raman evidence for an all-trans to 13-cis isomerization in the proton-pumping cycle of bacteriorhodopsin. Biochemistry. 1980 Nov 11;19(23):5421–5428. doi: 10.1021/bi00564a042. [DOI] [PubMed] [Google Scholar]
- Braiman M., Mathies R. Resonance Raman spectra of bacteriorhodopsin's primary photoproduct: evidence for a distorted 13-cis retinal chromophore. Proc Natl Acad Sci U S A. 1982 Jan;79(2):403–407. doi: 10.1073/pnas.79.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbison G. S., Smith S. O., Pardoen J. A., Winkel C., Lugtenburg J., Herzfeld J., Mathies R., Griffin R. G. Dark-adapted bacteriorhodopsin contains 13-cis, 15-syn and all-trans, 15-anti retinal Schiff bases. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1706–1709. doi: 10.1073/pnas.81.6.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig B., Ebrey T., Callender R. H., Dinur U., Ottolenghi M. Photoisomerization, energy storage, and charge separation: a model for light energy transduction in visual pigments and bacteriorhodopsin. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2503–2507. doi: 10.1073/pnas.76.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang K. S., Radhakrishnan R., Bayley H., Khorana H. G. Orientation of retinal in bacteriorhodopsin as studied by cross-linking using a photosensitive analog of retinal. J Biol Chem. 1982 Nov 25;257(22):13616–13623. [PubMed] [Google Scholar]
- Lewis A., Spoonhower J., Bogomolni R. A., Lozier R. H., Stoeckenius W. Tunable laser resonance raman spectroscopy of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4462–4466. doi: 10.1073/pnas.71.11.4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pande J., Callender R. H., Ebrey T. G. Resonance Raman study of the primary photochemistry of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7379–7382. doi: 10.1073/pnas.78.12.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J., Zagaeski M., Cantore W. A. Conformational changes of bacteriorhodopsin detected by Fourier transform infrared difference spectroscopy. Biochem Biophys Res Commun. 1981 Nov 30;103(2):483–489. doi: 10.1016/0006-291x(81)90478-2. [DOI] [PubMed] [Google Scholar]
- Stoeckenius W., Bogomolni R. A. Bacteriorhodopsin and related pigments of halobacteria. Annu Rev Biochem. 1982;51:587–616. doi: 10.1146/annurev.bi.51.070182.003103. [DOI] [PubMed] [Google Scholar]
- Tsuda M., Glaccum M., Nelson B., Ebrey T. G. Light isomerizes the chromophore of bacteriorhodopsin. Nature. 1980 Sep 25;287(5780):351–353. doi: 10.1038/287351a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Bicycle-pedal model for the first step in the vision process. Nature. 1976 Apr 22;260(5553):679–683. doi: 10.1038/260679a0. [DOI] [PubMed] [Google Scholar]
- Warshel A. Conversion of light energy to electrostatic energy in the proton pump of Halobacterium halobium. Photochem Photobiol. 1979 Aug;30(2):285–290. doi: 10.1111/j.1751-1097.1979.tb07148.x. [DOI] [PubMed] [Google Scholar]
- Warshel A., Karplus M. Calculation of pi-pi excited state conformations and vibronic structure of retinal and related molecules. J Am Chem Soc. 1974 Sep 4;96(18):5677–5689. doi: 10.1021/ja00825a001. [DOI] [PubMed] [Google Scholar]



