Abstract
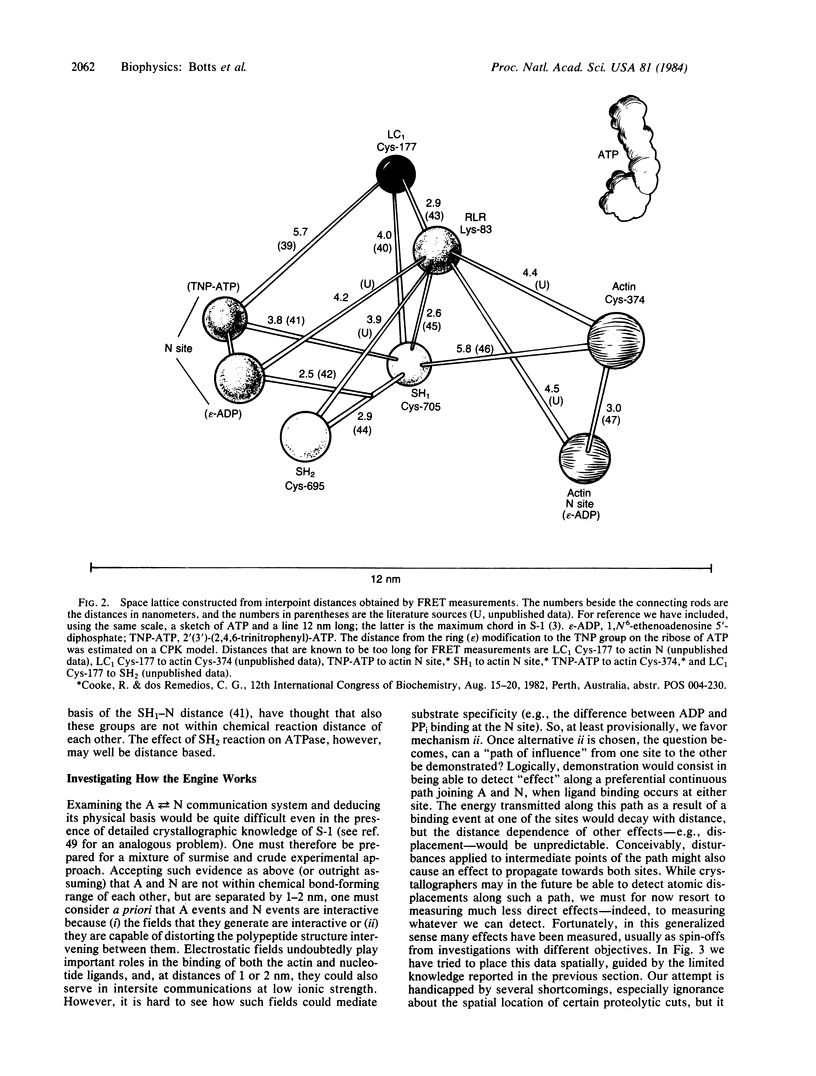
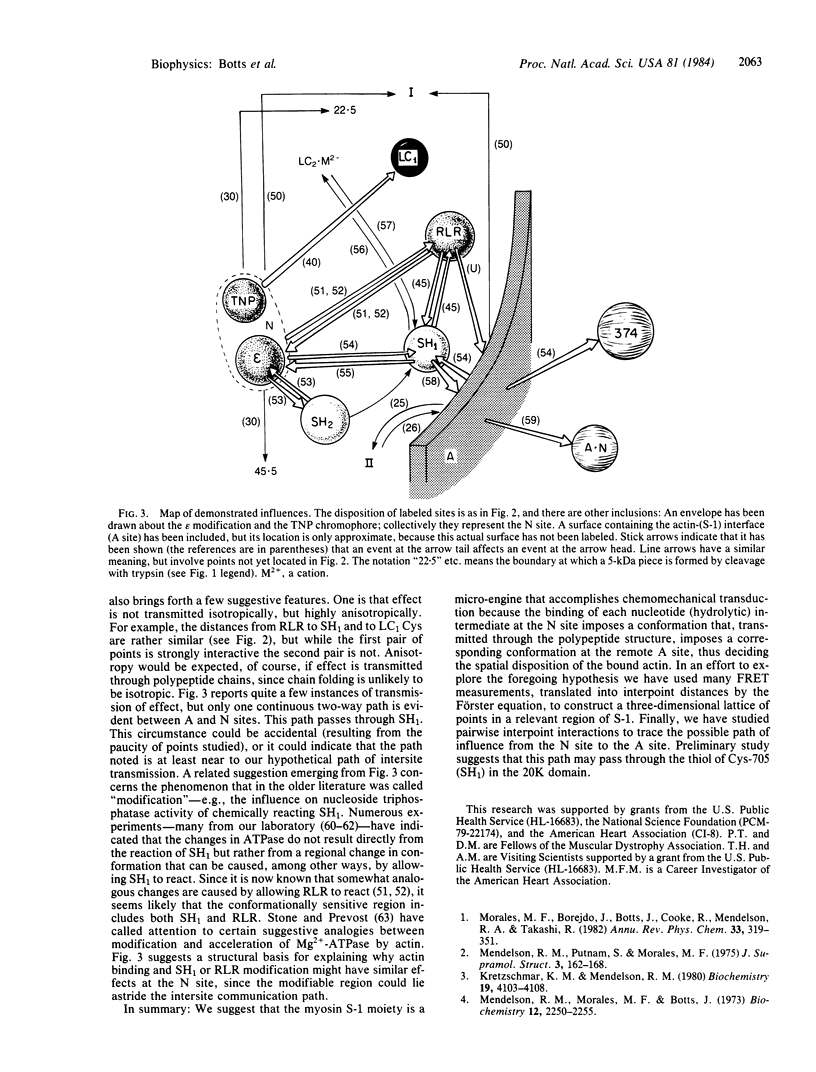
It is proposed that the myosin subfragment 1 moiety of the muscle contractile apparatus is a self-contained "engine" whose operational plan is based on the interactive nature of ATP (or degradation intermediate) binding and actin binding, made possible by an intersite communication system. It is suggested that the spatial information required for examining this engine can, at least provisionally, be derived from fluorescence resonance energy transfer measurements interpreted by the Förster equation and that the existence of an intersite communication system can be deduced from piece-wise detection of interacting pairs of points.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando T., Asai H. Conformational change in actin filament induced by the interaction with heavy meromyosin: effects of pH, tropomyosin and deoxy-ATP. J Mol Biol. 1979 Apr 5;129(2):265–277. doi: 10.1016/0022-2836(79)90280-8. [DOI] [PubMed] [Google Scholar]
- BARANY M., BARANY K. Studies on "active centers" of L-myosin. Biochim Biophys Acta. 1959 Oct;35:293–309. doi: 10.1016/0006-3002(59)90378-6. [DOI] [PubMed] [Google Scholar]
- Borejdo J., Putnam S., Morales M. F. Fluctuations in polarized fluorescence: evidence that muscle cross bridges rotate repetitively during contraction. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6346–6350. doi: 10.1073/pnas.76.12.6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botts J., Muhlrad A., Takashi R., Morales M. F. Effects of tryptic digestion on myosin subfragment 1 and its actin-activated adenosinetriphosphatase. Biochemistry. 1982 Dec 21;21(26):6903–6905. doi: 10.1021/bi00269a043. [DOI] [PubMed] [Google Scholar]
- Botts J., Ue K., Hozumi T., Samet J. Consequences of reacting the thiols of myosin subfragment 1. Biochemistry. 1979 Nov 13;18(23):5157–5163. doi: 10.1021/bi00590a020. [DOI] [PubMed] [Google Scholar]
- Burghardt T. P., Ando T., Borejdo J. Evidence for cross-bridge order in contraction of glycerinated skeletal muscle. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7515–7519. doi: 10.1073/pnas.80.24.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bálint M., Wolf I., Tarcsafalvi A., Gergely J., Sréter F. A. Location of SH-1 and SH-2 in the heavy chain segment of heavy meromyosin. Arch Biochem Biophys. 1978 Oct;190(2):793–799. doi: 10.1016/0003-9861(78)90339-9. [DOI] [PubMed] [Google Scholar]
- Chothia C., Lesk A. M., Dodson G. G., Hodgkin D. C. Transmission of conformational change in insulin. Nature. 1983 Apr 7;302(5908):500–505. doi: 10.1038/302500a0. [DOI] [PubMed] [Google Scholar]
- Collins J. H. Homology of myosin DTNB light chain with alkali light chains, troponin C and parvalbumin. Nature. 1976 Feb 26;259(5545):699–700. doi: 10.1038/259699a0. [DOI] [PubMed] [Google Scholar]
- Duke J. A., McKay R., Botts J. Conformational change accompanying modification of myosin ATPase. Biochim Biophys Acta. 1966 Nov 8;126(3):600–603. doi: 10.1016/0926-6585(66)90022-7. [DOI] [PubMed] [Google Scholar]
- Duke J., Takashi R., Ue K., Morales M. F. Reciprocal reactivities of specific thiols when actin binds to myosin. Proc Natl Acad Sci U S A. 1976 Feb;73(2):302–306. doi: 10.1073/pnas.73.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank G., Weeds A. G. The amino-acid sequence of the alkali light chains of rabbit skeletal-muscle myosin. Eur J Biochem. 1974 May 15;44(2):317–334. doi: 10.1111/j.1432-1033.1974.tb03489.x. [DOI] [PubMed] [Google Scholar]
- Fábián F., Mühlrad A. Effect of trinitrophenylation on myosin ATPase. Biochim Biophys Acta. 1968 Nov 26;162(4):596–603. doi: 10.1016/0005-2728(68)90065-0. [DOI] [PubMed] [Google Scholar]
- Hozumi T. Structure and function of myosin subfragment 1 as studied by tryptic digestion. Biochemistry. 1983 Feb 15;22(4):799–804. doi: 10.1021/bi00273a014. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Simmons R. M., Faruqi A. R., Kress M., Bordas J., Koch M. H. Millisecond time-resolved changes in x-ray reflections from contracting muscle during rapid mechanical transients, recorded using synchrotron radiation. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2297–2301. doi: 10.1073/pnas.78.4.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIELLEY W. W., BRADLEY L. B. The relationship between sulfhydryl groups and the activation of myosin adenosinetriphosphatase. J Biol Chem. 1956 Feb;218(2):653–659. [PubMed] [Google Scholar]
- KUBO S., TOKURA S., TONOMURA Y. On the active site of myosin A-adenosine triphosphatase. I. Reaction of the enzyme with trinitrobenzenesulfonate. J Biol Chem. 1960 Oct;235:2835–2839. [PubMed] [Google Scholar]
- Kiely B., Martonosi A. Kinetics and substrate binding of myosin adenosine triphosphatase. J Biol Chem. 1968 May 10;243(9):2273–2278. [PubMed] [Google Scholar]
- Labbé J. P., Mornet D., Roseau G., Kassab R. Cross-linking of F-actin to skeletal muscle myosin subfragment 1 with bis(imido esters): further evidence for the interaction of myosin-head heavy chain with an actin dimer. Biochemistry. 1982 Dec 21;21(26):6897–6902. doi: 10.1021/bi00269a042. [DOI] [PubMed] [Google Scholar]
- Langer J. A., Engelman D. M., Moore P. B. Neutron-scattering studies of the ribosome of Escherichia coli: a provisional map of the locations of proteins S3, S4, S5, S7, S8 and S9 in the 30 S subunit. J Mol Biol. 1978 Mar 15;119(4):463–485. doi: 10.1016/0022-2836(78)90197-3. [DOI] [PubMed] [Google Scholar]
- Marsh D. J., Lowey S. Fluorescence energey transfer in myosin subfragment-1. Biochemistry. 1980 Feb 19;19(4):774–784. doi: 10.1021/bi00545a025. [DOI] [PubMed] [Google Scholar]
- Mendelson R. A., Morales M. F., Botts J. Segmental flexibility of the S-1 moiety of myosin. Biochemistry. 1973 Jun 5;12(12):2250–2255. doi: 10.1021/bi00736a011. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Kretzschmar K. M. Structure of myosin subfragment 1 from low-angle X-ray scattering. Biochemistry. 1980 Aug 19;19(17):4103–4108. doi: 10.1021/bi00558a031. [DOI] [PubMed] [Google Scholar]
- Mendelson R., Putnam S., Morales M. Time-dependent fluorescence depolarization and lifetime studies of myosin subfragment-one in the presence of nucleotide and actin. J Supramol Struct. 1975;3(2):162–168. doi: 10.1002/jss.400030209. [DOI] [PubMed] [Google Scholar]
- Miki M., Mihashi K. Fluorescence energy transfer between epsilon-ATP at the nucleotide binding site and N-(4-dimethylamino-3,5-dinitrophenyl)-maleimide at Cys-373 of G-actin. Biochim Biophys Acta. 1978 Mar 28;533(1):163–172. doi: 10.1016/0005-2795(78)90560-3. [DOI] [PubMed] [Google Scholar]
- Morales M. F., Botts J. On the molecular basis for chemomechanical energy transduction in muscle. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3857–3859. doi: 10.1073/pnas.76.8.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M. F. Calculation of the polarized fluorescence from a labeled muscle fiber. Proc Natl Acad Sci U S A. 1984 Jan;81(1):145–149. doi: 10.1073/pnas.81.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mornet D., Bertrand R. U., Pantel P., Audemard E., Kassab R. Proteolytic approach to structure and function of actin recognition site in myosin heads. Biochemistry. 1981 Apr 14;20(8):2110–2120. doi: 10.1021/bi00511a007. [DOI] [PubMed] [Google Scholar]
- Mornet D., Bertrand R., Pantel P., Audemard E., Kassab R. Structure of the actin-myosin interface. Nature. 1981 Jul 23;292(5821):301–306. doi: 10.1038/292301a0. [DOI] [PubMed] [Google Scholar]
- Mornet D., Pantel P., Audemard E., Kassab R. The limited tryptic cleavage of chymotryptic S-1: an approach to the characterization of the actin site in myosin heads. Biochem Biophys Res Commun. 1979 Aug 13;89(3):925–932. doi: 10.1016/0006-291x(79)91867-9. [DOI] [PubMed] [Google Scholar]
- Mornet D., Ue K., Morales M. F. Proteolysis and the domain organization of myosin subfragment 1. Proc Natl Acad Sci U S A. 1984 Feb;81(3):736–739. doi: 10.1073/pnas.81.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. J., Trentham D. R. Distance measurement between the active site and cysteine-177 of the alkali one light chain of subfragment 1 from rabbit skeletal muscle. Biochemistry. 1983 Nov 8;22(23):5261–5270. doi: 10.1021/bi00292a004. [DOI] [PubMed] [Google Scholar]
- Muhlrad A., Hozumi T. Tryptic digestion as a probe of myosin S-1 conformation. Proc Natl Acad Sci U S A. 1982 Feb;79(4):958–962. doi: 10.1073/pnas.79.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad A., Morales M. F. Isolation and partial renaturation of proteolytic fragments of the myosin head. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1003–1007. doi: 10.1073/pnas.81.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern S. A., Eisenberg E. Interaction of spin-labeled and N-(iodacetylaminoethyl)-5-naphthylamine-1-sulfonic acid SH1-blocked heavy meromyosin and myosin with actin and adenosine triphosphate. Biochemistry. 1978 Oct 17;17(21):4419–4425. doi: 10.1021/bi00614a010. [DOI] [PubMed] [Google Scholar]
- RAINFORD P., HOTTA K., MORALES M. EXPERIMENTAS ON THE MODIFICATION OF MYOSIN NUCLEOSIDETRIPHOSPHATASE. Biochemistry. 1964 Sep;3:1213–1220. doi: 10.1021/bi00897a005. [DOI] [PubMed] [Google Scholar]
- Sivaramakrishnan M., Burke M. The free heavy chain of vertebrate skeletal myosin subfragment 1 shows full enzymatic activity. J Biol Chem. 1982 Jan 25;257(2):1102–1105. [PubMed] [Google Scholar]
- Srivastava S., Wikman-Coffelt J. An investigation into the role of SH1 and SH2 groups of myosin in calcium binding and tension generation. Biochem Biophys Res Commun. 1980 Feb 27;92(4):1383–1388. doi: 10.1016/0006-291x(80)90439-8. [DOI] [PubMed] [Google Scholar]
- Stone D. B., Prevost S. C. Characterization of modified myosin at low ionic strength. Enzymatic and spin-label studies. Biochemistry. 1973 Oct 9;12(21):4206–4211. doi: 10.1021/bi00745a026. [DOI] [PubMed] [Google Scholar]
- Sutoh K. Identification of myosin-binding sites on the actin sequence. Biochemistry. 1982 Jul 20;21(15):3654–3661. doi: 10.1021/bi00258a020. [DOI] [PubMed] [Google Scholar]
- Szilagyi L., Balint M., Sreter F. A., Gergely J. Photoaffinity labelling with an ATP analog of the N-terminal peptide of myosin. Biochem Biophys Res Commun. 1979 Apr 13;87(3):936–945. doi: 10.1016/0006-291x(79)92047-3. [DOI] [PubMed] [Google Scholar]
- Takashi R. Fluorescence energy transfer between subfragment-1 and actin points in the rigor complex of actosubfragment-1. Biochemistry. 1979 Nov 13;18(23):5164–5169. doi: 10.1021/bi00590a021. [DOI] [PubMed] [Google Scholar]
- Takashi R., Muhlrad A., Botts J. Spatial relationship between a fast-reacting thiol and a reactive lysine residue of myosin subfragment 1. Biochemistry. 1982 Oct 26;21(22):5661–5668. doi: 10.1021/bi00265a042. [DOI] [PubMed] [Google Scholar]
- Takashi R., Putnam S. A fluorimetric method for continuously assaying ATPase: application to small specimens of glycerol-extracted muscle fibers. Anal Biochem. 1979 Jan 15;92(2):375–382. doi: 10.1016/0003-2697(79)90674-2. [DOI] [PubMed] [Google Scholar]
- Tao T., Lamkin M. Excitation energy transfer studies on the proximity between SH1 and the adenosinetriphosphatase site in myosin subfragment 1. Biochemistry. 1981 Aug 18;20(17):5051–5055. doi: 10.1021/bi00520a035. [DOI] [PubMed] [Google Scholar]
- Tong S. W., Elzinga M. The sequence of the NH2-terminal 204-residue fragment of the heavy chain of rabbit skeletal muscle myosin. J Biol Chem. 1983 Nov 10;258(21):13100–13110. [PubMed] [Google Scholar]
- Trentham D. R., Eccleston J. F., Bagshaw C. R. Kinetic analysis of ATPase mechanisms. Q Rev Biophys. 1976 May;9(2):217–281. doi: 10.1017/s0033583500002419. [DOI] [PubMed] [Google Scholar]
- Wagner P. D., Giniger E. Hydrolysis of ATP and reversible binding to F-actin by myosin heavy chains free of all light chains. Nature. 1981 Aug 6;292(5823):560–562. doi: 10.1038/292560a0. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Saraste M., Runswick M. J., Gay N. J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1(8):945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren J. C., Stowring L., Morales M. F. The effect of structure-disrupting ions on the activity of myosin and other enzymes. J Biol Chem. 1966 Jan 25;241(2):309–316. [PubMed] [Google Scholar]
- Watterson J. G., Kohler L., Schaub M. C. Evidence for two distinct affinities in the binding of divalent metal ions to myosin. J Biol Chem. 1979 Jul 25;254(14):6470–6477. [PubMed] [Google Scholar]



