Abstract
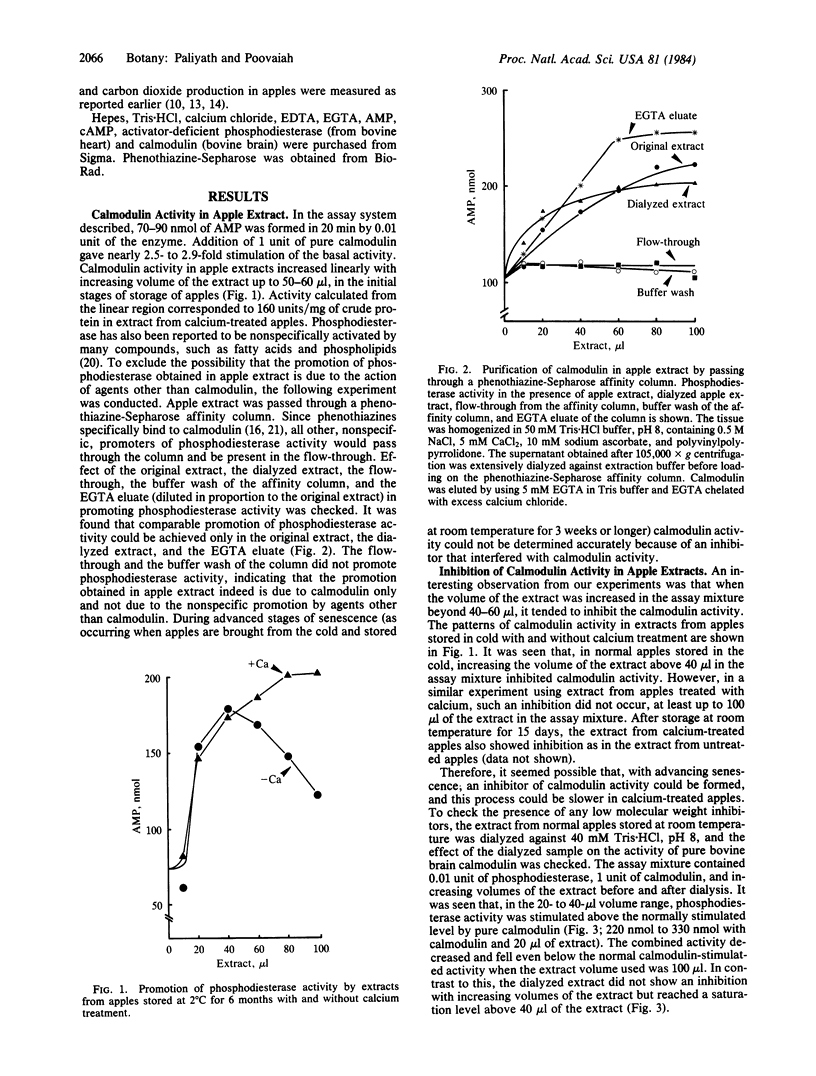
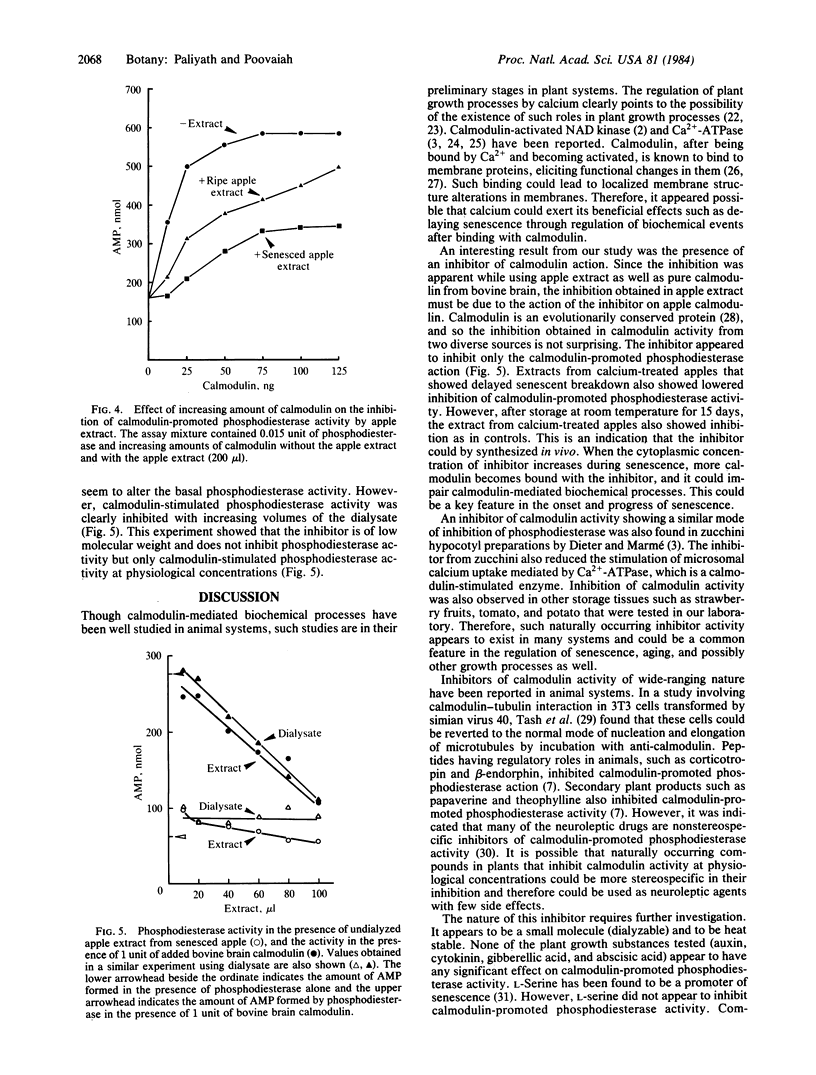
While assaying calmodulin activity in senesced apple extracts by using its property of promoting the activity of activator-deficient 3′,5′-cyclic AMP 5′-nucleotidohydrolase (phosphodiesterase, EC 3.1.4.17) from bovine heart, we detected a heat-stable, dialyzable, low molecular weight component that inhibited calmodulin activity. Specific activity of calmodulin as calculated from the linearly increasing portion of the activity curve was in the range of 150 to 160 units/mg of protein in crude extracts from apples stored at 2°C for a period of 6 months with or without calcium treatment. Apple extract that was passed through a phenothiazine-Sepharose affinity column did not promote phosphodiesterase activity, whereas the EGTA eluate of the column promoted phosphodiesterase activity similar to the original extract. The inhibition of calmodulin activity appeared to be lower in extracts from apples stored at 2°C after calcium treatment. The inhibition was found to increase after storage of apples at room temperature for 30 days. Activity of purified bovine brain calmodulin was also inhibited by the inhibitor present in apple extracts, which indicated that the inhibition was not specific to plant calmodulin alone and could have wide applications. The importance of the inhibitor in relation to senescence/aging and its possible pharmacological applications are discussed.
Keywords: calcium, calmodulin activity, senescence, aging, plants
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Charbonneau H., Jones H. P., McCann R. O., Cormier M. J. Characterization of the plant nicotinamide adenine dinucleotide kinase activator protein and its identification as calmodulin. Biochemistry. 1980 Jun 24;19(13):3113–3120. doi: 10.1021/bi00554a043. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Charbonneau H., Cormier M. J. Purification of plant calmodulin by fluphenazine-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1039–1047. doi: 10.1016/0006-291x(79)91931-4. [DOI] [PubMed] [Google Scholar]
- Dieter P., Marmé D. Calmodulin activation of plant microsomal Ca uptake. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7311–7314. doi: 10.1073/pnas.77.12.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinstein S., Furuya W. Calmodulin binding to platelet plasma membranes. Biochim Biophys Acta. 1982 Mar 23;686(1):55–64. doi: 10.1016/0005-2736(82)90151-1. [DOI] [PubMed] [Google Scholar]
- Jamieson G. A., Jr, Vanaman T. C. Calcium-dependent affinity chromatography of calmodulin on an immobilized phenothiazine. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1048–1056. doi: 10.1016/0006-291x(79)91932-6. [DOI] [PubMed] [Google Scholar]
- Kubowicz B. D., Vanderhoef L. N., Hanson J. B. ATP-Dependent Calcium Transport in Plasmalemma Preparations from Soybean Hypocotyls : EFFECT OF HORMONE TREATMENTS. Plant Physiol. 1982 Jan;69(1):187–191. doi: 10.1104/pp.69.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman J. A. Neuroleptic drugs are nonstereospecific inhibitors of calmodulin-stimulated phosphodiesterase activity. Ann N Y Acad Sci. 1980;356:415–416. doi: 10.1111/j.1749-6632.1980.tb29654.x. [DOI] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Effects of inorganic salts on tissue permeability. Plant Physiol. 1976 Aug;58(2):182–185. doi: 10.1104/pp.58.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R. K., Desai R., Thompson T. R., Wang J. H. Purification of the heat-stable inhibitor protein of the Ca2+-activated cyclic nucleotide phosphodiesterase by affinity chromatography. Can J Biochem. 1978 Jun;56(6):598–604. doi: 10.1139/o78-090. [DOI] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium-promoted protein phosphorylation in plants. Science. 1984 Jan 13;223(4632):167–169. doi: 10.1126/science.223.4632.167. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Waisman D. M. Calmodulin and its role in the second-messenger system. Curr Top Cell Regul. 1979;15:47–107. doi: 10.1016/b978-0-12-152815-7.50006-5. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Iverson D. B., Van Eldik L. J. Rapid separation and quantitation of 3',5'-cyclic nucleotides and 5'-nucleotides in phosphodiesterase reaction mixtures using high-performance liquid chromatography. J Biochem Biophys Methods. 1980 Mar;2(3):139–146. doi: 10.1016/0165-022x(80)90021-4. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Iverson D. B., Van Eldik L. J. Spinach calmodulin: isolation, characterization, and comparison with vertebrate calmodulins. Biochemistry. 1980 Dec 9;19(25):5762–5768. doi: 10.1021/bi00566a015. [DOI] [PubMed] [Google Scholar]
- Weiss B., Prozialeck W., Cimino M., Barnette M. S., Wallace T. L. Pharmacological regulation of calmodulin. Ann N Y Acad Sci. 1980;356:319–345. doi: 10.1111/j.1749-6632.1980.tb29621.x. [DOI] [PubMed] [Google Scholar]



