Abstract
Numerous CCT domain genes are known to control flowering in plants. They belong to the CONSTANS-like (COL) and PREUDORESPONSE REGULATOR (PRR) gene families, which in addition to a CCT domain possess B-box or response-regulator domains, respectively. Ghd7 is the most recently identified COL gene to have a proven role in the control of flowering time in the Poaceae. However, as it lacks B-box domains, its inclusion within the COL gene family, technically, is incorrect. Here, we show Ghd7 belongs to a larger family of previously uncharacterized Poaceae genes which possess just a single CCT domain, termed here CCT MOTIF FAMILY (CMF) genes. We molecularly describe the CMF (and related COL and PRR) gene families in four sequenced Poaceae species, as well as in the draft genome assembly of barley (Hordeum vulgare). Genetic mapping of the ten barley CMF genes identified, as well as twelve previously unmapped HvCOL and HvPRR genes, finds the majority map to colinear positions relative to their Poaceae orthologues. Combined inter-/intra-species comparative and phylogenetic analysis of CMF, COL and PRR gene families indicates they evolved prior to the monocot/dicot divergence ∼200 mya, with Poaceae CMF evolution described as the interplay between whole genome duplication in the ancestral cereal, and subsequent clade-specific mutation, deletion and duplication events. Given the proven role of CMF genes in the modulation of cereals flowering, the molecular, phylogenetic and comparative analysis of the Poaceae CMF, COL and PRR gene families presented here provides the foundation from which functional investigation can be undertaken.
Introduction
The control of flowering time is a crucial environmental adaptation in plants, as well as a major determinant of grain yield in cereal crops [1]. Numerous environmental and endogenous signals combine via intricate molecular pathways to determine flowering time, with seasonal changes in photoperiod representing major cues for floral transition [2]. Studies of photoperiod pathway mutants in the long day (LD) photoperiod responsive dicot, arabidopsis (Arabidopsis thaliana), have shown the transcription factor CONSTANS (CO) promotes flowering under LDs [3]. CO is an output of the circadian clock, showing circadian oscillations of expression regulated by the circadian clock and light-induced degradation, such that coincidence of peak expression with light only occurs under LD photoperiods [4]–[5]. Light-stabilized CO induces transcription of downstream genes such as the phytohormone FLOWERING LOCUS T (FT), which controls vegetative-to-reproductive transitioning of the shoot apical meristem by integrating inputs from various pathways [6]–[8]. The photoperiodic pathway is highly conserved within the plant kingdom, from the unicellular green alga Chlamydomonas reinhardtii [9] to tree species such as Populus tremula [10]. The observation that green micro-algae, but not earlier photosynthetic microorganisms such as diatoms and euglenoids, possess CO-like (COL) genes suggests that the gene family appeared during or soon after the endosymbiotic event in the photosynthetic lineage [5] and has evolved to regulate flowering in response to inductive daylengths in higher plants. In arabidopsis, CO belongs to a larger family of 17 COL genes, subdivided into three broad subgroups [11]. They encode proteins with a conserved region of 43 amino acids towards their carboxy-terminus, termed the CCT domain, which in arabidopsis interacts with CONSTITUITIVE PHOTOMORPHOGENIC 1 (COP1) protein to control CO stability [12]. In addition to the CCT domain, COL proteins contain one or two zinc-finger B-box domains towards the amino terminus, thought to be involved in protein-protein interactions [5]. While COL genes have been identified in various plant species [11], [13]–[14], comparatively little is known about their function. As well as COL genes, an additional CCT domain gene family has been shown to play a role in the photoperiod pathway. Termed PSEUDO RESPONSE REGULATORs (PRRs), they contain a response regulator domain towards the amino-terminus, as well as a CCT domain at the carboxy-terminus. PRR genes were first identified in arabidopsis, where PRR1 (TOC1) acts within the central oscillator of the circadian clock [15]. Although five PRR genes are found in arabidopsis, the precise function of the remaining four genes has not been determined [16]. PRR genes are also found in the Poaceae, with rice (Oryza sativa L.), barley (Hordeum vulgare ssp. vulgare L.) and sorghum (Sorghum bicolour L. Moench) all possessing five members [17]–[20].
In cereals, map-based cloning shows that natural variation within CCT domain genes have been critical in the control of flowering and spread of cereal domestication. Orthologous Poaceae PRR genes are known to encode LD photoperiod responsive loci in barley (PPD-H1, encoded by HvPRR37), sorghum (Ma1, encoded by SiPRR37) and hexaploid bread wheat Triticum aestivum L. (PPD-D1, encoded by TaPRR37-D) [18], [20]–[21]. Recessive alleles at all loci result in a delay in flowering under LDs, and have aided their spread of domestication into new agricultural environments. Furthermore, OsPRR37 has been mapped to the rice Heading date 2 (Hd2) quantitative trait locus (QTL) interval, with the photoperiod insensitive Kasalath allele associated with a severe mutational lesion in OsPRR37 [17]. Similarly, COL genes have been shown to control natural variation in flowering time in rice, where Heading date1 (Hd1, homologous to CO) induces the transcription of Hd3a (homologous to FT) promoting flowering under short day (SD) photoperiods [22]–[24]. Genes belonging to a Poaceae-specific diverged COL subgroup, previously termed the group IV COL genes [13], have been shown to control flowering time in temperate cereal species. The first to be identified were the ZCCT genes that underlie colinear VRN-2 vernalization response loci in temperate cereals. Their predicted proteins encode a CCT domain and a putative single amino zinc finger, divergent from the B-box domains found in other COL genes [25]. In the diploid wheat T. monococcum, the VRN-Am2 locus was found to be encoded by VRN2 (also termed ZCCT1, and used hereafter) [26]. Subsequently, orthologous ZCCT genes were found to co-segregate with the VRN-H2 locus in barley [27] and the VRN-A1/VRN-B1 loci in the tetraploid wheat, T. turgidum [28]. Recessive mutant alleles at colinear cereal VRN-2 genetic loci abolish vernalization response, resulting in spring-sown crop ideotypes that allowed flexibility of cultivation and adaptation to new agricultural environments. In spring barley, the three ZCCT-H genes underlying the VRN-H2 locus have been deleted in all accessions investigated to date [29]–[32]. Similarly, ZCCT copy number variation is associated with flowering time in tetraploid wheat [28]. Interestingly, no ZCCT genes are present at the colinear regions of the SD tropical plant rice, or in the sequenced rapid cycling accession of brachypodium [33]. Recently, map-based cloning of the rice flowering time locus Ghd7 (previously termed, OsI [13]) has found it to be encoded by a circadianly regulated group IV COL gene [34]. Enhanced expression of Ghd7 under non-inductive LD photoperiods delays flowering. Furthermore, naturally occurring Ghd7 deletions/mutations alleviate floral repression under LDs. Thus, differential retention/deletion/mutation of genes containing CCT domains appears to have been critical in the domestication and adaptation of cereal crops. The Triticeae ZCCT genes, along with Ghd7 and cereal orthologues of the rice gene OsH form a distinct grouping within the COL genes [26], [28]–[29]. By comparing inter- and intra-species microcolinearity between rice, brachypodium and barley, we previously showed that the ZCCT genes and OsH are evolutionarily related [33], forming a paralogous gene-pair that arose during the whole genome duplication event that occurred in the ancestral cereal genome [35]–[36].
While sixteen COL genes (OsA-OsP) have been identified in rice [13], the total should strictly be reduced to fourteen, due to the lack of B-box domains in OsH and Ghd7. Recently, eight members homologous to arabidopsis Activator of Spomin::LUC2 (ASML2) have been identified, which also encode proteins possessing just a single CCT domain [37]. However, no systematic investigation of this class of gene has been undertaken in any Poaceae species, or indeed, in arabidopsis. Preliminary investigation in rice shows that such genes are relatively common, with thirteen members (including OsH and Ghd7) identifiable in the current genome assembly. Accordingly, we name this group of genes the ‘CCT MOTIF FAMILY’ (CMF), following the protein annotation in the rice genome assembly. Thus, while analyses of COL or PRR genes and gene families have been previously undertaken to varying extents in barley, rice, sorghum and brachypodium [13], [19]–[20], comprehensive analysis of all CCT domain gene families known to possess members that regulate flowering in the Poaceae, and specifically the largely unrecognised CMF gene family, is yet to be undertaken in the grasses. To determine if CMF genes arose independently by species-specific 5′ truncation of COL or PRR genes, or whether they represent a more ancient gene family, we undertake molecular, phylogenetic and comparative genomic analysis of the CMF, COL and PRR gene families in sequenced Poaceae genomes, as well the crop species barley, which lacks a sequenced physical map. Finally, we propose a systematic nomenclature for Poaceae CMF genes, based on orthology between species.
Materials and Methods
Bioinformatic analysis
CMF, COL and PRR gene families were determined in the current rice genome assembly (Oryza sativa ssp. japonica cv. Nipponbare, MSU Osa1 assembly v6.1, http://rice.plantbiology.msu.edu/) using BLASTn searches (match / mismatch scores = 2,3; gap costs: existence = 5, extension = 2) and an expectation (e)-value threshold of 1.0e-30. For the COL family, coding regions (CDS) for each of the fourteen known rice genes [13] were used as queries, while for the PRR family, CDS of the five genes listed by [18] were used. CMF genes were identified in rice and arabidopsis (TAIR annotation release 10 of the A. thaliana genome, release 9) by BLAST analysis of CDS from OsH, OsI [13] and arabidopsis homologues [37]. Unless otherwise stated, primary transcripts were used throughout. Predicted proteins were translated using the VectorNTI Advance package v10.1.1 (Invitrogen) and protein domains determined using Pfam v25.0 [38] and Prosite v20.79 (http://prosite.expasy.org/). Amino acid sequence representation was undertaken using WebLogo [39]. Proteins identified as containing a CCT motif (and lacking additional domains) were annotated as CMF genes. Proteins additionally containing B-box and pseudoresponse regulator domains towards their N-termini were classified as COL or PRR genes, respectively. Following the approaches listed above, COL, PRR and CMF gene families were determined in B. distachyon accession BD21 (assembly v1.0, using sequence data produced by the US Department of Energy Joint Genome Institute, http://modelcop.org/), S. bicolour L. Moench accession BTx623 (v1.0, http://phytozome.net/), and Setaria italica cv. Yugu1 (foxtail millet assembly v1.0, using sequence data produced by the US Department of Energy Joint Genome Institute, http://phytozome.net/). Barley CMF, COL and PRR sequences were identified by BLASTn searches of the rice CDS versus a 28× NGS genomic survey of cv. Morex (generated by the International Barley Sequencing Consortium, available at http://mips.helmholtz-muenchen.de/plant/triticeae/) or fl-cDNAs [40]. De novo gene predictions and reassessments were conducted using FGENESH (http://www.softberry.com/) using ‘monocot’ as the basis of gene prediction. Homology groupings between CMF, COL and PRR genes identified in brachypodium, sorghum, foxtail millet and barley were verified by back-BLASTn searches of the rice genome, to ensure highest sequence similarity to the original rice query sequence. Genes are prefixed with the genus and species initials.
Phylogenetic analysis
CCT domain protein sequences were aligned using ClustalW [42] and manually edited using GENEDOC v2.6 (http://www.nrbsc.org/gfx/genedoc/). Phylogenetic analysis was conducted on the resulting alignments using the PHYLIP package v3.5 [43]. Unrooted phylogenies were determined using the distance matrix method, with tree topographies supported by bootstrapping (1,000 replicates).
Genetic mapping
Genomic DNA was extracted from leaf material using the DNeasy 96 Plant Kit (Qiagen). Genetic mapping was undertaken in the barley OWB doubled haploid population [44]. Primers for SNP identification in parental lines and genetic mapping in the complete population were designed using Primer3 v0.4.0 (http://primer3.sourceforge.net/) (Table 1). PCR amplification was carried out in 10 µl reactions using the reagents shipped in the FastStart Taq DNA polymerase kit (Roche). PCR cycling was carried out using a Veriti 96 well Thermo Cycler Thermocycler (Applied Biosystems) with the parameters: 5 min at 96°C, followed by 35 cycles of 50 sec at 96°C, 50 sec annealing temperature, 90 sec at 72°C, final extension of 7 min at 72°C. Annealing temperatures are listed in Table 1. Sequencing in parental lines used a minimum of three independent PCRs as templates for sequencing using BigDye kit v3.1 (Applied Biosystems), following the protocols described by [45]. Sequence traces were manipulated using VectorNTI. DNA polymorphisms were genotyped in the mapping population by direct sequencing of PCR amplicons using the polymorphisms listed in Table 1. Genetic mapping was conducted using JoinMap v3.0 [46]. Genetic map positions of COL, CMF, PRR and ZCCT genes in the OWB population were integrated into the barley consensus map [47] based on co-segregation with genetic markers common to both maps. Genomic nucleotide sequences derived from OWB-D and OWB-R parental lines have been deposited in GenBank under accession numbers JQ791213–JQ791252.
Table 1. Genetic mapping of barley genes.
| Gene | Rice orthologue | Primer sequence (5′→3′)1 | A/GC2 | GenBank accession3 | Mapped SNP | Chromosome (cM)4 |
| HvCO10 | Os03g50310 | F: CACCTCCTCCCTTCAGCAC | 60°C/+ | D: JQ791236 | G68/T | 4Hs (32.5) |
| R: CGTATCTTCTTGGCGAACAG | R: JQ791237 | |||||
| HvCO11 | Os02g49880 | F: CGTTCTACATGGAGGCCCTA | 60°C/+ | D: JQ791238 | G93/A | 6Hl (93.1) |
| R: TTCTGTTCCCGGTCATTCTC | R: JQ791239 | |||||
| HvCO12 | Os06g15330 | F: AGCACAAGTGTGACGTGGAG | 60°C/+ | D: JQ791240 | A285/G | 7H (74.1) |
| R: GATGCACCATACCGTGACTG | R: JQ791241 | |||||
| HvCO13 | Os06g19444 | F: CTTCCGAAGCTGGTCTGAAC | 58°C/+ | D: JQ791242 | G300/T | 7H (74.1) |
| R: TGTTGCATATGCCAGTCCAT | R: JQ791243 | |||||
| HvCO14 | Os02g49230 | F: GCCGCATCTAGCTTTTTGTC | 60°C/+ | D: JQ791244 | C3824/T | 6Hl (89.7) |
| R: CCACGTTTTGAGTTGTGGT | R: JQ791245 | |||||
| HvCO15 | Os08g424405 | F: TTCTCCGATCGGTTTTTCAC | 60°C/+ | D: JQ791246 | T92/G | 7H (80.7) |
| R: AGCTGAGAGGCCTACCACAA | R: JQ791247 | |||||
| HvCO16 | Os03g22770 | F: CATGGAGAAGCCGTTCTTGT | 60°C/+ | D: JQ791248 | A128/G | 4Hl (54.4) |
| R: TTGCAACCTCATGGCTGTTA | R: JQ791249 | |||||
| HvCO18 | Os07g47140 | F: CAACAACAAGGTGGACGAGA | 58°C/+ | D: JQ791250 | C640/G | 2Hs (55.2) |
| R: CAAGATCAGCATGCCCAGTA | R: JQ791251 | |||||
| HvCMF1 | Os01g61900 | F: GACCCATCCATCGCCTACTA | 60°C/− | D: JQ791213 | C340/G | 3Hl (127.9) |
| R: GCATTCTTCTCGTCCTCGTC | R: JQ791214 | |||||
| HvCMF3 | Os02g05470 | F: GGCTTTTCTGCAATTTGCTC | 60°C/− | D: JQ791215 | A232/T | 6Hs (60.9) |
| R: CAGCGAGGCTTAGTGAATCC | R: JQ791216 | |||||
| HvCMF4 | Os03g04620 | F: CAATGCAAATGAGCAGCACT | 60°C/+ | D: JQ791217 | A148/T | 4Hl (101.9) |
| R: TCAGCTGAAAGACGCACAAC | R: JQ791218 | |||||
| HvCMF5 | Os05g38990 | F: GAGGTCGTTGTATGGGCAGT | 60°C/− | D: N/A | InDel | 1Hl (94.2) |
| R: CAGACACACCAGAGGGGATT | R: JQ791219 | |||||
| HvCMF6a | Os05g51690 | F: CATAGTCGAGGAACCGCTG | 60°C/− | D: JQ791220 | In322/Del | 1Hl (156.3) |
| R: TTCTAGACGCAGACAAGCCA | R: JQ791221 | |||||
| HvCMF6b | Os05g51690 | F: CATAGTCGAGGAACCGCTG | 59°C/+ | D: JQ791252 | InDel | 1Hl (156.3) |
| R: CACTCGTAGACTCTATGCTCTCA | R: N/A | |||||
| HvCMF7 | Os06g48610 | F: GGAAATGCTTCTTTGGGTGA | 58°C/− | D: JQ791222 | T505/C | 7Hl (114.6) |
| R: AGCCGTCATCTGCTTCACTT | R: JQ791223 | |||||
| HvCMF10 | Os10g32900 | F: GCGGACCCATTGTAAAAGAA | 60°C/− | D: JQ791224 | T1155/A | 1H (57.7) |
| R: AGAGTGGGTAGGGTGGCTTT | R: JQ791225 | |||||
| HvCMF13 | Os12g01080 | F: CATCCATCCTGCCTTCATCT | 58°C/+ | D: JQ791226 | T635/C | 5Hl (64.9) |
| R: AGCGCAACTTCCAAAAGAAA | R: JQ791227 | |||||
| HvPRR59 | Os11g05930 | F: GAGGCGCCACTCTGTAAGTC | 60°C/+ | D: JQ791228 | A143/G | 4Hl (52.3) |
| R: AGAACGTTGCTGCTTCCCTA | R: JQ791229 | |||||
| HvPRR73 | Os03g17570 | F: CTTCAGACCCAGCTCTTTGG | 60°C/+ | D: JQ791230 | G1104/A | 4Hl (54.4) |
| R: AGCCCACAAAACCCACTATG | R: JQ791231 | |||||
| HvPRR95 | Os09g36220 | F: CTCAGGGAAAGCTCCAACTG | 60°C/+ | D: JQ791232 | T983/A | 5Hl (131.5) |
| R: GTGTCAAGGCCTGGGAATTA | R: JQ791233 | |||||
| HvTOC1 | Os02g40510 | F: TGAAGTGCCATGCTATGCTC | 60°C/+ | D: JQ791234 | G936/T | 6Hl (68.5) |
| R: CGAAGGAGACGGATGGTAAA | R: JQ791235 |
Forward (F) and reverse (R) primer; primers highlighted in bold were used for direct sequencing for detection of polymorphisms.
A/GC = anealing temperature/GC buffer (+ = GC buffer added, − = no GC buffer added).
GenBank accessions for genomic sequences from OWB-D (D) and OWB-R (R) parental lines are indicated. SNP positions are relative to OWB-D.
Barley chromosome arm: short (s), long (l). HvCMF5 and HvCMF6b were genetically mapped using presence/absence PCR/agarose gel InDel assays.
Due to lack of polymorphism in the genomic fragment investigated, HvCO15 was mapped using a SNP in an adjacent predicted gene (HvOs08g42430) located on the same sequence contig (Table S4).
Poaceae colinearity and nomenclature
Colinearity between rice (Mbp) and the barley consensus genetic map (cM) was determined as described by [48]. Rice chromosomal regions duplicated during the ancestral WGD were identified in the current rice genome assembly as previously described [49], and plotted using Circos [50]. Microcolinearity between orthologous Poaceae CMF genes was determined using ±10 genes on each side of each OsCMF gene for BLASTn query of the sequenced genomes of brachypodium, sorghum and foxtail millet, using the BLAST parameters listed above. Poaceae macro-colinearity follows that previously described [51]–[52]. Previously mapped COL, PRR and ZCCT genes [13], [18], [29] are integrated into the barley consensus map [47] based on common markers and established inter-specific barley-rice colinearity. For clarity when comparing orthologous genetic loci and genes, we use the vernalization locus nomenclature described by [41], and ZCCT1 (also called VRN2) to denote the gene underlying the VRN-Am2 locus in T. monococcum. All gene synonyms are listed in Tables S1–S5. The updated Poaceae COL nomenclature presented here builds on the numerical numbering system previously introduced for brachypodium, sorghum and barley in these species. However, to avoid confusion, the reclassification of HvCO9 as CMF11 (due to lack of B-boxes) has meant we have had to miss out CO9 in the otherwise consecutive ordering of Poaceae CO genes identified here. Rice COL nomenclature continues the alphabetical system previously used [13].
Results
Using CDS from the eight previously reported arabidopsis CMF genes [37] a total of fifteen arabidopsis family members were identified (Table S1). Towards understanding the evolution of Poaceae CCT domains genes, we then determined the CMF, COL, PRR and ZCCT gene families in the sequenced genomes of rice, brachypodium, sorghum, foxtail millet, as well as in the preliminary draft barley genome. Results are summarised in Tables S2–S5.
Poaceae COL genes
With the recognition of the CMF gene family, the rice COL gene family [13] should be reduced from sixteen (OsA-OsP) to fourteen members, due to the lack of B-box domains in OsH and Ghd7 (OsI). To determine COL copy number in the current rice genome assembly, we used OsCOL CDS for BLASTn analyses. Three additional OsCOL genes were identified (termed OsQ, OsR and OsS, respectively), resulting in a total of seventeen COL genes, distributed across chromosomes Os2–Os4 and Os6–Os9 (Table S2). All three novel OsCOL genes were predicted to possess one B-box domain, located towards the N-terminus of their predicted proteins. Analysis of the brachypodium genome returned sixteen COL genes. No homologues OsB and OsQ were identified, although one BdCOL gene lacking a rice homologue rice was identified (BdCO2 [19]). Sorghum and foxtail millet were fond to possess sixteen and seventeen COL genes, respectively. As well as lacking homologues of OsB and OsQ, both species possessed a single additional COL gene (SbCO20 and SiCO20), absent in the temperate grasses investigated here. Similarly, sorghum and foxtail millet contained homologues of BdCO2, although in sorghum, the predicted protein did not contain a B-box domain, and is therefore classified as a CMF gene (SbCMF16). Three of the foxtail millet COL genes (SiCO1, SiCO5 and SiCO19) were identified by gene prediction reanalysis of genomic regions identified by BLASTn analysis. In addition to the previously identified barley orthologues of OsA (HvCO1), OsB-OsG (HvCO3-HvCO8) and BdCO2 (HvCO2) [13], [19], searches of the preliminary draft barley genome and full length (fl) cDNAs identified eight additional full-length COL genes homologous to OsJ-OsP and OsR. Barley OsL-OsN homologues were identified on genomic contigs of size 4,069–6,808 bp, with gene prediction software identifying just one gene per contig. Remaining HvCOL genes were identified from fl-cDNA sequences. No barley homologues were identified for OsQ and OsS.
Poaceae PRR genes
Five PRR genes were identified in the genomes of rice and brachypodium (Table S3), agreeing with previous reports [18]–[19]. Similarly, five PRR genes were found in foxtail millet (chromosomes Si1–2, Si8–9) and sorghum (chromosomes Sb1–2, Sb4–6). Although no sorghum gene models homologous to OsPRR95 were identified, an unannotated genomic region with high sequence homology (4.3e-60) was identified. Closer inspection found a sequencing gap spanning predicted SbPRR95 exon 6 and part of exon 7, indicating complete sequencing would resolve an SbPRR95 gene model. For SbPRR37, the single annotated splice transcript contains seven exons. However, it should more correctly be annotated according to the photoperiod insensitive sbprr37-2 ma1 allele, previously reported to possess 8 exons [20]. Searches in barley identified five PRR genes, including the barley OsPRR37 orthologue that encodes the photoperiod locus PPD-H1 [18]. Three of the remaining four HvPRR genes were predicted to encode full length CDS, while lack of available genomic sequence corresponding to HvPRR59 means that the resulting predicted protein is truncated upstream of exon 2.
Poaceae CMF genes
Using CDS for OsH and Ghd7 (OsI) (both previously grouped within the COL gene family) and the arabidopsis CMF genes, an additional twelve members of the rice CMF gene family were identified, resulting in fourteen members distributed between ten chromosomes (Table S4). All contained a single CCT domain as the only identifiable domain within their predicted proteins, and are named here in chromosome order as OsCMF1-OsCMF14. In all but three CMF genes (OsCMF4, OsCMF10, OsCMF11), the CCT domain is encoded within regions spanning the penultimate and last exon (Figure S1). OsCMF physical map locations show they are located in distinct genomic regions relative to OsCOL genes (Table S2,S4), indicating the two gene families did not arise due to tandem duplication and subsequent loss of B-box domains in the rice lineage. Brachypodium was found to contain eleven CMF members. While homologues for OsCMF2, OsCMF8, OsCMF12 and OsCMF14 are absent, one additional brachypodium member was identified: BdCMF15 (for which no homologues were identified in any of the other Poaceae species investigated). CMF homologues for twelve of the fourteen rice members were identified in sorghum, with CDS ranging from 678–1374 bp. Foxtail millet was found to possess eleven CMF genes, lacking homologues of rice genes OsCMF2, OsCMF13 and OsCMF14. Although three SiCMF genes were not annotated as S. italica gene models, gene-prediction reanalysis of genomic regions identified by BLASTn analysis resolved CMF genes in all three instances (Table S4). Barley CMF genes were identified for nine of the thirteen rice members (HvCMF1, HvCMF3-HvCMF7, HvCMF10–11, HvCMF13), with no barley homologues of OsCMF2, OsCMF8, OsCMF9, OsCMF12 and OsCMF14 identified. All but two of these (HvCMF1 and HvCMF3) were predicted to encode full length transcripts. Two fl-cDNA sequences with high homology to OsCMF6 were identified, and are named HvCMF6a (AK250075) and HvCMF6b (AK355694). Finally, previous studies show that while the rice paralogue of OsH (chromosome Os10) has been lost on chromosome Os03 after whole genome duplication (WGD) in the ancestral cereal genome, the paralogous gene has been retained on the long arm of the temperate cereal group 4 chromosomes [33]. In barley, three cosegregating copies of the ancestral paralogue (ZCCT-Ha, ZCCT-Hb and ZCCT–Hc) are present in vernalization sensitive varieties [30]. As expected, BLASTn analysis of the vernalization insensitive barley varieties ‘Morex’ and ‘Haruna Nijo’ did not return any ZCCT-H genes. Accordingly, previously published sequences identified in vernalization sensitive lines [29] are used in subsequent molecular and phylogenetic analyses.
CMF, COL, PRR and ZCCT protein domain and coding region analysis
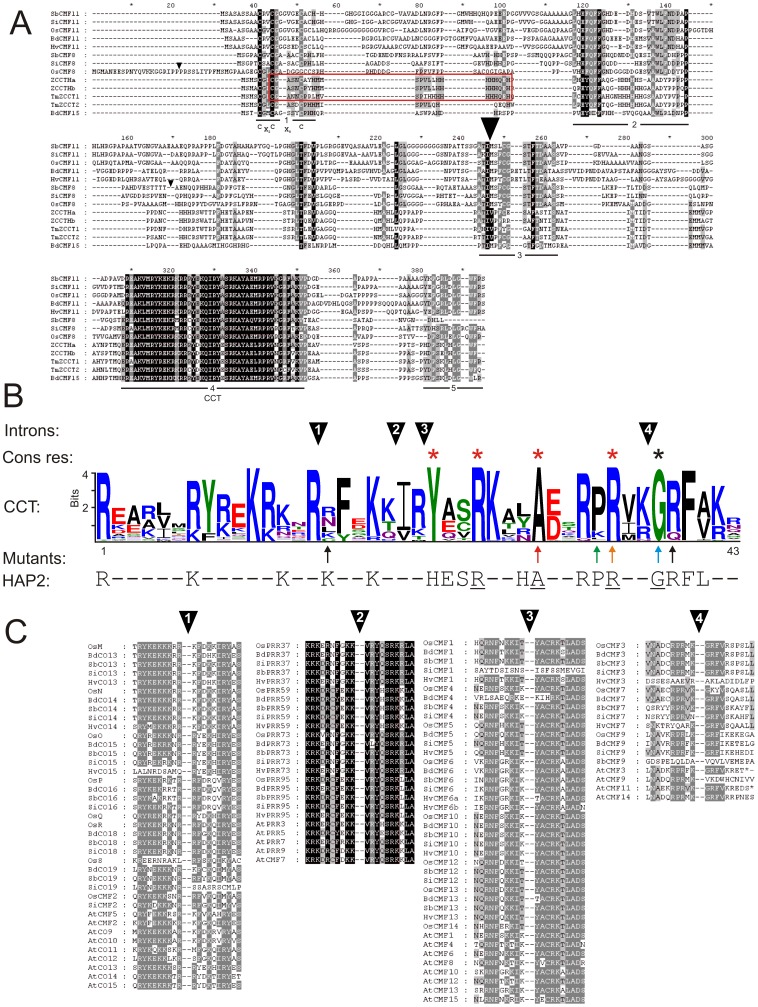
COL proteins possess either one or two B-boxes, in addition to a CCT domain. All Poaceae COL proteins identified here possess protein domain configurations identical to their closest rice homologues (Table S2). The three novel rice COL proteins (OsR-OsS) and their Poaceae homologues all possess a single B-box, while SbCO20/SiCO20 and BdCO2 homologues in sorghum and foxtail millet contain two B-boxes. Three of the four ZCCT genes investigated (ZCCT1, ZCCT-Ha, ZCCT-Hb) were predicted to encode zinc-finger domains towards the N-terminus, as previously reported [26], [29], which shows some similarity to the conserved C and H residues of COL B-box1 (Figure 1a). Alignment of CCT domains from Poaceae CMF, COL, PRR and ZCCT CCT proteins investigated in subsequent phylogenetic analysis, finds perfect amino acid conservation at four positions: Tyr23-Arg26-Ala30-Arg35 (Figure 1b). In addition, Gly38 was conserved in the proteins of all but one (HvCMF3) of the Poaceae genes investigated. Excluding the CCT domain, Poaceae CMF proteins did not share additional common conserved regions, nor were further protein domains identified. However, dispersed regions of conservation were evident between smaller sub-groups of CMF proteins (Figure 1a, Figure S2). Exon numbers for homologous genes commonly varied between species, although nine COL (homologues of rice genes OsA,OsC-OsD,OsG,OsJ-OsN), three CMF (CMF1,CMF5,CMF11) and three PRR (PRR59,PRR73,TOC1) genes shared identical numbers in all four of the sequenced Poaceae species investigated (Table S2–S4). To explore gene family structure and evolution in more detail, protein sequences immediately adjacent to introns bisecting CCT domains were aligned, resolving four major groups (Figure 1c). The first contains proteins with an intron after CCT residue 15, and is composed predominantly of COL genes (homologues of rice genes OsM-OsS), along with four CMF genes (OsCMF2, SiCMF2, AtCMF2, AtCMF5). The second group possess an intron after residue 20, and contains Poaceae PRR genes (excluding TOC1 homologues, which lack a CCT domain intron), as well as AtCMF7. Group 3 has an intron located after residue 22, and consists exclusively of Poaceae and arabidopsis CMF genes (CMF1, CMF4-CMF6, CMF10, CMF12-CMF14). Similarly, the final group exclusively contains CMF genes from the Poaceae (CMF3, CMF7, CMF9) and arabidopsis CMF (AtCMF3, AtCMF9, AtCMF11, AtCMF14), which all possess an intron after residue 37. While all remaining genes lack introns within the CCT domain (CMF8, CMF11, ZCCT, BdCMF15), alignment of their protein sequences find all to possess an intron at a conserved position, distinct from those observed in other COL genes (Figure S3,S4), with additional regions of conservation identified across their peptides (Figure S1).
Figure 1. Protein domain analysis.
Intron positions are indicated by triangles. (a) Alignment of CMF8, CMF11, ZCCT and BdCMF14 proteins. Conserved regions are underlined. The Zinc finger is boxed in red, and the conserved residues present at the N-terminus end of Poaceae COL B-box1 are indicated. (b) WebLogo representation of CCT domains from Poaceae CMF, COL, PRR and ZCCT sequences. Residues conserved in all proteins, and all proteins except HvCMF3, are indicated by red and black asterisks, respectively. Arrows indicate the position of mutated CCT domain residues in T. monococcum ZCCT1 (TmZCCT1, black), Arabidopsis thaliana CO (AtCO, green), PPD-H1 (blue), AtCO and ZCCT1 (orange), AtCO and AtTOC1 (red) [11], [18], [26], [59]. HAP2 amino acid sequence conserved with the Poaceae CCT domains is illustrated, and common residues underlined. (c) Conservation of intron/exon boundaries in Poaceae CMF, COL and PRR genes which possess an intron within the CCT domain.
Phylogenetic analysis of Poaceae CMF, COL, PRR and ZCCT gene families
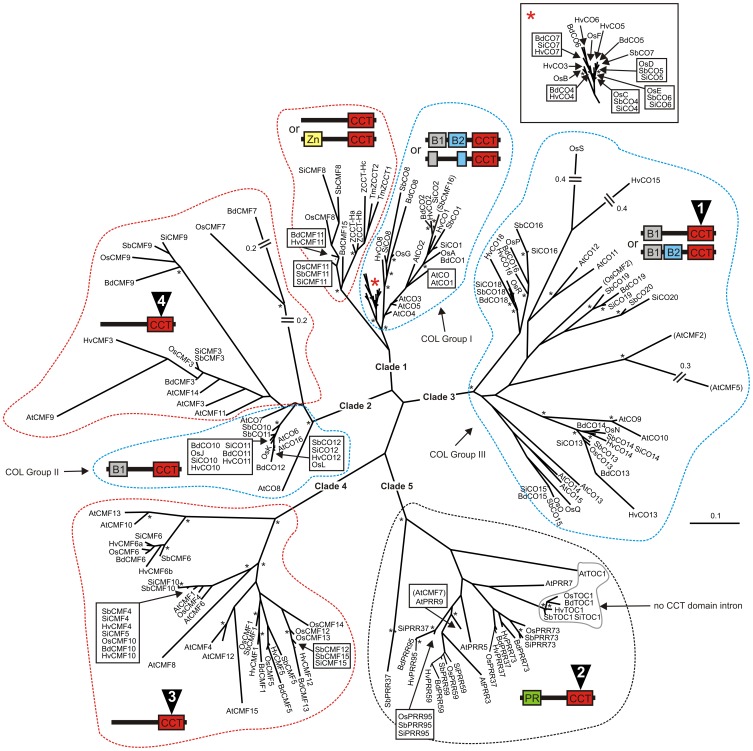
To help understand the evolution of the CMF gene family, protein sequence from the CCT domains encoded by Poaceae and arabidopsis CMF, COL, PRR and ZCCT genes were used to construct a Neighbour-Joining phylogenetic tree. Analysis resolved five major groupings, named Clade 1–5 (Figure 2). All Poaceae PRR genes clustered together within Clade 5, along with a single arabidopsis CMF gene (AtCMF7), indicating it may represent a recently truncated PRR gene. All Poaceae CMF, COL and ZCCT genes were partitioned within the four remaining phylogenetic groupings. Clade 4 compromised exclusively of Poaceae and arabidopsis CMF genes, with sub-clustering evident for gene pairs CMF1-CMF5, DMF4-CMF10 and CMF12-CMF13. Poaceae CMF6 genes formed a distinct sub-cluster within Clade 4, with the CCT domain encoded by HvCMF6b the most diverged. In addition, eight of the fifteen arabidopsis CMF homologues clustered within Clade 4. All but three of the forty-two Clade 3 genes belong to the COL gene family, and possess a single zinc finger domain towards their N-terminus. This class of rice gene has previously been termed the COL Group III gene family (OsM-OsP) [13], and also includes the three additional Poaceae COL genes identified in this study (homologous to rice genes OsQ-OsS). Sub-clustering of Poaceae COL gene pairs homologous to OsM-OsN, OsO-OsQ, OsP-OsR and OsS-OsT was evident, while arabidopsis genes AtCMF2 and AtCMF5 formed a distinct grouping off the S-T branch, as did a single CMF gene (OsCMF2). No OsCMF2 homologues were identified in any other species investigated, suggesting it represents a recently truncated COL gene. Clade 2 consists of two separate sub-clades. The first is made up of the Poaceae and arabidopsis Group II COL genes, while the second consists of a cluster of Poaceae CMF3 and CMF9 members, four arabidopsis CMF genes (AtCMF3,9,11,14), and the Poaceae CMF7 genes (SbCMF7, SiCMF7 and HvCMF7 were excluded from phylogenetic analysis due to deletions within the genomic region encoding the CCT domain). Clade 1 consists of two sub-groupings, and with the exception of SbCMF16, the first consists of the rice Group I rice COL genes (OsA-OsG) and their Poaceae homologues, with clustering of gene pairs OsC-OsD and OsE-OsF. The second sub-grouping contains fourteen CMF genes split between two main branches. The first branch contains CMF11 homologues from each of the five Poaceae species investigated, as well as CMF8 genes from rice, sorghum and foxtail millet. The first member to split from the base of the second branch is BdCMF15, for which no homologues were identified in any other species. The remainder of the branch consists exclusively of the barley ZCCT-Ha, -Hb, -Hc genes that underlie the VRN-H2 flowering time genetic locus, as well as the orthologous ZCCT1 and ZCCT2 genes, located at the colinear VRN-Am2 locus in T. monococcum (detailed in Table S5).
Figure 2. Phylogenetic analysis of CMF, COL, PRR and ZCCT proteins, based on the CCT domain.
Dashed lines enclose predominantly CMF (red), COL (blue) and PRR (black) families, within which the predominant protein domain configurations within full length proteins are indicated (proteins lacking the predominant pattern are indicated in brackets). Numbered triangles indicate CCT domain intron position (Figure 1). COL clades [following 11,13] are indicated with COL I and II proteins possessing two or one B-box domains, respectively. Genes are prefixed with genus and species initials. Gene synonyms as listed in Supplementary Tables S1–S5. Although Group III COL genes are previously described as posessing two B-boxes, domain prediction software used here identified variously one or two B-boxes. Arabidopsis CMF (Table S1), COL [13] and PRR [19] members are included, as are ZCCT1 (also termed VRN2, AAS60241) and ZCCT2 (AAS60248) from T. monococcum. Black asterisks indicate nodes with bootstrap values >60%. SiCMF1, BdCMF4, HvCMF7, SbCMF7 and SiCMF7 are excluded, due to deletions within the CCT domain.
Genetic mapping of barley CMF, COL and PRR genes
As nine COL genes and HvPRR37 (PPD-H1) have previously been genetically mapped [13], [18], their map locations in the barley consensus map are estimated utilizing comparative approaches (Text S1). Of the additional eight HvCOL and four PRR genes identified here, all were found to contain genetic polymorphisms between parental Oregon Wolfe Barley-Dominant (OWB-D) and OWB-Recessive (OWB-R) lines, allowing subsequent genetic mapping (Table 1, Text S2). Similarly, polymorphisms identified in all ten barley CMF genes allowed all to be genetically mapped. Using SNP C340/G (Gln → Glu), HvCMF1 was mapped to the long arm of chromosome 1H at 127.9 cM, between SNP markers 11_21081 and 11_21277. Using a SNP in the 3′UTR HvCMF3 was found to cosegregate with 17 genetic markers at 60.9 cM on 6H, while HvCMF4 cosegregated with four genetic markers at 101.9 cM on the long arm of 4H. A presence/absence polymorphism mapped HvCMF5 to 94.2 cM on the long arm of 1H, cosegregating with 12_30072 and bPb-8477. Two OsCMF6 homologues were identified in barley: an 11 bp intronic InDel allowed mapping of HvCMF6a to the distal end of chromosome 1H at 156.3 cM, where it cosegregated with nine previously mapped markers. HvCMF6b was found to cosegregate with HvCMF6a, using a presence (OWB-D)/absence (OWB-R) polymorphism. Intronic SNP T505/C allowed HvCMF7 to be mapped to 114.6 cM, on the long arm of 7H. Polymorphisms downstream of predicted stop codons were used for the remaining HvCMF genes, allowing HvCMF10 and HvCMF13 to be mapped to chromosome 1H (57.7 cM) and 5H (64.9 cM), respectively. We previously mapped HvCMF11 (previously known as HvCO9) to the long arm of 1H, were it was found to cosegregate with five gene-based markers [33], allowing integration into the consensus map (Text S1).
Comparative genomic analysis of Poaceae CMF, COL and PRR gene families
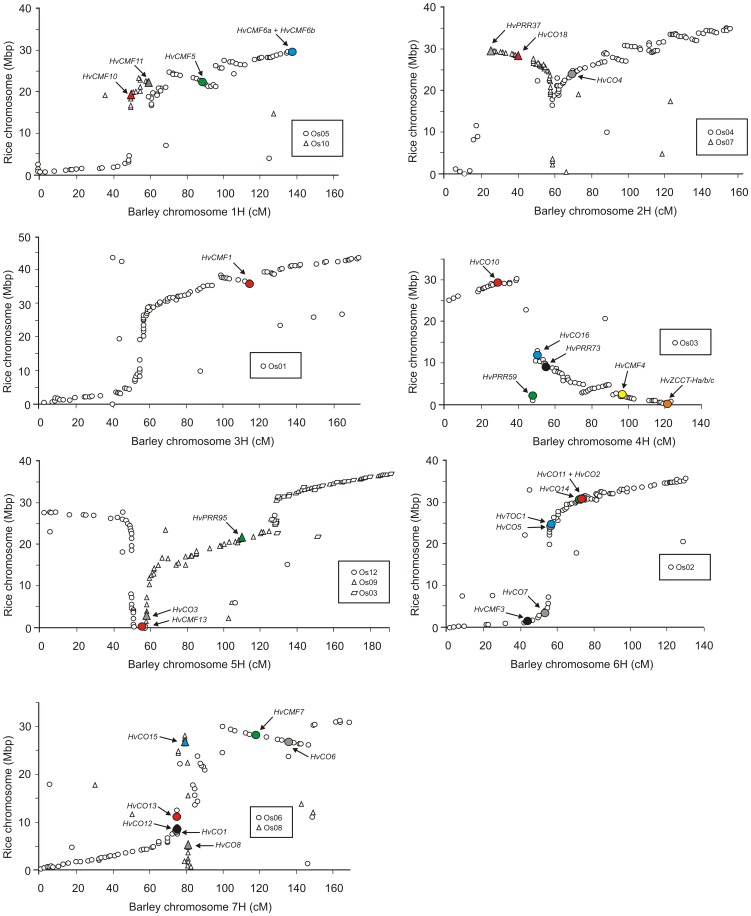
Comparative mapping allows the genomes of rice, sorghum, brachypodium, foxtail millet and barley to be aligned, and blocks of conserved synteny to be established [49], [51]–[54]. Initially, integration of barley CMF, COL, PRR and ZCCT genetic map locations into the consensus map (Text S1,S3) allowed investigation of their chromosomal locations within the framework of barley-rice colinearity (Figure 3). All twenty of the mapped barley genes were located within broader regions of established inter-specific colinearity (Text S4). Integration of the ten previously mapped CMF, COL, PRR and ZCCT genes allowed subsequent comparative analysis all barley gene families to be conducted across five Poaceae species. With the exception of genes present in just one species (OsCMF14, BdCMF15, OsQ), colinear cross-species genomic locations of all remaining CMF, COL and PRR genes was established (Figure 4). Patterns of macro-colinearity were confirmed by investigation of microcolinearity around CMF, COL and PRR genes in the four sequenced grass genomes (Table S6).
Figure 3. Barley-rice colinearity, indicating CCT domain genes.
The position of barley gene-based genetic markers in the barley consensus genetic map [47] are indicated on the x-axis (cM). The locations of their orthologues in the rice physical map are indicated on the y-axis (Mbp). Genes mapped in the OWB population, as well as previously mapped genes included within the figure (highlighted in grey), are integrated into the consensus genetic map as described in Text S1,S3.
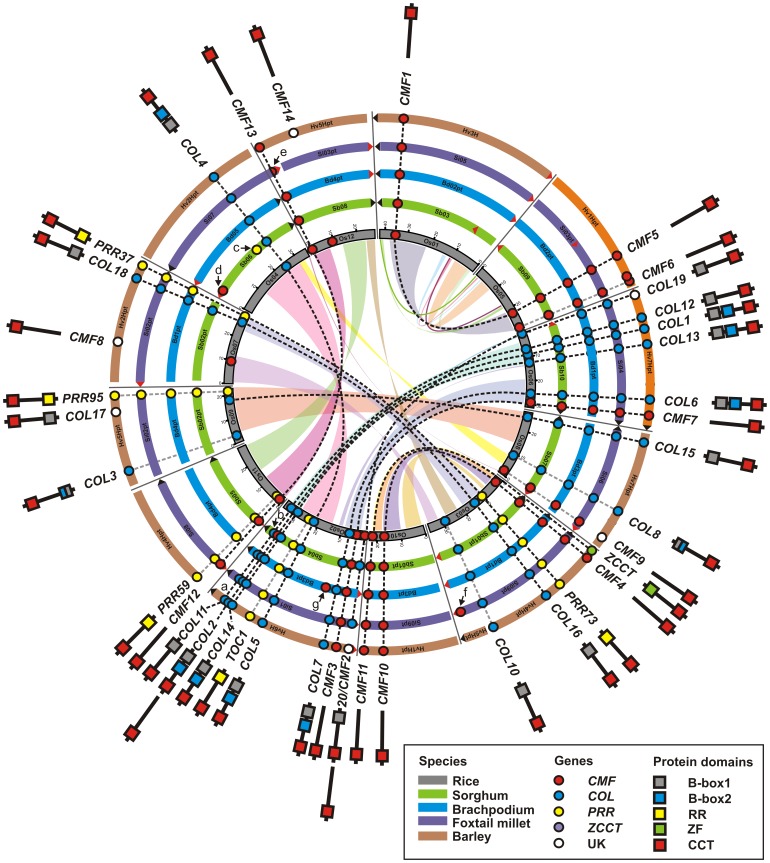
Figure 4. Location of Poaceae CMF, COL, PRR and ZCCT genes within the framework of Poaceae inter- and intra-specific colinearity.
Rice chromosomal regions duplicated during the ancestral WGD are linked by coloured bands. Paralogous gene pairs arising from WGD are connected by black dashed lines, while colinear genes lacking paralogues are connected by grey dashed lines. As instances where full-length barley orthologues were not identified in the draft genomic sequence and fl-CDNAs does not mean they are absent in barley, their predicted positions are indicated tentatively here as unknown (UK) genes. Protein domain configurations are indicated. Localised segmental chromosomal inversions are not shown. a Poaceae orthologues for HvCO2 orthologous are present in all genomes except rice [19]. b SbCMF16. c SbPRR37. d SbCMF8. e Si011862m, truncated CCT motif. f SiCMF8. g BdCMF15.
To investigate the gene evolution in the context of intra-specific genome duplication that arose due to WGD in the ancestral grass genome [35]–[36], [49], [52]–[54], the positions of rice CMF, COL and PRR genes relative to WGD blocks was analysed (Figure 4). The presence of corresponding regions of segmental duplication in each of the sequenced grass species was verified at the micro-synteny level by BLASTn analysis of gene content surrounding all CCT domain genes (Table S6). Combined analysis of inter- and intra-specific colinearity allowed identification of paralogous gene pairs that arose due to ancestral WGD. Of the five PRR genes found in each Poaceae species, PRR37 and PRR73 appear to have originated from a common ancestor prior to WGD. While the remaining three PRR genes originate from regions involved in the WGD, paralogous genes have been lost in all Poaceae species investigated. Furthermore, no CMF genes (which could have evolved from PRR genes by truncation of the pseudo-receiver domain at the N-terminus) are located in within these genomic locations. Of the seventeen rice COL genes and their Poaceae orthologues, the majority form paralogous gene pairs arising from the ancestral WGD (OsC-OsD, OsE-OsF, OsK-OsL, OsM-OsN, OsO-OsQ, OsP-OsR). With one exception (due to the absence of OsQ orthologues), these paralogous pairs are conserved in all Poaceae species investigated. Of the remaining five OsCOL genes, two are located within duplicated genomic regions. OsA (chromosome Os06) resides within a chromosomal region displaying intra-specific colinearity with a region of chromosome Os02. OsA orthologues are present in all Poaceae species, and while no OsA paralogue is present on rice chromosome Os02, brachypodium, sorghum, foxtail millet and barley all retain a paralogue at colinear positions within their genomes. The second COL gene located with a region of WGD is OsS and its Poaceae orthologues (COL19). Although COL genes are absent at the corresponding region of chromosomal duplication, CMF2 genes are present in all species investigated, indicating that Poaceae OsS and CMF2 orthologues are related by evolutionary descent. The remaining Poaceae COL genes orthologous to OsG and OsJ are not located within duplicated chromosomal regions. Of the fourteen members of the rice CMF gene family, OsCMF2 has no orthologous Poaceae CMF genes, as while in rice it appears to have lost the N-terminus B-box domain, this motif is retained in colinear COL genes in all other grasses investigated here. All remaining OsCMF genes have orthologues located at colinear chromosomal positions in ≥2 grass species. Four CMF gene pairs appear to have evolved as a result of the ancestral WGD (CMF1–5, CMF3–7, CMF4–10, CMF12–13). In addition, comparative analysis indicates that Poaceae CMF11 and Triticeae ZCCT genes form a paralogous pair originating by ancestral WGD, confirming previous reports in rice/brachypodium/barley [33]. The five remaining grass CMF genes (CMF6, CMF8, CMF9, CMF11, CMF14) are located in non-duplicated genomic regions.
Discussion
Evolution of CCT domain gene families
The role of CCT domain genes in the control of flowering is well documented. However, the recent identification of Poaceae CO-like flowering time genes which lack B-boxes [26], [34], and the delimitation of an extended family of related plant CMF genes in this study, raises questions concerning their evolutionary origin: are they species-specific independent truncations/mutations of existing COL genes, or do they represent a more ancient gene lineage? Here, combined phylogenetic, molecular and comparative analysis identifies predominantly distinct CMF, COL and PRR gene families in arabidopsis and the Poaceae, showing they predate the monocot/dicot divergence ∼200 million years ago (mya). COL [5] and PRR [55] genes are present in the moss Physcomitrella patens, with BLAST searches showing CMF genes to be absent. Mosses within the streptophyte lineage such as the ancestor of P. patens separated from the cormophytes ∼500 mya, with the cormophyte lineage (to which the ancestor of the spikemoss Selaginella moellendorffii belongs) diverging from seed plants ∼400 mya [56]. Analysis of the S. moellendorffii genome (release v1.0) finds both COL and CMF genes to be present, with ≥1 CMF gene(s) within each of the CMF clades identified in this study (data not shown). Thus, parsimonious analyses indicate the CMF family may have evolved from COL genes after the divergence of the cormophyte and streptophyte lineages, around 500−400 mya. Characteristic regions of sequence conservation outside the identified protein domains within and between gene families indicate a hierarchy of evolutionary relatedness resulting from genetic mutation following processes such as WGD, inter-species hybridization events and localized gene duplication or deletion over their evolutionary history. Inter- and intra-species comparative analysis shows that the CMF, COL and PRR gene families consist largely of arrays of paralogous gene-pairs located at colinear genomic locations across the Poaceae that evolved from common ancestors prior to WGD in the ancestral grass genome. This hypothesis is supported by clustering of Poaceae paralogues within each of the five phylogenetic clades, as well as regions of conservation throughout their proteins. Additionally, phylogenetic and protein/gene structure analyses indicates evolutionary relatedness between groups of COL and CMF genes prior to monocot/dicot divergence. Within Clade 2, the CMF and COL Group II sub-branches are phylogeneticly linked, indicating Clade 2 CMF genes evolved from Group II COL genes, between 400−200 mya. The remaining CMF genes are distributed between phylogenetic Clades 4 and 1. While the former consists exclusively of arabidopsis and Poaceae CMF genes with a conserved CCT domain intron, the latter contains sub-branches possessing COL, CMF and ZCCT genes (discussed subsequently). While the majority of CMF genes predate monocot/dicot divergence, a minority appear to have evolved more recently (AtCMF2, AtCMF5, AtCMF7, OsCMF2, SbCMF16). Such genes cluster unambiguously within COL phylogenetic clades, with comparative mapping in the Poaceae indicating they mutated from COL genes after separation of the lineages investigated.
All gene families investigated possess a CCT domain in their predicted proteins, with greater amino acid conservation towards the C-terminus. CCT domains possess regions of amino acid conservation with HEME ACTIVATOR PROTEIN2 (HAP2), a component of the HA2/HAP3/HAP5 protein complex that binds to promoter CCAAT DNA motifs in eukaryotic genes, and modifies their transcription [57]. Indeed, arabidopsis CO and COL15 have been shown to interact with several HAP proteins in vitro and in vivo [57]–[58]. Of the five highly conserved CCT domain residues identified (Tyr23-Arg26-Ala30-Arg35-Gly38), all but Try23 are located in the NF-YA2 sub-domain of HAP2, though to mediate the interaction of HAP2 with CCAAT DNA sequences [59]. These highly conserved CCT/HAP2 residues are likely sites for point mutations resulting in altered function in CMF, COL and PRR proteins. Indeed, independent mutations at three of these conserved residues have been shown to result in novel alleles in several plant species [11], [18], [26], [28], [60] (Figure 1b), Recently, the CCT domain of arabidopsis CO has been shown to mediate protein-protein interaction with the E3 ubiquitin ligases COP1 and HOS1 [60]–[62], both of which regulate CO stability. Taking such data into account, CMF gene families are likely to encode transcription factors that modify floral gene expression through DNA-binding or DNA-binding complexes, mediated by the CCT motif.
Evolution of Poaceae Clade 1 CCT domain genes
Clade 1 contains CMF, COL and ZCCT genes, distributed between two sub-branches. The first contains COL Group I genes from arabidopsis and the grasses, while the second contains CMF and ZCCT genes, unique to the Poaceae. Common ancestry was demonstrated for four Clade 1 paralogous gene-pairs, which evolved as a result of ancestral cereal WGD (COL genes orthologous to OsA-OsU, OsC-OsD, OsE-OsF and CMF11-ZCCT). Interestingly, Clade 1 contains the greatest number of protein domain configurations, including proteins encoded COL genes with two B-boxes, or chimeric B-box1/B-box2 domains, as well as CMF and ZCCT genes. The trend of degradation or complete loss of B-box domains within Clade 1 appears to have resulted in the evolution of CCT domain genes lacking B-boxes within the Poaceae-specific CMF/ZCCT sub-branch. The absence of arabidopsis genes within this sub-branch, and the clear grouping (and conserved intron structure) of arabidopsis COL genes within the COL sub-branch, indicates the CMF/ZCCT branch evolved from common ancestors of the COL Group I genes after the monocot/dicot divergence. Two examples of Poaceae-specific B-box degradation are evident. In the case of Poaceae OsB and OsG orthologues in the COL sub-branch, the two B-boxes found in all other COL Group I proteins have been fused into one chimeric domain. The presence of chimeric B-boxes in orthologous Poaceae genes shows that truncation occurred prior to the divergence of the grasses, around 60 mya [53]. It has been suggested that COL gene evolution is leading to proteins encoding just one B-box domain [13]. The presence of tandemly arrayed B-box domains containing the short stretches of sequence conservation necessary for non-homologous end joining (NHEJ) following double-stranded DNA breaks [63], provides a possible mechanism for B-box contraction. Such flanking motifs have previously been observed in plants surrounding NHEJ deletions within transposable elements [64] and similar deletions within plant genes are responsible for the creation of novel phenotypic variation [49], [65]. As chimeric or degraded B-box domains are absent in arabidopsis COL proteins [13], [26], [29], it appears that the evolutionary process of B-box elimination is prominent in Poaceae Clade 1. Indeed, Clade 1 members illustrate the mechanism by which CMF genes may have evolved from COL genes after the divergence of the Bryophyta and flowering plants, by creation of non-functional chimeric B-boxes and their subsequent degradation/mutation, as evidenced by the proteins encoded by the paralogous ZCCT-CMF11 gene pair. The significance of this evolutionary trend in Clade 1 is highlighted by the occurrence of B-box degradation/elimination in two of the three known Poaceae CCT domain flowering time genes, affecting floral response to vernalization in cereals (ZCCT1 orthologues) and to LD photoperiods in rice (Ghd7/OsI). While Ghd7 orthologues (Poaceae CMF8 genes) are present in all three tropical SD species investigated, they are not found in either of the two temperate LD cereals investigated (barley and brachypodium). Deletions/mutations causing truncation of Ghd7, and the resulting lack of floral delay under LDs, is one of the key variants that allowed rice to be grown in temperate LD climates [34]. The ancestral cereal is hypothesised to have been a SD plant of tropical environment [1]. Thus, absence of Ghd7 orthologues in temperate cereals could be explained by their mutation/deletion after the split of temperate and tropical cereal lineages. The resulting removal of LD floral delay would allow growth and reproduction in the warm LD conditions of temperate summers.
It has been suggested that COL proteins act by replacing HAP2 in the HAP2/HAP3/HAP5 transcriptional complex, thus affecting binding to promoter CCAAT motifs of target genes [25], [57]. If the CCT domains of CMF proteins retain similar function, the absence of B-box domains (which mediate protein-protein interactions) could further alter the binding, composition and function of such protein complexes, and their resulting transcriptional modulation. We note that copy number variation for additional Poaceae CCT domain genes has also been critical in the domestication and spread of cereal cultivation, including the ZCCT genes at cereal vernalization loci [29]-[30] and TaPRR at the hexaploid wheat PPD-B1 photoperiod locus [21]. Accordingly, the occurrence of species-specific genes (OsB, OsQ, OsCMF13, BdCMF14), as well as gene absences (rice BdCO2, brachypodium CMF8 and Poaceae OsCMF14 orthologues), translocations (SbCMF8, SiCMF8, SbPRR37) and duplications (HvCMF6a/b), represent genes of potential significance for Poaceae floral control.
Analysis of CCT domain genes relative to flowering time loci
Three flowering time QTL have been identified in the OWB mapping population used here. The first maps across the VRN-H2 locus [66], encoded by the ZCCT-Ha, -Hb, -Hc genes [29]. Additional QTL have been mapped towards the telomeric region of chromosome 1HL [63]–[64], and between the Nud and Lks2 loci on chromosome 7H [67]. The 1H QTL peak is associated with markers ABG387A and 11_20840, orthologous to Os05g51530 and Os05g51450, respectively [66]–[67]. We find HvCMF6a and HvCMF6b (orthologous to Os05g51630) to cosegregate with ABG387A and 11_20840 on the long arm of chromosome 1H. Flowering time QTL have been previously mapped in this region in other barley populations [68]–[71], as has the early flowering mutant early maturity 8 (eam8) [67]. Furthermore, QTL for related morphological traits including final leaf number and grain yield are also found at this location (154–158 cM, between markers 11_11509 and 11_20840) [66]. Interestingly, a flowering time QTL is located at a colinear position on the long arm of chromosome 1D in hexaploid wheat [72], based on comparative marker XBJ544902. This wheat marker, and barley SNP 11_20840 (which cosegregates with HvCMF6a/HvCMF6b and the flowering time QTL in barley), originate from orthologous genes. Environmentally robust flowering time QTL towards the telomeric region of chromosome 1BL and 1DL have been identified in three additional hexaploid wheat mapping populations, leading to the classification of these genomic regions as flowering time meta-QTL, putatively controlled by homeoalleles [72]. Based on known flowering time genes in model species, no additional candidate genes were identified in this region of rice chromosome Os05 (29.00–29.95 Mbp). However, eam8 has recently been found to encode a homologue of the arabidopsis circadian clock regulator EARLY FLOWERING 3 (ELF3), absent in the colinear region of rice [73]. Nevertheless, it is unclear whether the naturally occurring flowering time QTL in barley and wheat correspond to ELF3 homologues, with genetic mapping suggesting eam8 is distal to the barley QTL. Although partial sequencing of HvCMF6a did not identify any non-synonymous mutations, HvCMF6b was mapped as a presence/absence polymorphism, indicating that deletions, or mutation within primer binding-sites, could result in an HvCMF6b allele of altered function in the OWB-R parental line. Further investigation is needed to evaluate the candidacy of CMF6 and ELF3 genes for the 1H, 1BL and 1DL meta-QTL. Although HvCMF7 was mapped to chromosome 7H, it is ∼5 cM distal to Lks2, indicating it is an unlikely candidate for the 7H flowering time QTL. However, we note that HvCO6 (orthologous to Os06g44450) has been mapped to this region in barley [13]. Comparative analysis predicts HvCO6 to map between HvCKX10 (Os06g37500) [44] and HvCMF7 (Os06g48610) in barley, indicating it is a candidate gene for the 7H QTL. Future genetic mapping of HvCO6 in the OWB population would help clarify its map position relative to Nud, Lks2 and the 7H flowering time QTL.
Supporting Information
Intron (line)/exon (box) structure of rice COL, PRR and CMF genes. Coding regions encoding protein domains are indicated: CCT (green), B-box1 (blue), B-box2 (red), PRR (orange). Non-significant B-box1 protein domains are indicated in light blue. Synonyms: OsCMF8 = OsI [13] and Ghd7 [34]. OsCMF11 = OsH [13]. OsA = Hd1 [24] and OsCO1. OsB = OsCO3 [74].
(TIF)
Alignment of Poaceae and arabidopsis CMF and ZCCT proteins. ZCCT proteins from T. monococcum and H. vulgare are included in the alignment. HvCMF1 and HvCMF3 are partial at the N-terminus end. AtCMF2, AtCMF5 and AtCMF7 are excluded.
(PDF)
Protein alignment of Poaceae COL Group I proteins. Intron positions are indicated by triangles and boxed. Positions of protein domains are indicated. Intron positions for HvCO1 – HvCO5 were determined from the sequences submitted by [13]. Intron positions for HvCO6, HvCO7 and HvCO8 were determined by gene prediction analysis of cv Morex RBCA contigs 6788, 2171376 and 143637, respectively.
(TIF)
Protein alignment of COL Group II proteins. Intron positions are indicated by triangles and boxed. Positions of protein domains are indicated. AtCO8 is excluded due to low sequence conservation with other Group II proteins.
(TIF)
Arabidopsis CMF genes. Chromosome (chr), amino acids (aa). Synonyms in brackets.
(DOCX)
Poaceae COL genes, and their homologues identified in the sequenced genomes of brachypodium, sorghum and foxtail millet. HvCO1 -HvCO8 are previously identified by [13]. Note, HvCO9 is re-named here as HvCMF11, due to lack of B-boxes. Alternative rice COL nomenclature shown in parentheses. Note, HvCO3 is orthologous to OsB – no OsB orthologues are found in brachypodium, sorghum or foxtail millet. B1 (B-box1), B2 (B-box2), B1/2 (B-box1/B-box2 chimeric domain), CCT (CONSTANS, CO-LIKE, TOC1). a relative to closest rice homologue. FGENESH reanalysis of: b scaffold_4, 12553835–12558834 bp; c scaffold_1, 30543240–30545239 bp; d scaffold_4:164743–169742 bp; e scaffold_6:33982724–33992723. f FGENESH reanalysis of genomic region around annotated gene.
(DOCX)
Poaceae PRR genes, and their homologues identified in the sequenced genomes of brachypodium, sorghum and foxtail millet. a relative to closest rice homologue. b Details according to Sbi1.4 gene set from the Sb1 assembly. Comparison with the allele from genotype ATx623 suggests that this allele corresponds to the Sbprr37-2 allele, which is predicted to possess a different intron/exon structure [20]. c No gene model (GM) predicted in the sorghum annotation. Analysis of the sorghum genomic region homologous to Os09g36220 (chromosome Sb2, 65858746–65868745 bp) indicated that a sequencing gap has likely resulted in failure of an appropriate gene model to be formed. Manual inspection shows of the eight exons present in Os09g36220, exon 6 and the 5′ end of exon 7 are predicted to lie within the sorghum sequencing gap. RR (response regulator), CCT (CONSTANS, CO-LIKE, TOC1).
(DOCX)
Poaceae CMF genes, and their homologues identified in the sequenced genomes of brachypodium, sorghum and foxtail millet. Full length barley CMF genes identified in the barley transcriptome/draft genome assembly are also shown. c_ (contig_), N/A (not applicable), B1 (B-box1), B2 (B-box2), B1/2 (B-box1-B-box2 chimeric domain), CCT (CONSTANS, CO-LIKE, TOC1). a relative to closest rice homologue. b FGENESH re-analysis of gene model. c FGENESH gene model. Note: e-value similarity with closest rice homologue is below the arbitrary experimental significance threshold. P truncated at 5′ end. d Although genes shown in brackets are most similar to OsCMF genes, they possess b-box domains and so belong to the COL gene family. Their details are described within the COL analyses (Table S2). e Si011862m, truncated CCT motif.
(DOCX)
Poaceae ZCCT genes investigated in this study. a E-values are relative to ZCCT1 CDS. b ZF = zinc-finger domain. c partial cDNA sequence only available. N/A = not applicable.
(DOCX)
Analysis of microsynteny. Rice CDS were used for BLASTn queries to identify genes with significant (≤1.0e-15) homology in the genomes of rice, brachypodium, sorghum and foxtail millet. Where WGD-derived colinear genomic regions are present, micro-colinearity with rice is investigated in both regions. Transposable elements are highlighted in grey. HP = hypothetical protein, CHP = conserved hypothetical protein, EP = expressed protein. N/A = not applicable. * = region of homology is located upstream of gene model. (A) Regions involved in the ancestral WGD. (B) Regions not involved in the ancestral WGD.
(XLSX)
Positioning of previously mapped CMF, COL and PRR genes within the barley consensus genetic map.
(DOCX)
Genetic mapping of barley genes.
(DOCX)
Integration of genes genetically mapped in the OWB population into the barley consensus genetic map.
(DOCX)
Comparative barley-rice analysis of mapped HvCMF, HvCOL and HvPRR genes.
(DOCX)
Acknowledgments
We thank Jeffrey Bennetzen for permission to analyse the CMF, COL and PRR gene families in the unpublished S. italica genome, and the International Barley Sequencing Consortium for permission to utilize genomic sequences from the preliminary draft barley genome sequence. We thank the anonymous reviewers for their constructive comments.
Funding Statement
This work was funded by the National Institute of Agricultural Botany (NIAB) Trust. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Cockram J, Jones H, Leigh FJ, O'Sullivan D, Powell W, et al. (2007) Control of flowering time in temperate cereals: genes, domestication and sustainable productivity. J Exp Bot 58: 1231–1244. [DOI] [PubMed] [Google Scholar]
- 2. Amasino R (2010) Seasonal and developmental timing of flowering. Plant J 61: 1001–1013. [DOI] [PubMed] [Google Scholar]
- 3. Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–858. [DOI] [PubMed] [Google Scholar]
- 4. Suárez-López P, Wheatley K, Robson F, Onouchi H, Valverde F, et al. (2001) CONSTANS mediates between circadian clock and the control of flowering in Arabidopsis . Nature 410: 1116–1120. [DOI] [PubMed] [Google Scholar]
- 5. Valverde F (2011) CONSTANS and the evolutionary origin of photoperiodic timing of flowering. J Ex Bot 62: 2453–2463. [DOI] [PubMed] [Google Scholar]
- 6. Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, et al. (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056. [DOI] [PubMed] [Google Scholar]
- 7. Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, et al. (2007) FT protein movement contributes to long-distance signalling in floral induction of Arabidopsis. Science 316: 1030–1033. [DOI] [PubMed] [Google Scholar]
- 8. Jaeger KE, Wiggle PA (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17: 1050–1054. [DOI] [PubMed] [Google Scholar]
- 9. Serrano G, Herrera-Palau R, Romero JM, Serrano A, Coupland G, et al. (2009) Chlamydomonas CONSTANS and the evolution of plant photoperiodic signalling. Curr Biol 19: 359–368. [DOI] [PubMed] [Google Scholar]
- 10. Zhang H, Harry DE, Ma C, Yuceer C, Hsu CY, et al. (2010) Precocious flowering in trees: the FLOWERING LOCUS T gene as a research and breeding tool in Populus . J Ex Bot 61: 2549–2560. [DOI] [PubMed] [Google Scholar]
- 11. Robson F, Costa MMR, Hepworth SR, Vizir I, Pine˜iro M, et al. (2001) Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J 28: 619–631. [DOI] [PubMed] [Google Scholar]
- 12. Jang S, Marchal V, Panigrahi KC, Wenkel S, Soppe W, et al. (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27: 1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griffiths S, Dunford RP, Coupland G, Laurie DA (2003) The evolution of CONSTANS-like gene families in barley, rice and Arabidopsis. Plant Physiol 131: 1855–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shin BS, Lee JH, Lee JH, Jeong HJ, Yun CH, et al. (2004) Circadian regulation of rice (Oryza sativa L.) CONSTANS-like gene transcripts. Mol Cells 17: 10–16. [PubMed] [Google Scholar]
- 15. Strayer C, Oyama T, Schultz TF, Raman R, Somers DE, et al. (2000) Cloning of the Arabidopsis clock gene TOC1, an autoregulatory response regulator homolog. Science 289: 768–711. [DOI] [PubMed] [Google Scholar]
- 16. Mizuno T, Nakamichi N (2005) Pseudo-response regulators (PRRs) or true oscillator components (TOCs). Plant Cell Physiol 46: 677–685. [DOI] [PubMed] [Google Scholar]
- 17. Murakami M, Matsushika A, Ashikari M, Yamashino T, Mizuno T (2005) Circadian-associated rice Pseudo Response Regulators (OsPRRs): insight into the control of flowering time. Biosci Biotechnol Biochem 69: 410–414. [DOI] [PubMed] [Google Scholar]
- 18. Turner A, Beales J, Faure S, Dunford RP, Laurie DA (2005) The pseudo-response regulator Ppd-H1 provides adaptation to photoperiod in barley. Science 310: 1031–1034. [DOI] [PubMed] [Google Scholar]
- 19. Higgins J, Bailey P, Laurie DA (2010) Comparative genomics of flowering time pathways using Brachypodium distachyon as a model for the temperate grasses. Plos One 5: e10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Murphy RL, Klein RR, Morishige DT, Brady JA, Rooney WL, et al. (2011) Coincident light and clock regulation of pseudoresponse regulator protein 37 (PRR37) controls photoperiodic flowering in sorghum. Proc Natl Acad Sci U S A 108: m16469–16474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beales J, Turner A, Griffiths S, Snape JW, Laurie DA (2007) A Pseudo-Response Regulator is misexpressed in the photoperiod insensitive Ppd-D1a mutant of wheat (Triticum aestivum L.). Theor Appl Genet 115: 721–733. [DOI] [PubMed] [Google Scholar]
- 22. Kojima S, Takahashi Y, Kobayashi Y, Monna L, Sasaki T, et al. (2002) Hd3a, a rice ortholog of the Arabidopsis FT gene, promotes transition to flowering downstream of Hd1 under short-day conditions. Plant Cell Physiol 43: 1096–1105. [DOI] [PubMed] [Google Scholar]
- 23. Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 24. Yano M, Katayose Y, Ashikara M, Yamanouchi U, Monna L, et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice is closely related to the Arabidopsis flowering time gene CONSTANS . Plant Cell 12: 2473–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Distelfeld A, Dubcovsky J (2009) Regulation of flowering in temperate cereals. Curr Opin Plant Biol 12: 178–184. [DOI] [PubMed] [Google Scholar]
- 26. Yan L, Loukoianov A, Blechl A, Tranquilli G, Ramarkrishana W, et al. (2004) The wheat VRN2 gene is a flowering repressor down-regulated by vernalization. Science 303: 1640–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Karsai I, Szűcs P, Mészáros K, Filichkina T, Hayes PM, et al. (2005) The Vrn-H2 locus is a major determinant of flowering time in a facultative x winter growth habit barley (Hordeum vulgare L.) mapping population. Theor Appl Genet 110: 1458–1466. [DOI] [PubMed] [Google Scholar]
- 28. Distelfeld A, Tranquilli G, Li C, Yan L, Dubcovsky J (2009) Genetic and molecular characterization of the VRN2 loci in tetraploid wheat. Plant Physiol 149: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dubcovsky J, Chen C, Yan L (2005) Molecular characterization of the allelic variation at the VRN-H2 vernalization locus in barley. Mol Bred 15: 395–407. [Google Scholar]
- 30. Cockram J, Chiapparino E, Taylor SA, Stamati K, Donini P, et al. (2007) Haplotype analysis of vernalization loci in European barley germplasm reveals novel VRN-H1 alleles and a predominant winter VRN-H1/VRN-H2 multi-locus haplotype. Theor Appl Genet 115: 993–1001. [DOI] [PubMed] [Google Scholar]
- 31. Cockram J, White J, Leigh FJ, Lea VJ, Chiapparino E, et al. (2008) Association mapping of partitioning loci in barley (Hordeum vulgare ssp. vulgare L.). BMC Genet 9: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cockram J, Norris C, O'Sullivan DM (2009) PCR markers diagnostic for seasonal growth habit in barley. Crop Sci 49: 403–410. [Google Scholar]
- 33. Cockram J, Howells R, O'Sullivan DM (2010) Segmental duplication harbouring group IV CONSTANS-like genes in cereals. Genome 53: 231–240. [DOI] [PubMed] [Google Scholar]
- 34. Xue W, Xing Y, Weng X, Zhao Y, Tang W, et al. (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767. [DOI] [PubMed] [Google Scholar]
- 35. Bolot S, Abrouk M, Masood-Quraishi U, Stein N, Messing J, et al. (2009) The ‘inner circle’ of the cereal genome. Curr Opin Plant Biol 12: 119–125. [DOI] [PubMed] [Google Scholar]
- 36. Paterson AH, Bowers JE, Bruggmann R, Dubchak I, Grimwood J, et al. (2009) The Sorghum bicolour genome and the diversification of grasses. Nature 457: 551–556. [DOI] [PubMed] [Google Scholar]
- 37. Masaki T, Tsukagoshi H, Mitsui N, Nishii T, Hattori T, et al. (2005) Activation of a gene for a protein with novel class of CCT-domain activates expression of a subset of sugar-inducible genes in Arabidopsis thaliana . Plant J 43: 142–152. [DOI] [PubMed] [Google Scholar]
- 38. Finn RD, Mistry J, Tate J, Finn RD, Hollich V, et al. (2010) The Pfam protein families database. Nucl Acids Res 38: D211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14: 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Matsumoto T, Tanaka T, Sakai H, Amano N, Kanamori H, et al. (2011) Comprehensive sequence analysis of 24,783 barley full-length cDNAs derived from 12 clone libraries. Plant Physiol 156: 20–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dubcovsky J, Lijavetzky D, Appendino L, Tranquilli G (1998) Comparative RFLP mapping of Triticum monococcum genes controlling vernalization requirement. Theor Appl Genet 97: 968–975. [Google Scholar]
- 42. Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position specific gap penalties and weight matrix choice. Nucleic Acids Res 22: 4673–4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Felsenstein J (1989) PHYLIP—phylogeny inference package (version 3.2). Cladistics 5: 164–166. [Google Scholar]
- 44. Costa JM, Corey A, Hayes PM, Jobet C, Kleinhofs A, et al. (2001) Molecular mapping of the Oregon Wolfe Barleys: a phenotypically polymorphic doubled-haploid population. Theor Appl Genet 103: 415–424. [Google Scholar]
- 45. Mameaux S, Cockram J, Thiel T, Steuernagel B, Stein N, et al. (2012) Molecular, phylogenetic and comparative genomic analysis of the cytokinin oxidase/dehydrogenase gene family in the Poaceae. Plant Biotec J 10: 67–82. [DOI] [PubMed] [Google Scholar]
- 46.Van Ooijen JW, Voorrips RE (2004) JoinMap 3.0: software for the calculation of genetic maps in experimental populations. CPRO-DLO, Wageningen.
- 47. Close TJ, Bhat PR, Lonardi S, Wu Y, Rostoks N, et al. (2009) Development and implementation of high-throughput SNP genotyping in barley. BMC Genomics 10: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cockram J, White J, Zuluaga DL, Smith D, Comadran J, et al. (2010) Genome-wide association mapping to candidate polymorphism resolution in the unsequenced barley genome. Proc Natl Acad Sci U S A 107: 21611–21616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thiel T, Graner A, Waugh R, Grosse I, Close TJ, et al. (2009) Evidence and evolutionary analysis of ancient whole-genome duplication in barley predating the divergence from rice. BMC Evol Biol 9: 209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Krzywinski M, Schein J, Birol I, Connors J, Gascoyne R, et al. (2009) Circos: an information aesthetic for comparative genomics. Genome Res 19: 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Devos KM (2005) Updating the ‘crop circle’. Curr Opin Plant Biol 8: 155–162. [DOI] [PubMed] [Google Scholar]
- 52. International Brachypodium Initiative (2010) Genome sequencing and analysis of the model grass Brachypodium distachyon . Nature 11: 763–768. [DOI] [PubMed] [Google Scholar]
- 53. Salse J, Bolot S, Throude M, Jouffe V, Piegu B, et al. (2008) Identification and characterization of shared duplications between rice and wheat provide new insights into grass genome evolution. Plant Cell 20: 11–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Paterson AH, Bowers JE, Chapman BA (2004) Ancient polyploidization predating the divergence of the cereals, and its consequences for comparative genomics. Proc Natl Acad Sci U S A 101: 9903–9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Holm K, Källman T, Gyllenstrand N, Hedman H, Lagercrantz U (2010) Does the core circadian clock in the moss Physcomitrella patens (Bryophyta) compromise a single loop? BMC Plant Biol 10: 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lang D, Zimmer AD, Rensing SA, Reski R (2008) Exploring plant biodiversity: the Physcomitrella genome and beyond. Trends Plant Sci 13: 542–549. [DOI] [PubMed] [Google Scholar]
- 57. Wenkel S, Turck F, Singer K, Gissot L, Le Gourrierec J, et al. (2006) CONSTANS and the CCAAT box binding complex share a functionally important domain and interact to regulate flowering of Arabidopsis . Plant Cell 18: 2971–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ben-Naim O, Eshed R, Parnis A, Teper-Bamnolker P, Shalit A, et al. (2006) The CCAAT binding factor can mediate interactions between CONSTANS-like proteins and DNA. Plant J 46: 462–476. [DOI] [PubMed] [Google Scholar]
- 59. Romier C, Cocchiarella F, Mantovani R, Moras D (2003) The NF-YB/NFYC structure gives insight into DNA binding and transcription regulation by CCAAT factor NF-Y. J Biol Chem 278: 1336–1345. [DOI] [PubMed] [Google Scholar]
- 60. Gendron JM, Pruneda-Paz JL, Doherty CJ, Gross AM, Kang SE, et al. (2012) Arabidopsis circadian clock protein, TOC1, is a DNA-binding transcription factor. Proc Natl Acad Sci U S A 109: 3167–3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lazaro A, Valverde F, Piñeiro M, Jarillo JA (2012) The Arabidopsis E3 Ubiquitin Lingase HOS1 negatively regulates CONSTANS abundance in the photoperiodic control of flowering. Natl Acad Sci U S A 24: 982–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jang S, Marchal V, Panigrahi KCS, Wenkel S, Soppe W, et al. (2008) Arabidopsis COP1 shapes the temporal pattern of CO accumulation conferring a photoperiodic flowering response. EMBO J 27: 1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Puchta H (2005) The repair of double-strand breaks in plants: mechanisms and consequences for genome evolution. J Exp Bot 56: 1–14. [DOI] [PubMed] [Google Scholar]
- 64. Bennetzen JL, J Ma, Devos KM (2005) Mechanisms of recent genome size variation in flowering plants. Annals Bot 95: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cockram J, Mackay IJ, O'Sullivan DM (2007c) The role of double-stranded break repair in the creation of phenotypic diversity at cereal VRN1 loci. Genetics 177: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Cistué L, Cuesta-Marcos A, Chao S, Echávari B, Chutimanitsakum Y, et al. (2011) Comparative mapping of the Oregon Wolfe Barley using doubled haploid lines derived from female and male gametes. Theor Appl Genet 122: 1399–1410. [DOI] [PubMed] [Google Scholar]
- 67. Börner A, Buck-Sorlin GH, Hayes PM, Malyshev S, Korzun V (2002) Molecular mapping of major genes and quantitative loci determining flowering time in response to photoperiod in barley. Plant Breed 121: 129–132. [Google Scholar]
- 68. Emebiri LC, Moody DB (2006) Heritable basis for some genotype-environment stability statistics: inferences from QTL analysis of heading date in two-rowed barley. Field Crops Res 96: 243–251. [Google Scholar]
- 69. Malosetti M, van Eeuwijk FA, Boer MP, Casas AM, Elía M, et al. (2010) Gene and QTL detection in a three-way barley cross under selection by a mixed model with kinship information using SNPs. Theor Appl Genet 122: 1605–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sameri M, Takeda K, Komatsuda T (2006) Quantitative trait loci controlling agronomic traits in recombinant inbred lines from a cross of oriental- and occidental-type barley cultivars. Breed Sci 56: 243–252. [Google Scholar]
- 71. Sameri M, Pourkheirandish S, Chen G, Tonooka T, Komatsuda T (2011) Detection of photoperiod responsive and non-responsive flowering time QTL in barley. Theor Appl Genet 61: 183–188. [Google Scholar]
- 72. Griffiths S, Simmonds J, Leverington M, Wang Y, Fish L, et al. (2009) Meta-QTL analysis of the genetic control of ear emergence in elite European winter wheat germplasm. Theor Appl Genet 119: 383–395. [DOI] [PubMed] [Google Scholar]
- 73. Faure S, Turner AS, Gruszka D, Christodoulou V, Davis SJ (2012) Mutation at the circadian clock gene EALRY MATURITY 8 adapts domesticated barley (Hordeum vulgare) to short growing seasons. Proc Natl Acad Sci U S A 109: 8328–8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kim S-K, Yun C-H, Lee JH, Hang YH, Park HY, et al. (2008) OsCO3, a CONSTANS-LIKE gene, controls flowering by negatively regulating the expression of FT-like genes under SD conditions in rice. Planta 228: 355–365. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Intron (line)/exon (box) structure of rice COL, PRR and CMF genes. Coding regions encoding protein domains are indicated: CCT (green), B-box1 (blue), B-box2 (red), PRR (orange). Non-significant B-box1 protein domains are indicated in light blue. Synonyms: OsCMF8 = OsI [13] and Ghd7 [34]. OsCMF11 = OsH [13]. OsA = Hd1 [24] and OsCO1. OsB = OsCO3 [74].
(TIF)
Alignment of Poaceae and arabidopsis CMF and ZCCT proteins. ZCCT proteins from T. monococcum and H. vulgare are included in the alignment. HvCMF1 and HvCMF3 are partial at the N-terminus end. AtCMF2, AtCMF5 and AtCMF7 are excluded.
(PDF)
Protein alignment of Poaceae COL Group I proteins. Intron positions are indicated by triangles and boxed. Positions of protein domains are indicated. Intron positions for HvCO1 – HvCO5 were determined from the sequences submitted by [13]. Intron positions for HvCO6, HvCO7 and HvCO8 were determined by gene prediction analysis of cv Morex RBCA contigs 6788, 2171376 and 143637, respectively.
(TIF)
Protein alignment of COL Group II proteins. Intron positions are indicated by triangles and boxed. Positions of protein domains are indicated. AtCO8 is excluded due to low sequence conservation with other Group II proteins.
(TIF)
Arabidopsis CMF genes. Chromosome (chr), amino acids (aa). Synonyms in brackets.
(DOCX)
Poaceae COL genes, and their homologues identified in the sequenced genomes of brachypodium, sorghum and foxtail millet. HvCO1 -HvCO8 are previously identified by [13]. Note, HvCO9 is re-named here as HvCMF11, due to lack of B-boxes. Alternative rice COL nomenclature shown in parentheses. Note, HvCO3 is orthologous to OsB – no OsB orthologues are found in brachypodium, sorghum or foxtail millet. B1 (B-box1), B2 (B-box2), B1/2 (B-box1/B-box2 chimeric domain), CCT (CONSTANS, CO-LIKE, TOC1). a relative to closest rice homologue. FGENESH reanalysis of: b scaffold_4, 12553835–12558834 bp; c scaffold_1, 30543240–30545239 bp; d scaffold_4:164743–169742 bp; e scaffold_6:33982724–33992723. f FGENESH reanalysis of genomic region around annotated gene.
(DOCX)
Poaceae PRR genes, and their homologues identified in the sequenced genomes of brachypodium, sorghum and foxtail millet. a relative to closest rice homologue. b Details according to Sbi1.4 gene set from the Sb1 assembly. Comparison with the allele from genotype ATx623 suggests that this allele corresponds to the Sbprr37-2 allele, which is predicted to possess a different intron/exon structure [20]. c No gene model (GM) predicted in the sorghum annotation. Analysis of the sorghum genomic region homologous to Os09g36220 (chromosome Sb2, 65858746–65868745 bp) indicated that a sequencing gap has likely resulted in failure of an appropriate gene model to be formed. Manual inspection shows of the eight exons present in Os09g36220, exon 6 and the 5′ end of exon 7 are predicted to lie within the sorghum sequencing gap. RR (response regulator), CCT (CONSTANS, CO-LIKE, TOC1).
(DOCX)
Poaceae CMF genes, and their homologues identified in the sequenced genomes of brachypodium, sorghum and foxtail millet. Full length barley CMF genes identified in the barley transcriptome/draft genome assembly are also shown. c_ (contig_), N/A (not applicable), B1 (B-box1), B2 (B-box2), B1/2 (B-box1-B-box2 chimeric domain), CCT (CONSTANS, CO-LIKE, TOC1). a relative to closest rice homologue. b FGENESH re-analysis of gene model. c FGENESH gene model. Note: e-value similarity with closest rice homologue is below the arbitrary experimental significance threshold. P truncated at 5′ end. d Although genes shown in brackets are most similar to OsCMF genes, they possess b-box domains and so belong to the COL gene family. Their details are described within the COL analyses (Table S2). e Si011862m, truncated CCT motif.
(DOCX)
Poaceae ZCCT genes investigated in this study. a E-values are relative to ZCCT1 CDS. b ZF = zinc-finger domain. c partial cDNA sequence only available. N/A = not applicable.
(DOCX)
Analysis of microsynteny. Rice CDS were used for BLASTn queries to identify genes with significant (≤1.0e-15) homology in the genomes of rice, brachypodium, sorghum and foxtail millet. Where WGD-derived colinear genomic regions are present, micro-colinearity with rice is investigated in both regions. Transposable elements are highlighted in grey. HP = hypothetical protein, CHP = conserved hypothetical protein, EP = expressed protein. N/A = not applicable. * = region of homology is located upstream of gene model. (A) Regions involved in the ancestral WGD. (B) Regions not involved in the ancestral WGD.
(XLSX)
Positioning of previously mapped CMF, COL and PRR genes within the barley consensus genetic map.
(DOCX)
Genetic mapping of barley genes.
(DOCX)
Integration of genes genetically mapped in the OWB population into the barley consensus genetic map.
(DOCX)
Comparative barley-rice analysis of mapped HvCMF, HvCOL and HvPRR genes.
(DOCX)