Abstract
Objective
To test whether a disclosure intervention improves subjective health and reduces medical consumption and sick leave in somatising patients in general practice.
Design
Non-blind randomised controlled trial.
Setting
10 general practices in the Netherlands.
Participants
161 patients who frequently attended general practice with somatising symptoms.
Intervention
Patients in the intervention group were visited two to three times and invited to disclose emotionally important events in their life. Control patients received normal care from their general practitioners.
Main outcome measures
Use of medical services (drugs and healthcare visits), subjective health, and sick leave assessed by self completion questionnaires after 6, 12, and 24 months.
Results
Of the 161 patients, 137 completed the trial (85%). Both groups were comparable at baseline. The intervention had no effect on the main outcome measures at any point. Intervention patients made one more visit to health care (95% confidence interval −4 to 6); the use of medicines did not change in both groups (−1 to 1); subjective health improved 3.6 points more in the control group (−11.2 to 4.3); and disclosure patients were on sick leave one more week (−1 to 3). Patients often had a depression or anxiety disorder for which they were not receiving adequate care.
Conclusion
Although the intervention was well received by patients and doctors, disclosure had no effect on the health of somatising patients in general practice.
What is already known on this topic
Up to 5% of patients in general practice attend frequently with somatising symptoms
Emotional expression techniques have been shown to have favourable effects on subjective health, visits to the doctor, and symptoms in healthy people
What this study adds
A disclosure intervention does not improve somatisation in primary care
About 45% of patients had an anxiety or depressive disorder, which was often unrecognised
Introduction
Patients presenting with functional complaints are common in general practice.1,2 Many patients present functional complaints only incidentally, whereas others have long term tendencies to seek medical attention for unexplained symptoms, often in relation to psychological stress, depression, or anxiety.3,4 A few patients are seriously disabled through a long history of many unexplained symptoms, defined as somatisation disorder.1,5 We adopted Lipowski's definition of somatisation: “A tendency to experience and express somatic distress and symptoms unaccounted for by pathological findings, to attribute them to physical illness, and to seek medical help for them. It is often assumed that somatisation becomes manifest in response to psychosocial stress brought about by life events that are personally stressful to the individual.”3
A purely medical approach is clearly insufficient when patients present with somatisation. Psychological methods are needed that are both feasible and acceptable to patients. Traumatic experiences in childhood can lead to later somatisation,6–8 and doctors often ask about life events and stressful circumstances when seeking a psychosocial explanation for unexplained symptoms.
For most patients sharing emotions with someone is acceptable, and self disclosure is culturally embedded.9 Use of emotional expression techniques, or disclosure, has been shown to improve subjective health, stress related immune measures, and physical symptoms in healthy people and to reduce the number of visits to general practitioners.10–12 However, the effectiveness of disclosure in somatisation has not been evaluated in a randomised controlled trial.
We developed an intervention focusing on disclosure of traumatic experiences for patients with somatisation in primary care. We studied the effect of adding the disclosure intervention to regular care on use of medical services, subjective health, and sick leave. In addition, we studied the effect on quality of life, severity of symptoms, patient's perception of support, and doctor's judgment of somatisation.
Participants and methods
We conducted a randomised controlled trial in 10 general practices cooperating in the Registration Network of General Practices around Maastricht in the Netherlands.13 We compared usual care with usual care plus the disclosure intervention. Randomisation was performed at the level of individual patients. Another nine randomly selected practices (five in Maastricht, and four in a sister project in Amsterdam) served as an extra control group to test for contamination of the effect of the trial as a whole; no intervention was introduced in these practices. The protocol was approved by the ethics committee of the Academic Hospital, Maastricht, and the University of Maastricht.
Recruitment of patients
We sent a postal questionnaire inquiring about somatisation symptoms to patients aged 20-45 years who frequently attended general practice. Frequent attendance was defined as 15 contacts or more with the doctor, on the patient's initiative, in the past three years. Routine examinations on the initiative of the doctor—for example, for cervical smears or checking blood pressure—were not counted. We estimated that this would select the 10% most frequently attending patients in this age group.14 The somatisation questionnaire, which we developed and validated in a pilot study, contained the 37 somatisation symptoms listed in the Diagnostic and Statistical Manual of Mental Disorders, third edition, revised (DSM-III-R), with the relevant follow up questions.5 Symptoms counted when not explained by organic disease and use of medicines, alcohol, or drugs (based on the physical examination of physicians, as reported by the patient in the questionnaire). Frequently attending patients with five or more somatisation symptoms were eligible for the study.1,2,15,16
We excluded patients with the following serious physical or mental diseases: cancer, AIDS, rheumatoid arthritis, multiple sclerosis, dementia, schizophrenia, mental disorder, and psychosis. We included patients with other chronic diseases, such as asthma, osteoarthritis, or cardiovascular diseases so that the sample would remain representative of patients in primary care. Patients with insufficient knowledge of the Dutch language to fill in questionnaires were excluded.
Treatment conditions
Control patients were treated by their doctor in the usual way. Intervention patients received, in addition to usual care, the disclosure intervention. This consisted of two meetings with a trained “disclosure doctor” at the patient's home. When important information was disclosed, and if the patient agreed, a joint consultation including the patient's doctor was planned as well. The first meeting had to be within two weeks after inclusion in the trial and lasted two hours; the second meeting, one week later, took one hour; and the optional joint consultation, another week later, took 30 minutes to one hour.
In the meetings, participants were invited to disclose emotionally important events in their life. If such events were not mentioned spontaneously, the disclosure doctor asked open questions about family life, health, work situation, and childhood. A structured, childhood trauma questionnaire was used.7 The disclosure doctor applied an open, evocative interview style, using non-directive consultation techniques such as open questions, reflection of emotions, inquiry on vague or unclear statements, and summarising. The disclosure doctor showed sincere interest in the patient's story and followed the patient's frame of reference.
Towards the end of the first meeting, the disclosure doctor asked a set of screening questions for depressive, anxiety, and somatoform disorders (DSM-IV). Between the two meetings, participants kept a diary for six days, in which they reported thoughts, emotions, physical sensations, and their activities throughout the day.17 In the second meeting, the results of the participant's life story, the DSM-IV screening, and the diary were discussed, and patients were asked to write a summary of the two meetings. Both the patient and the disclosure doctor rated the degree of disclosure. The three disclosure doctors were general practitioners who had had a training session in the disclosure intervention.
Randomisation
Eligible patients received information on the trial and were randomised when they agreed to participate. The randomisation was stratified (one stratum per practice) by using a sequence of labelled cards in opaque, sealed, numbered envelopes. An independent person produced the randomisation envelopes, and the research assistant (MBFL), who did not apply the intervention, executed the randomisation procedure. Although the general practitioners knew which patients received the intervention, they were not told which patients participated as controls. The allocation scheme was broken after the two year follow up.
Outcome measures
At baseline, six months, one year, and two years after entry in the trial, patients completed a questionnaire on the outcome measures. We calculated use of medical services (over the preceding six months) from the total number of visits to the general practitioner and other healthcare workers and the number of different drugs taken daily for at least a week. Subjective health in the previous month (0=very bad, 100=excellent) was operationalised as the average of a direct question on health and a combined score of six questions on the influence of symptoms on work, sleep, sports activities, social life, mood, and ruminating about being ill. Sick leave (inability to work or do household chores) was expressed in number of weeks over the preceding six months.
We assessed severity of symptoms with the somatisation, depression, and anxiety and agoraphobia subscales of the symptom check list-90.18 Patients rated their quality of life on a visual analogue scale (0=very low, 100=very high). Doctors rated the somatisation of each patient on a 5 point Likert scale (1=no somatisation, 5=severe somatisation) that had been validated in another study.16 We determined social support as the average support received from the five most important people in the patient's life (0= support, 100=high support).19 We also assessed the number of life events in the past year,19 problems in childhood,7 chronic difficulties,19 and attitude towards illness at baseline for comparison.
Statistical analysis
We calculated the sample size required to detect a difference of at least 25% (a worthwhile difference for a time intensive treatment) between the intervention and control condition in use of medical services. With a two sided significance of 5%, a power of 80%, and taking into account a dropout rate of up to 25%, we needed 80 patients per group. The analysis was based on intention to treat and included all patients who completed baseline and 24 month follow up questionnaires. We obtained missing data in returned questionnaires by telephone contact with the patient. The relative effectiveness of the intervention was determined by comparing the change in primary outcome variables between baseline and two years (Hodges-Lehman estimate of shift of the difference between intervention and control groups in change of outcome variables, based on a two sided Mann-Whitney U test with 95% confidence interval).
We planned the following subgroup analyses before the study: high versus low DSM-III-R somatisation score, high versus low somatisation according to general practitioner, presence or absence of major childhood problems, presence or absence of depression and anxiety, disclosure of emotionally important information during the intervention, and participation in the joint consultation.
Results
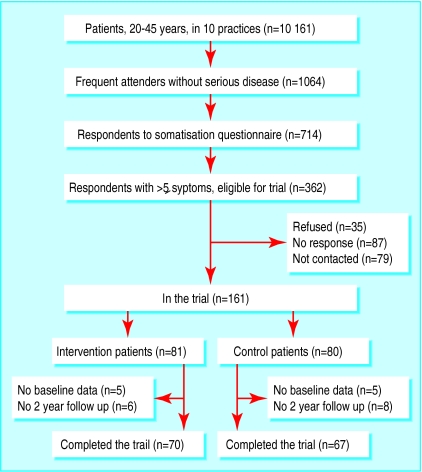
The figure shows the progress of patients through the study. Of the 10 161 patients aged 20-45 years in the 10 practices, 1064 patients (10.5%) were frequent attenders without serious disease. The somatisation questionnaire was returned by 714 frequent attenders (67%), of whom 362 (51%) reported five or more somatisation symptoms. Non-respondents (350) were more likely to be unmarried men, living alone, and have psychological problems, particularly addiction to alcohol and hard drugs. Of the 362 patients eligible for the trial, 87 did not answer telephone calls or letters, and 35 declined to participate. We did not contact 79 patients because enough patients had already been enrolled. Accordingly, 161 patients were included in the trial.
Altogether 137 patients completed the trial; 24 patients (15%) dropped out and were not included in the final analyses. Ten patients (five in each group) did not return the baseline questionnaire, and 14 (six in the intervention group and eight in the control group) did not complete the two year follow up. Of these 14, two control patients were admitted to a psychiatric clinic, two died (one control, one intervention), three patients moved with no forwarding address (one intervention, two control), and the remaining seven did not return the two year questionnaire despite several reminders (four intervention, three control).
Of the 81 intervention patients, 77 received the disclosure intervention and completed the two meetings with the disclosure doctor; the remaining four dropped out before receiving the intervention. Twenty two patients participated in an additional joint consultation with their own doctor. Of the remaining 55 patients, 30 did not disclose important information in the two meetings, nine had previously discussed the disclosed information with their doctor, 11 did not want to share the disclosed information with their doctor, and five gave no clear reason why they did not want to participate in the joint consultation.
According to the patients and disclosure doctors, 47 patients disclosed emotionally important information during the intervention. Topics of disclosure were childhood abuse (sexual, physical, or mental), alcohol dependency of parents, and loss of a parent or sibling at young age. Quite often patients reported that they had borne onerous household responsibilities as young children. A range of problems in adulthood was disclosed, including physical or sexual abuse, alcoholism, problems in relationships or at work, and social isolation. Patients commonly disclosed combinations of problems—for example, sexual abuse in childhood and depression in later life with marital problems. According to the DSM-IV screening, 34 of the 77 patients had an active depressive or anxiety disorder (16 a depressive disorder, 30 an anxiety disorder). Two patients fulfilled the criteria for hypochondriasis and 18 for the chronic benign pain syndrome.
At the start of the study, both groups had similar demographic and clinical characteristics (table 1). Changes in main outcome measures during two years of follow up were not significantly different between the two groups (table 2). Visits to health care increased by one more visit in the disclosure group at 24 months; the use of medicines did not change in either group; subjective health improved 3.6 points more in the control group; and disclosure patients were on sick leave one more week.
Table 1.
Comparison of intervention and control groups at baseline. Values are median (interquartile range) unless stated otherwise
| Scale range | Disclosure (n=76) | Usual care (n=75) | |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | 20-45 | 38 (33-41) | 39 (36-41) |
| No (%) of women | — | 61 (80) | 39 (78) |
| No (%) married or cohabiting | — | 56 (74) | 62 (83) |
| Educational status (No (%)): | |||
| Low | — | 36 (48) | 41 (55) |
| Middle | — | 36 (47) | 32 (43) |
| High | — | 4 (5) | 2 (2) |
| Professional status (No (%)): | |||
| Low | — | 44 (58) | 48 (64) |
| Middle | — | 28 (37) | 26 (35) |
| High | — | 4 (5) | 1 (1) |
| No (%) in paid employment | — | 42 (55) | 39 (52) |
| No (%) of immigrants | — | 2 (3) | 5 (7) |
| No (%) with public insurance | — | 59 (78) | 64 (86) |
| Main outcome measures | |||
| Use of medical services in past 6 months: | |||
| No of visits to healthcare professionals | — | 5 (3-14) | 7 (3-14) |
| No of different medicines | — | 2 (1-3) | 2 (1-2) |
| Subjective health in past month | 0-100 | 44 (27-60) | 47 (33-63) |
| Sick leave in past 6 months (weeks) | 0-26 | 2 (0-5) | 2 (0-6) |
| Subsidiary outcome measures | |||
| Quality of life score | 0-100 | 50 (36-75) | 51 (40-73) |
| Symptom check list score (past week): | |||
| Somatisation | 0-48 | 20 (16-25) | 22 (17-27) |
| Depression | 0-64 | 22 (13-33) | 22 (15-35) |
| Anxiety and agoraphobia | 0-68 | 15 (9-23) | 17 (10-31) |
| Somatisation according to GP* | 1-5 | 3.7 (3.0-5.0) | 3.7 (3.0-5.0) |
| Social support | 0-100 | 66 (59-71) | 65 (58-70) |
| Other measurements | |||
| No of consultations with GP in past 3 years | 15-77 | 22 (18-28) | 20 (17-28) |
| Somatisation symptoms (DSM-III-R) | 4-37 | 8 (6-11) | 8 (6-11) |
| No of life events in preceding year | 0-30 | 3 (1-4) | 2 (1-3) |
| Chronic difficulties | 0-60 | 10 (5-17) | 7 (2-14) |
| No of childhood problems | 0-8 | 2 (1-3) | 2 (0-3) |
| Illness attitude scale: | |||
| Total score | 0-96 | 32 (26-47) | 37 (27-46) |
| Score on health anxiety subscale | 0-44 | 16 (11-23) | 17 (12-25) |
| General practice records: | |||
| No (%) with depression | 5 (6) | 2 (3) | |
| No (%) with anxiety disorder | 3 (4) | 4 (5) | |
| No (%) with chronic disease† | 34 (45) | 23 (31) | |
Mean instead of median because of scale size.
44 International Code of Primary Care coded, prevalent chronic somatic disease conditions, such as diabetes mellitus, asthma, osteoarthritis, or cardiovascular diseases.
Table 2.
Change in outcome variables between baseline and follow up at two years. Values are medians (interquartile range)
| Scale range | Disclosure (n=70)
|
Usual care (n=67)
|
Difference* (95%CI) | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Change at 2 years | Baseline | Change at 2 years | ||||
| Main outcome variables | |||||||
| Use of medical services: | |||||||
| No of visits all health care | — | 5 (3 to 14) | 1 (−5 to 9) | 7 (3 to 14) | 0 (−6 to 7) | 1 (−4 to 6) | |
| No of different medicines | — | 2 (1 to 3) | 0 (−1 to 1) | 2 (1 to 2) | 0 (−1 to 1) | 0 (−1 to 1) | |
| Subjective health | 0-100 | 44 (27 to 60) | 0 (−16 to 12) | 47 (33 to 63) | 5 (−10 to 19) | −3.6 (−11.2 to 4.3) | |
| Sick leave | 0-26 | 2 (0 to 5) | 0 (−2 to 4) | 2 (0 to 6) | 0 (−2 to 2) | 1 (−1 to 3) | |
| Subsidiary variables | |||||||
| Quality of life | 0-100 | 50 (36 to 75) | 0 (−14 to 24) | 51 (40 to 73) | 4 (−8 to 19) | −1 (−10 to 8) | |
| Symptom check list score: | |||||||
| Somatisation | 0-48 | 20 (16 to 25) | 0 (−6 to 5) | 22 (17 to 27) | 0 (−5 to 5) | 0 (−3 to 3) | |
| Depression | 0-64 | 22 (13 to 33) | −2 (−8 to 4) | 22 (15 to 35) | −2 (−10 to 5) | 1 (−4 to 5) | |
| Anxiety and agoraphobia | 0-68 | 15 (9 to 23) | −2 (−5 to 2) | 17 (10 to 31) | 0 (−5 to 3) | −1 (−3 to 2) | |
| GP's assessment of somatisation | 1-5 | 3.7 (3.0 to 5.0) | 0.0 (−1.0 to 0.0) | 3.7 (3.0 to 5.0) | 0.0 (−1.0 to 1.0) | 0.0 (−1.0 to 1.0) | |
| Social support | 0-100 | 66 (59 to 71) | −1 (−7 to 8) | 65 (58 to 70) | 2 (−5 to 10) | −0.9 (−5.0 to 2.9) | |
Hodges-Lehman estimate of shift of difference between intervention and control groups in change of outcome variables (2 years to baseline) based on Mann-Whitney U test.
Detailed analyses of visits to specific healthcare professionals and use of different kinds of drugs showed no significant differences (table 3). In addition, changes in subsidiary outcome measures (quality of life, symptom check list-90, social support, and doctor's judgment of somatisation) did not differ between the groups (table 2). The general pattern was the same at the six and 12 month follow ups (data not shown). We found no significant differences in the subgroup analyses. Neither successful disclosure nor participation in the joint consultation affected the results.
Table 3.
Visits to health care and use of drugs at baseline and two year follow up (n=137)
| Disclosure (n=70)
|
Usual care (n=67)
|
Odds ratio* (95% CI) | ||||
|---|---|---|---|---|---|---|
| Baseline | 2 years | Baseline | 2 years | |||
| Mean No of visits to GP | 2.9 | 2.4 | 3.4 | 3.0 | 0 (−1 to 1)† | |
| No (%) who visited‡: | ||||||
| Specialist | 31 (44) | 29 (41) | 28 (42) | 24 (36) | 1.3 (0.6 to 2.7) | |
| Physiotherapist | 19 (27) | 20 (29) | 16 (24) | 22 (33) | 0.8 (0.4 to 1.7) | |
| Psychologist | 10 (14) | 8 (11) | 10 (15) | 9 (13) | 0.8 (0.3 to 2.4) | |
| Social worker | 8 (11) | 5 (7) | 2 (3) | 3 (4) | 1.1 (0.2 to 5.4) | |
| Complementary medicine | 11 (16) | 6 (9) | 5 (7) | 7 (10) | 0.5 (0.1 to 1.9) | |
| Other health care | 4 (6) | 6 (9) | 3 (4) | 6 (9) | 0.9 (0.3 to 3.1) | |
| No (%) taking§: | ||||||
| Antidepressants | 8 (11) | 8 (11) | 8 (12) | 11 (16) | 0.6 (0.2 to 1.8) | |
| Sedatives | 8 (11) | 11 (16) | 7 (10) | 8 (12) | 1.4 (0.5 to 3.9) | |
| Pain killers | 36 (51) | 41 (59) | 30 (45) | 29 (43) | 1.8 (0.9 to 3.8) | |
Logistic regression model; odds ratio for intervention patients at two years adjusted for baseline.
Hodges-Lehman estimate of shift of difference between intervention and control groups in change of outcome variables (two years−baseline) based on Mann-Whitney U test.
In past six months.
Drugs taken for at least a week daily in past six months.
In general, the disclosure intervention was well received: 57 out of 77 patients judged it positive, and at two years of follow up more intervention than control patients thought participation in the study had changed their health favourably (mean score 51 in intervention patients versus 47 in control patients (scale 0-100), P=0.11). However, at six and 12 months there was no difference between the groups on this measure. One patient criticised the disclosure intervention; she refused to be confronted with her childhood story again.
We did two checks for possible contamination between the two branches of the trial. General practitioners were asked to identify the control patients in their practice from a larger list towards the end of the trial. Fourteen patients (17%) were correctly identified, 21 patients (26%) were falsely identified as intervention patients, and 45 were incorrectly thought to be non-participants (κ=0.08). As a second check, we followed 98 patients in nine practices in which no intervention was introduced (data not presented). These 98 patients showed similar characteristics at baseline to the intervention and control patients, and changes on primary outcome measures at the two year follow up were not significantly different from the intervention and control group. The only difference was that patients in the intervention group were judged by their doctors to somatise less than patients in the external practices at the two year follow up (median score 3 in intervention patients v 3.5 in control patients (scale 1-5), P=0.01).
Discussion
The disclosure intervention had no effect on use of medical services, subjective health, or sick leave in somatising patients in general practice. We found no effect in patients who disclosed important information or in subgroups of patients with strong somatisation tendencies, depression, anxiety, or a traumatic childhood. However, a positive effect of disclosure in somatisation cannot be completely ruled out because the 95% confidence intervals were wide.
Design of intervention and study
The technique, duration, and frequency of our intervention were in accordance with the recommendations in a recent meta-analysis on emotional expression.10 The review found that short writing tasks may improve subjective health and have a smaller effect on health behaviours,10 although this last finding has been disputed.11
Although our trial was not blinded, the general practitioners did not identify most of their control patients. We cannot rule out, however, that some control patients may have benefited from their doctor's participation in the trial, thereby masking the effect of disclosure. If such contamination existed, it must have been small, since patients in the extra control practices had comparable results on all main outcome measures.
Explanation for findings
The effect of emotional expression in long term somatising patients may differ from that in student populations or other groups of patients, such as holocaust survivors.10,12 Somatising patients have an established pattern of medical consumption, which may be hard to alter with a short disclosure intervention. Their symptoms may be independent of earlier life experiences. A more sustained disclosure intervention, allowing patients to link symptoms to earlier traumatic events and process this trauma, might give better results.
About 40% of patients in the intervention group did not disclose information. These patients may not have had anything to disclose or were not ready to share their experiences. In addition, some patients had already shared their traumatic experiences with their general practitioner, and the intervention may have had limited effect in this group. All participants knew the aim and method of the study through the informed consent procedure, and patients not willing to share their traumatic experiences may have refused to participate. It remains unclear whether non-participating patients would benefit from a disclosure intervention if offered at another moment or in a different way.
We found an alarmingly high prevalence of depressive and anxiety disorders in the intervention group. The participating general practitioners had rarely registered these psychiatric disorders, and few patients had received adequate treatment. Instead, patients tended to visit specialists and physiotherapists and were taking painkillers.
Other studies
We compared our findings with those of studies using other techniques in somatisation. Management strategies focused on structuring general practitioner or specialist care through psychiatric consultations show positive20 as well as neutral21 results. Strategies aimed at “medical behaviour” and illness attributions of patients in primary and secondary care have been partially effective.22,23 Strategies focusing on the other elements of Lipowski's definition of somatisation,3 such as illness attributions and use of health care, may give better results than disclosure of traumatic experiences. Personal interest of doctors in their patients' life stories remains crucial to communication24–26 but will probably not in itself reduce use of medical services or sick leave or improve subjective health.
We conclude that, although valued by both general practitioners and patients, disclosure of emotionally important events has no effect in somatising patients in general practice. The patients did not improve, on average, in main outcome measures during the two year follow up. As up to 5% of patients in general practice are frequent attenders with somatising symptoms, further research is needed to determine effective strategies for treatment.
Figure.
Profile of trial
Acknowledgments
We thank the patients and their general practitioners for participating, Frenk Peeters for advice on psychiatry, and Hubert Schouten and Arnold Kester for statistical advice. We also thank members of the European General Practice Work Group for their constructive advice.
Footnotes
Funding: Netherlands Organisation for Scientific Research and Council for Medical and Health Research (grant No 940-33-016).
Competing interests: None declared.
References
- 1.Escobar JI, Rubio-Stipec M, Canino G, Karno M. Somatic symptom index (SSI): a new and abridged somatization construct. Prevalence and epidemiological correlates in two large community samples. J Nerv Ment Dis. 1989;177:140–146. doi: 10.1097/00005053-198903000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Portegijs PJM, van der Horst FG, Proot IM, Kraan HF, Gunther NC, Knottnerus JA. Somatization in frequent attenders of general practice. Soc Psychiatry Psychiatr Epidemiol. 1996;31:29–37. doi: 10.1007/BF00789119. [DOI] [PubMed] [Google Scholar]
- 3.Lipowski ZJ. Somatization: the concept and its clinical application. Am J Psychiatry. 1988;145:1358–1368. doi: 10.1176/ajp.145.11.1358. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg D, Bridges K. Somatic presentations of psychiatric illness in primary care setting. J Psychosom Res. 1988;32:137–144. doi: 10.1016/0022-3999(88)90048-7. [DOI] [PubMed] [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 3rd edition, revised. Washington, DC: APA; 1987. pp. 261–264. [Google Scholar]
- 6.Arnold RP, Rogers D, Cook DA. Medical problems of adults who were sexually abused in childhood. BMJ. 1990;300:705–708. doi: 10.1136/bmj.300.6726.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Portegijs PJM, Jeuken FM, van der Horst FG, Kraan HF, Knottnerus JA. A troubled youth: relations with somatization, depression and anxiety in adulthood. Fam Pract. 1996;13:1–11. doi: 10.1093/fampra/13.1.1. [DOI] [PubMed] [Google Scholar]
- 8.Craig TK, Boardman AP, Mills K, Daly Jones O, Drake H. The south London somatisation study. I: Longitudinal course and the influence of early life experiences. Br J Psychiatry. 1993;163:579–588. doi: 10.1192/bjp.163.5.579. [DOI] [PubMed] [Google Scholar]
- 9.George E. A cultural and historical perspective on confession. In: Pennebaker JW, editor. Emotions, disclosure, and health. Washington, DC: American Psychological Association; 1995. pp. 11–22. [Google Scholar]
- 10.Smyth JM. Written emotional expression: effect sizes, outcome types, and moderating variables. J Cons Clin Psychol. 1998;66:174–184. doi: 10.1037//0022-006x.66.1.174. [DOI] [PubMed] [Google Scholar]
- 11.Pennebaker JW. Writing about emotional experiences as a therapeutic process. Psychol Sci. 1997;8:162–166. [Google Scholar]
- 12.Pennebaker JW, Barger SD, Tiebout J. Disclosure of traumas and health among Holocaust survivors. Psychosom Med. 1989;51:577–589. doi: 10.1097/00006842-198909000-00009. [DOI] [PubMed] [Google Scholar]
- 13.Metsemakers JFM, Höppener P, Knottnerus JA, Kocken RJJ, Limonard CBG. Computerized health information in the Netherlands: a registration network of family practices. Br J Gen Pract. 1992;42:102–106. [PMC free article] [PubMed] [Google Scholar]
- 14.De Bakker D, Kulu-Glasgow I, Abrahamse H. Annual report of Information Network for General Practitioners 1997 [in. Dutch]. Utrecht: Dutch Institute for Primary Care Research; 1998. [Google Scholar]
- 15.Escobar JI, Waitzkin H, Silver RC, Gara M, Holman A. Abridged somatization: a study in primary care. Psychosom Med. 1998;60:466–472. doi: 10.1097/00006842-199807000-00012. [DOI] [PubMed] [Google Scholar]
- 16.Schilte AF, Portegijs PJM, Blankenstein AH, Knottnerus JA. Somatisation in primary care: clinical judgement and standardised measurement compared. Soc Psychiatry Psychiatr Epidemiol. 2000;35:276–282. doi: 10.1007/s001270050239. [DOI] [PubMed] [Google Scholar]
- 17.De Vries MW, editor. The experience of psychopathology: investigating mental disorders in their natural settings. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- 18.Derogatis L, Cleary P. Confirmation of the dimensional structure of the SCL-90: a study in construct validation. J Clin Psychol. 1977;33:981–989. [Google Scholar]
- 19.Portegijs PJM. Maastricht: University of Maastricht; 1996. Somatization in frequent attenders of general practice [PhD thesis] [DOI] [PubMed] [Google Scholar]
- 20.Smith GR, Rost K, Kashner TM. A trial of the effect of a standardized psychiatric consultation on health outcomes and costs in somatizing patients. Arch Gen Psychiatry. 1995;52:238–243. doi: 10.1001/archpsyc.1995.03950150070012. [DOI] [PubMed] [Google Scholar]
- 21.Katon WJ, Von Korff M, Lin E, Bush T, Russo J, Lipscomb P, et al. A randomized trial of psychiatric consultation with distressed high utilizers. Gen Hosp Psychiatry. 1992;14:86–98. doi: 10.1016/0163-8343(92)90033-7. [DOI] [PubMed] [Google Scholar]
- 22.Speckens AE, van Hemert AM, Spinhoven P, Hawton KE, Bolk JH, Rooijmans HG. Cognitive behavioural therapy for medically unexplained physical symptoms: a randomised controlled trial. BMJ. 1995;311:1328–1332. doi: 10.1136/bmj.311.7016.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morriss RK, Gask L, Ronalds C, Downes Grainger E, Thompson H, Goldberg D. Clinical and patient satisfaction outcomes of a new treatment for somatized mental disorder taught to general practitioners. Br J Gen Pract. 1999;49:263–267. [PMC free article] [PubMed] [Google Scholar]
- 24.Salmon P, Peters S, Stanley I. Patients' perception of medical explanations for somatisation disorders: qualitative analysis. BMJ. 1999;318:372–376. doi: 10.1136/bmj.318.7180.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cape J, McCulloch Y. Patients' reasons for not presenting emotional problems in general practice consultations. Br J Gen Pract. 1999;49:875–879. [PMC free article] [PubMed] [Google Scholar]
- 26.Di Blasi Z, Harkness E, Ernst E, Georgiou A, Kleijnen J. Influence of context effects on health outcomes: a systematic review. Lancet. 2001;357:757–762. doi: 10.1016/s0140-6736(00)04169-6. [DOI] [PubMed] [Google Scholar]