Abstract
Background
Diffusion tensor imaging (DTI) study revealed reduced fractional anisotropy (FA) values in the corpus callosum (CC) in migraine patients without aura. Abnormalities in white matter integrity, particularly in the CC, may affect inter-hemispheric resting state functional connectivity (RSFC). Unfortunately, relatively little is known about the alterations in functional interactions between the cerebral hemispheres during resting state in migraine patients without aura, and even less about how the inter-hemispheric RSFC are affected by the abnormalities of the CC.
Methods and findings
Twenty-one migraine patients without aura and 21 healthy controls participated in this study, age-, sex-, and education-matched. Tract-based spatial statistics (TBSS) was employed to investigate the white matter alterations of the CC. Meanwhile, voxel-mirrored homotopic connectivity (VMHC) was used to compare the inter-hemispheric RSFC differences between the patients and controls. TBSS analysis revealed reduced FA values in the genu and the splenium of CC in patient group. VMHC analysis showed decreased inter-hemispheric RSFC of anterior cingulate cortex (ACC) in migraine patients without aura relative to that of the controls. Furthermore, in migraine patients without aura, the reduced FA values of the genu of CC correlated with the decreased inter-hemispheric RSFC of the ACC.
Conclusions
Our findings demonstrated that the migraine patients without aura showed reduced FA values of the genu of CC and decreased inter-hemispheric RSFC of the ACC. The correlation between the above structural and functional changes suggested that the reduced fractional anisotropy (FA) of CC modulates inter-hemispheric VMHC in migraine patients without aura. Our results demonstrated that the VMHC alterations of ACC can reflect the FA changes of the genu of CC in migraine patients without aura.
Introduction
Migraine is an idiopathic headache disorder, characterized as moderate to severe, often unilateral and pulsating headache attacks [1], [2]. With the advance in neuroimaging techniques, it has recently been regarded as a central nervous system disorder rather than merely a vascular, to a neurovascular disorder [1], [2]. Researchers have identified the probably migraine generator which is responsible for initiation of the migraine attack, i.e. the dorsal rostral pons [3], [4], [5], [6]. Apart from these lines, in patients with migraine structural [7], [8], [9], [10], [11] and functional [3], [4], [5], [6], [12], [13], [14] alterations have been observed in brain regions implicated with pain processing between migraine attacks, such as the anterior cingulate cortex (ACC), the orbitofrontal cortex (OFC), the insula, the temporal lobe, the posterior cingulate cortex (PCC), the supplementary motor area (SMA), the cerebellum and the thalamus. The reduced gray matter of the frontal lobes, brainstem, and the cerebellum were found to correlate with disease duration and severity, suggesting that more severe disease could result in more abnormalities that persist among migraines [1], [2], [15], [16].
Among previous findings, it is worthy to note that diffusion tensor imaging (DTI) method revealed the reduced fractional anisotropy (FA) values of the corpus callosum (CC) (genu, body, and splenium) in migraine patients without aura compared with the control group [17]. As the largest white matter structure in the brain, the CC is a wide bundle of neural fibers beneath the cortex in the brain at the longitudinal fissure, connecting the left and right cerebral hemispheres. The CC provides the main route of communication between the two hemispheres of the brain and facilitates inter-hemispheric communication [18], [19]. Abnormalities in white matter integrity, particularly in CC, may affect inter-hemispheric resting state functional connectivity (RSFC), which is fundamental to integrative attention processing and cognitive control [20], [21]. Unfortunately, relatively little is known about the alterations in functional interactions between the cerebral hemispheres during resting state in migraine patients without aura, and even less about the association between the inter-hemispheric RSFC changes and the abnormalities of the CC.
RSFC measures spontaneous brain activity of low-frequency fluctuations in blood oxygen level–dependent (BOLD) signals [22], which offers a route to directly quantify the inter-hemispheric functional interactions. During resting state, highly correlated spontaneous fluctuations are often observed within spatially separated but functionally related groups of cortical and subcortical regions, which consisted of the human brain’s intrinsic functional networks [23]. Vast majority of functional connections identified in these intrinsic functional networks are bilateral [24], [25]. Strong RSFC is even observable between homotopic regions with few monosynaptic callosal connections [26], [27], [28], which suggest that functional homotopy reflects an essential aspect of brain function [29]. Consistent with this conclusion, homotopic RSFC exhibits regional variation congruent with the brain’s functional hierarchy [30]. In addition, the developmental trajectories of homotopic RSFC show regional and hierarchical specificity across the life span [31], and homotopic RSFC is disrupted in autism [32] and cocaine addiction [33]. Therefore, homotopic RSFC may provide a sensitive indicator, which can reflect the effects of frequent nociceptive input on the pain processing circuits in migraine patients without aura.
To investigate that whether the abnormal white matter integrities of CC modulates inter-hemispheric RSFC in migraine patients without aura, multimodal MRI approach was employed in the current study. Firstly, tract-based spatial statistics (TBSS) was used to detect the FA differences of CC between the migraine patients and controls. Secondly, we examined inter-hemispheric RSFC in migraine patients without aura, using a novel approach, voxel-mirrored homotopic connectivity (VMHC) [31], [32], [33]. VMHC assesses the RSFC between each voxel and its mirrored counterpart in the other hemisphere. We compared VMHC differences between migraine patients without aura and controls and hypothesized that migraine patients would show abnormal inter-hemispheric RSFC. Thirdly, for the regions showed abnormal inter-hemispheric RSFC, seeding-based RSFC was employed to investigate the RSFC patterns within corresponding networks. Finally, we assessed the relationship between the white matter density of CC and abnormal inter-hemispheric RSFC in migraine patients without aura.
Materials and Methods
This study was approved by the Medical Ethics Committee of the West China Hospital of Sichuan University and was conducted in accordance with the Declaration of Helsinki. All participants gave their written informed consent after the experimental procedure was fully explained.
Participants
The diagnostic criteria of International Headache Society (IHS) for migraine without aura consists of the occurrence of at least 5 headache attacks that fulfill the following including criteria: 1) Headache attacks lasting 4–72 hours (untreated or unsuccessfully treated); 2) featuring at least two of the following characteristics: unilateral location, pulsating quality, moderate-to-severe pain intensity, and aggravation by causing avoidance of routine physical activity (e.g., walking or climbing stairs); 3) during the headache, they must have at least one of the following: nausea and/or vomiting, photophobia and phonophobia and 4) headache is not attributed to another disease [34]. According to the IHS criteria, migraine patients without aura were screened in our hospital. Twenty-one migraine patients without aura (16 females, aged 21–53 years, 32.4±10.3) were enrolled in our hospital. Patients rated the average pain intensity as 5.3±1.5 on a 0–10 scale derived from attacks in the past 4 weeks, with 10 being the most intense pain imaginable. In addition, twenty-one age- and gender-matched healthy controls (16 females, aged 22–54 years, 31.6±9.5) participated in our study. The controls either had no headache days per year or had family members who suffered regularly from a migraine or had other headaches. All of the participants were right-handed. Exclusion criteria for both groups were: 1) existence of a neurological disease; 2) alcohol, nicotine or drug abuse; 3) pregnancy or menstrual period in women; 4) any physical illness such as a brain tumor, hepatitis, or epilepsy as assessed according to clinical evaluations and medical records; and 5) claustrophobia. Patients were not having a migraine attack at least 72 hours prior to testing and no patient had a migraine precipitated during or on the day following the scan [12], [14]. The clinical characteristics of migraine patients without aura are shown in Table 1.
Table 1. Clinical information of migraine patients without aura and healthy controls (mean ± SD).
| Items | migraine patients (N = 21) | healthy controls (N = 21) |
| Age (years) | 32.4±10.3 | 31.6±9.5 |
| Gender (female/male) | 16/5 | 16/5 |
| Duration of disease (years) | 10.6±6.6 | N/A |
| Attack frequency (times) | 4.5±1.7 | N/A |
| Duration of a migraine attack (hours) | 13.4±5.8 | N/A |
| Average pain intensity (0–10) | 5.3±1.5 | N/A |
Data Acquisitions
This experiment was carried out in a 3.0 Tesla Signa GE scanner with an 8-channel phase-array head coil at the Huaxi MR Research Center. For each subject, a high-resolution structural image was acquired by using a three-dimensional MRI sequence with a voxel size of 1 mm3 using an axial Fast Spoiled Gradient Recalled sequence (FSGPR) with the following parameters: repetition time (TR) = 1,900 ms; echo time (TE) = 2.26 ms; data matrix = 256×256; field of view (FOV) = 256 mm×256 mm. The resting-state functional images were obtained with an echo-planar imaging (EPI) with the following parameters: 30 contiguous slices with a slice thickness = 5 mm; TR = 2,000 ms; TE = 30 ms; flip angle = 90°; FOV = 240 mm×240 mm; data matrix = 64×64, and total volumes = 180. During the 6 minute functional scan, subjects were instructed to keep their eyes closed, not to think about anything, and to stay awake during the entire session. After the scan, the subjects were asked whether or not they remained awake during the whole procedure. Diffusion tensor images were acquired with 2 averages. The diffusion sensitizing gradients were applied along 30 non-linear directions (b = 1000 s/mm2) together with an acquisition without diffusion weighting (b = 0 s/mm2). The imaging parameters were 45 continuous axial slices with a slice thickness of 3 mm and no gap, FOV = 240 mm×240 mm; TR = 6800 ms; TE = 93 ms; data matrix = 128×128.
DTI Data Analysis
DTI data preprocessing was carried out using FSL 4.1.9 (www.fmrib.ox.ac.uk/fsl/). First of all, correction for eddy-currents and head motion was done by means of affine registration on the first no-diffusion weighted volume of each subject. FA images were created by fitting the diffusion tensor to the raw diffusion data after brain extraction using BET [35]. Then, a voxel-wise statistical analysis of the FA data was carried out using the TBSS of FSL [36], [37]. FA images from all the subjects were realigned into an FMRIB58_FA standard-space image by FNIRT [38], [39] using a b-spline representation of the registration warp field [40]. The mean FA image was then created and thinned to create a mean FA skeleton (threshold of 0.2) representing the centers of all of the tracts common to the group. Each subject’s aligned FA data was then projected back onto this skeleton. Analysis of covariance (ANCOVA) was employed for statistical analysis, with age and gender effects as covariates. White matter FA value changes were assessed using permutation-based non-parametric testing [41] with 10000 random permutations. The threshold for significance was p<0.05, using threshold-free cluster enhancement (TFCE) method with family wise-error (FWE) correction for multiple comparisons [42]. The FA values of the brain regions showed abnormal white matter diffusion properties were extracted, averaged and correlated with the duration of migraine in patients group.
Resting State Imaging Data Preprocessing
Data processing was performed using Analysis of Functional NeuroImages (AFNI) and FSL software according to previous studies [31], [33]. Preprocessing comprised the following steps: 1) remove first 5 volumes; 2) slice timing correction; 3) three-dimensional motion correction; 4) temporal despiking; 5) mean-based intensity normalization; 6) temporal band-pass filtering (0.0009–0.1 Hz); 7) linear and quadratic detrending; 8) nuisance signal removal (white matter, cerebrospinal fluid, global signal and 6 motion parameters) via multiple regression; 9) linear registration of functional to structural images (with intermediate registration to a low-resolution image and b0 unwarping); 10) nonlinear registration of structural images to the Montreal Neurological Institute (MNI) 152 template.
Voxel-Mirrored Homotopic Connectivity
To account for geometric differences between hemispheres, we refined the registration from individual anatomic to MNI152 template space using the group-specific symmetrical template. In detail, all 42 registered structural images were averaged to create a mean image, which was then averaged with its left–right mirror to generate a group-specific symmetrical template using FSLUTILS. Nonlinear registration to this symmetrical template was performed for each participant, and the resultant transformation was applied to each participant’s preprocessed functional data. Homotopic RSFC was computed as the Pearson correlation (Fisher Z-transformed) between every pair of symmetrical inter-hemispheric voxels’ time series. The resultant correlations constitute VMHC. Since x = 0 defines the brain midline and there are no voxels medial of this plane, we excluded voxels medial of x = 0.
Global and regional group differences in VMHC were examined between the migraine and control group. Global VMHC was calculated by averaging VMHC values across all brain voxels within a unilateral hemispheric gray matter mask (there is only one correlation for each pair of homotopic voxels), which was created using the MNI152 gray matter tissue prior in FSL (threshold = 25% tissue-type probability). Group comparisons of global VMHC were performed using t tests. The significance threshold was p<0.05 and Bonferroni correction was used for multiple comparisons. With regard to the regional group differences in VMHC, the Permutation-based non-parametric testing with 10000 random permutations controlling for age and gender effects was employed. The threshold for significance was p<0.05, using TFCE method with FWE correction for multiple comparisons. The VMHC values of the brain regions showed abnormal inter-hemispheric connectivity were extracted, averaged and correlated with the duration of migraine in patients group.
Seeding-Based RSFC
We examined the RSFC associated with areas exhibiting significantly different VMHC between groups. Specifically, we computed whole-brain voxelwise correlations associated with mean time series derived separately for two regions of interest (ROIs), comprising all voxels within the ACC area exhibiting reduced VMHC for migraine patients. Fisher Z–transformed correlation maps were then entered into a group-level voxelwise t test analysis including age and gender as covariates. Whole-brain correction for multiple comparisons was performed (p<0.05, FWE correction).
Functional-Structural Relationship
To investigate how the inter-hemispheric RSFC are affected by the abnormalities of the CC, we assessed the relationship between the abnormal FA of CC and VMHC of the ACC in the migraine patients without aura. In detail, Pearson correlation was carried out between the FA value of the genu of CC and VMHC value of ACC in migraine patients.
Results
DTI Results
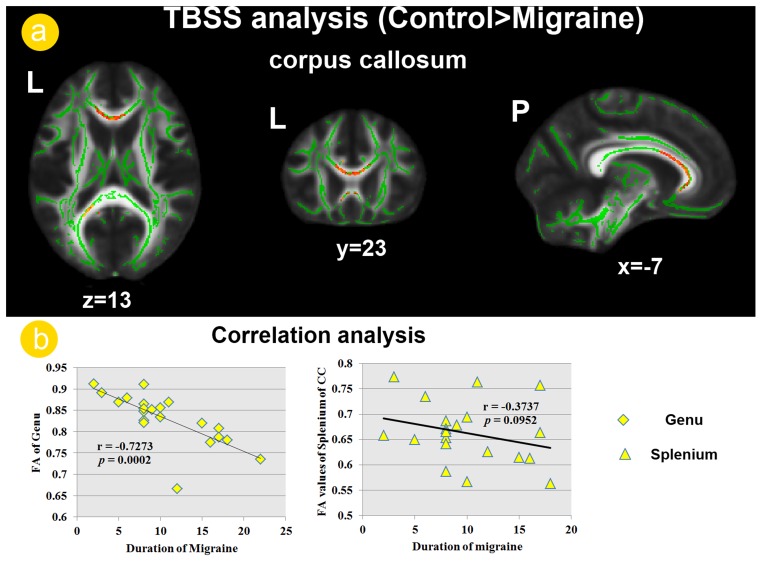
In this study, we focused on the diffusion measurements of CC and only the findings of CC were reported here. TBSS analysis revealed that the migraine patients showed decreased FA values in the left splenium and bilateral genu of CC relative to the controls (Figure1. a). The correlation analysis revealed a significant negative correlation between the FA values of the genu of CC and duration of migraine in patients group (r = −0.7273; p = 0.0002), although the FA values of splenium of CC were not significant correlated with duration of migraine (r = −0.3737; p = 0.0952) (Figure1. b).
Figure 1. White matter abnormalities of the corpus callosum (CC) in migraine patients.
a). Tract-based spatial statistics (TBSS) analysis revealed decreased fractional anisotropy (FA) values in the genu and the splenium of CC among patient group. b). the decreased FA values of the genu of CC were negatively correlated with duration of disease in migraine patients without aura.
Voxel-Mirrored Homotopic Connectivity
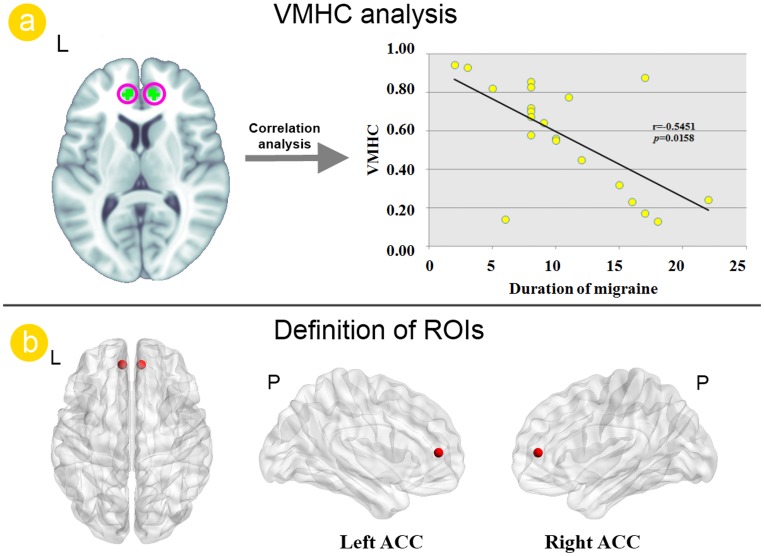
The global VMHC were not significant different (t(39) = 1.6501; p = 0.1149) between migraine (0.3794±0.0221) and control groups (0.4107±0.0236). However, the local comparisons revealed the ACC in which migraine exhibited reduced VMHC than controls after controlling for the age and sex (Figure2. a). No brain regions showed stronger VMHC in the migraine, relative to the control group. The correlation analysis indicated that the VMHC value of ACC negatively correlated with the duration of migraine in patients group (r = −0.5451; p = 0.0158) (Figure2. a).
Figure 2. Voxel-mirrored homotopic connectivity (VMHC) analysis results between migraine patients and healthy controls.
a). Resting state analysis revealed the reduced VMHC value of the ACC in migraine patients without aura. The VMHC value of the anterior cingulate cortex (ACC) was correlated with duration of migraine. b). The bilateral ACC were chosen as the region of interest (ROI) for subsequent seeding-based resting state functional connectivity (RSFC).
Seeding-Based RSFC
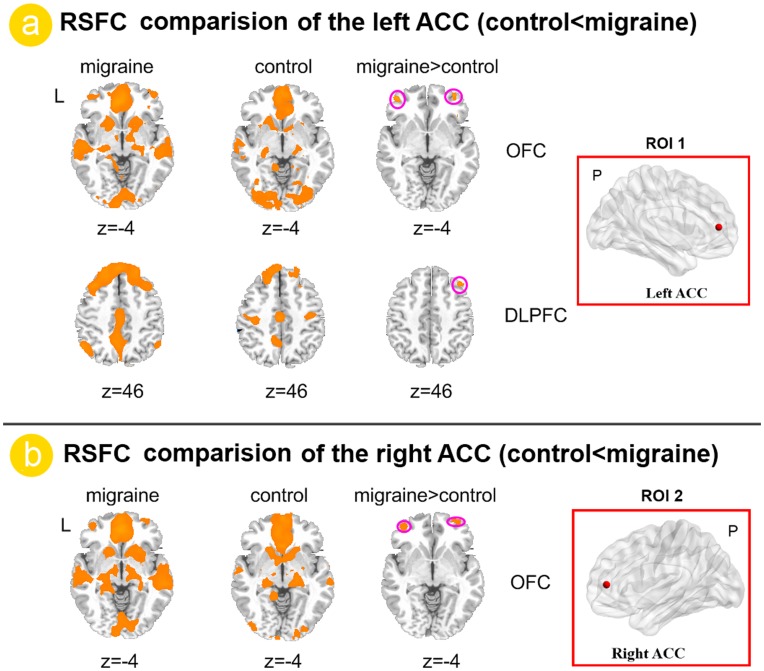
We examined whole brain RSFC associated with the two ROIs (Figure1b), i.e. the left and right ACC, which exhibited reduced VMHC for migraine patients. Relative to healthy controls, migraine patients showed increased RSFC between the left ACC and the bilateral OFC and right dorsolateral prefrontal cortex (DLPFC) (Figure3. a). With regards to the right ACC, the migraine patients showed increased RSFC with the bilateral OFC (Figure3. b).
Figure 3. Functional connectivity analysis results between migraine patients and healthy controls.
a). Relative to healthy controls, migraine patients without aura showed increased RSFC between the left anterior cingulate cortex (ACC) and the left dorsolateral prefrontal cortex (DLPFC) and right orbitofrontal cortex (OFC) (Figure3. a). With regards to the right ACC, the migraine patients without aura showed increased RSFC in the bilateral OFC.
Function-structure Relationship
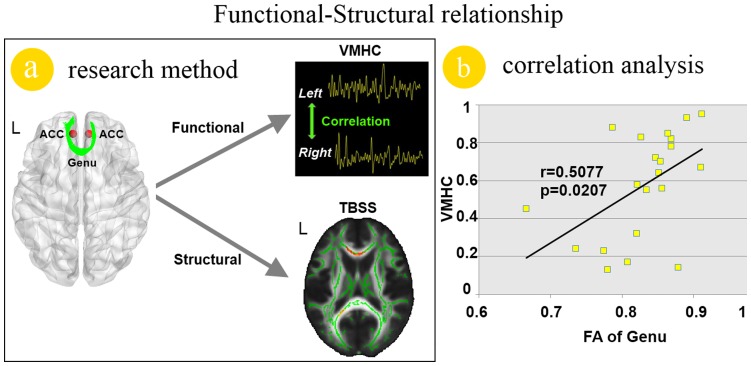
To assess the effect of structural abnormalities of the genu of CC on inter-hemispheric RSFC of the ACC in migraine patients, the person correlation was introduced. Our results revealed a significant positive correlation (r = 0.5077; p = 0.0207) between the VMHC of the ACC and FA values of the genu of CC in the migraine patients (Figure4. b).
Figure 4. Functional and structural relationship analysis results in migraine patients.
a). The demonstration of the methods in this study. b). The reduced voxel-mirrored homotopic connectivity (VMHC) value of the anterior cingulate cortex (ACC) was significant correlated with the decreased fractional anisotropy (FA) value of the genu of orpus callosum (CC) in migraine patients without aura.
Discussion
Migraine is the most common neurological disorder and cause significant individual and social burden due to chronic and unexpected pain attack [1]. In the past few decades, advanced neuroimaging had led to our evolution in our understanding of migraine pathophysiology [1], [2]. Brain structural and functional changes were both observed in patients with migraine and these alterations were negatively associated with longer migraine duration and attack frequency [1], [2], [7], [14], [15], [16]. Consistent with previous migraine study findings [17], our TBSS analysis revealed the genu and splenium part of CC showed decreased FA values in migraine patients without aura, relative to the healthy controls (Figure1. a). Furthermore, the reduced FA values of genu of CC were negatively correlated with the duration of disease (Figure1. b). As the largest white matter structure in the brain, the CC facilitates inter-hemispheric communication by providing the main route of communication between the two hemispheres of the brain [18], [19]. Abnormalities in white matter integrity in CC may influence inter-hemispheric RSFC. Therefore, we further investigated the inter-hemispheric RSFC between the migraine patients and healthy controls.
In the present study, we found reduced inter-hemispheric RSFC of ACC in migraine patients without aura (Figure2. a) and left ACC and right ACC networks both showed abnormal RSFC patterns (Figure3). The brain’s intrinsic functional architecture constitutes the foundation on which momentary neuronal responses underlying cognition and behavior are built [43], [44]. Thus, although we detected decrements in inter-hemispheric RSFC in ACC while participants were at rest, those decrements is likely to contribute to impaired function when that network is engaged in pain processing. As one of the most salient characteristics of the brain’s intrinsic functional architecture, homotopic functional connectivity can reflect the importance of inter-hemispheric communication to integrate brain function underlying coherent cognition and behavior [20], [30]. Altered inter-hemispheric functional interactions have been observed in several brain disorders [32], [33] and in normal aging [45], [46]. Our results provided further evidence of an association between migraine and disruptions between bilateral hemispheres, the inter-hemispheric communication of ACC especially (Figure2). Our findings suggested that attention should be paid to the roles of the disrupted functional circuitry as well.
Modern neuroimaging methods have explicated the neural substrate undying pain processing, namely pain matrix. It involves several cortical and subcortical brain regions, such as the thalamus, the amygdala, the insula cortex, the SMA, the posterior parietal cortex, the prefrontal cortex, the ACC, the periaqueductal gray, the basal ganglia, cerebellar cortex, the primary and secondary sensory cortex [15], [47]. As a key component of pain matrix, both structural and functional studies explicates ACC’s pivotal role in pain processing. Previous studies revealed that the affective responses of pain, such as unpleasantness, suffering and other negative effects, may be principally integrated in ACC [48], [49], [50], [51]. ACC is also involved in endogenous pain control, which is mediated by the endogenous opioid systems [52], [53]. The role of the ACC in migraine has been underpinned by recent neuroimaging studies. Positron emission tomography (PET) studies have shown an association between pain and abnormal activation of the ACC in migraine patients [5], [6]. Reduced regional homogeneity (ReHo) value of the BOLD signal in ACC was also observed in migraine patients without aura [14]. On the other hand, researchers using voxel-based morphormetry (VBM) method detected the decreased gray matter of ACC in migraine patients and significant correlations between the structural changes in ACC and disease duration [8], [9]. Therefore, we suggest that the VMHC abnormalities of ACC in migraine patients without aura (Figure2. a) validated the role of ACC in migraine.
Acknowledging that ACC dysfunction alone cannot explain the pathophysiology of migraine, therefore the bilateral ACC were chosen as the ROIs for whole brain RSFC analysis. Our results demonstrated that the left ACC showed increased RSFC with the right DLPFC and bilateral OFC (Figure3. a) and the right ACC showed increased RSFC with the bilateral OFC (Figure3. b) in migraine patients. The DLPFC has been shown to be involved with the cognitive dimension of pain processing, i.e. localization and encoding of the attended stimulus [54]. The OFC was involved in sensory integration, decision-making, expectation and planning behavior associated with sensitivity to reward and punishment [55]. All received sensations are modulated and related affective responses are assigned accordingly by the OFC [56], which is associated with the learning of the affective and motivational value of stimulation [57]. In addition, pain and pleasurable stimuli have been shown to elicit opioid release in the OFC [58], [59]. The increased functional connectivity between the ACC and DLPFC, between the ACC and OFC may explain the abnormal pain processing and executive function in migraine patients [12], [15], [60]. We suggested that frequent nociceptive input modified the frontal cortex resting state connectivity patterns and these changes may explain the functional impairments in migraine patients.
The correlation analysis demonstrated that the reduced inter-hemispheric RSFC of ACC in migraine patients without aura correlated with the duration of disease (Figure2. a), which suggested that longer duration of disease could result in more serious effects on the inter-hemispheric RSFC of ACC. Furthermore, the VMHC value in the ACC was positively correlated with the FA values of the genu of CC in the migraine patients (Figure4). Anatomically, the thinner axons in the genu connect the prefrontal cortex between the two hemispheres of the brain [18], [19]. The bilateral ACC communicated with one and the other by information transferring through the genu of CC (Figure4. a). Therefore, the white matter integrity abnormalities of the genu of CC probably affect the VMHC of ACC. Given that VMHC changes of the ACC were correlated with the FA changes of the genu of CC, we suggest that there is the possibility that the white matter changes of the genu of CC modulate the VMHC between bilateral ACC. In addition, previous studies had provided scientific evidence for that the RSFC can reflects structural connectivity in brain networks [61], [62], [63]. Our results demonstrated that the VMHC alterations of ACC can reflect the FA changes of the genu of CC in migraine patients without aura.
In the current study, it is worth noting that the VMHC changes were not randomly distributed, but concerned defined and highly pain related brain areas, i.e. ACC. It is indeed remarkable that the structural [1],[2],[15],[16] and functional [5], [6], [14] alterations seen in the ACC in migraine patients are similar to the changes reported in chronic back pain [64] and chronic phantom pain [65]. As most changes correlate to disease duration, it is plausible to suggest that the alteration of this region is a consequence, rather than a cause, of frequent nociceptive input. Our study used cross-sectional design and the question that whether these differences were consequence and precondition of migraine remains unclear. Although, correlation analysis indicated that the disease duration significant correlation with the reduced VMHC of the ACC (Figure2. a) and the reduced FA values of the genu of the CC (Figure1. b) in migraine patients without aura, this question could only be answered by investigating the temporal characteristics of experience-induced plasticity changes using a longitudinal design in the future. A comprehensive study design is also needed to investigate the exact relationship between the ACC functional changes during resting state and the CC structural changes.
In conclusion, we employed multimodal imaging methods to test the hypothesis that whether the abnormal white matter integrities of CC modulates inter-hemispheric RSFC in migraine patients without aura (Figure4. a). Our results demonstrated that the ACC showed reduced VMHC in migraine patients without aura and the inter-hemispheric RSFC changes of ACC correlated with the reduced FA value of the genu of the CC. Our findings provide further evidence of an association between the functional and structural alterations in migraine patients without aura. We suggested that the reduced FA value in genu of CC modulates inter-hemispheric RSFC in migraine patients without aura. It is hoped that combination of the functional and structural information may provide deeper insights into the migraine effects on the brain. However, more comprehensive experiment design with pain processing task are needed to investigate the accurate role of the VMHC abnormalities of ACC in migraine patients without aura.
Funding Statement
This paper is supported by the Project for the National Key Basic Research and Development Program (973) under Grant Nos. 2011CB707702, 2012CB518501, the National Natural Science Foundation of China under Grant Nos. 30930112, 30970774, 60901064, 30873462, 81000640, 81000641, 81071217, 81101036, 81101108, 31150110171, 30901900, 81271644, 31200837, 61179019, the Fundamental Research Funds for the Central Universities, the Natural Science Foundation of Inner Mongolia under Grant Nos. 2012MS0908 and the Innovation Fund Project of Inner Mongolia University of Science and Technology Nos. 2010NC030, 2010NC037.
References
- 1. Schwedt TJ, Dodick DW (2009) Advanced neuroimaging of migraine. The Lancet Neurology 8: 560–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiapparini L, Ferraro S, Grazzi L, Bussone G (2010) Neuroimaging in chronic migraine. Neurological Sciences 31: 19–22. [DOI] [PubMed] [Google Scholar]
- 3. Afridi SK, Giffin NJ, Kaube H, Friston KJ, Ward NS, et al. (2005) A positron emission tomographic study in spontaneous migraine. Archives of neurology 62: 1270–1275. [DOI] [PubMed] [Google Scholar]
- 4. Afridi S, Matharu M, Lee L, Kaube H, Friston K, et al. (2005) A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain 128: 932–939. [DOI] [PubMed] [Google Scholar]
- 5. Weiller C, May A, Limmroth V, Jüptner M, Kaube H, et al. (1995) Brain stem activation in spontaneous human migraine attacks. Nature Medicine 1: 658–660. [DOI] [PubMed] [Google Scholar]
- 6. Bahra A, Matharu M, Buchel C, Frackowiak R, Goadsby P (2001) Brainstem activation specific to migraine headache. The lancet 357: 1016–1017. [DOI] [PubMed] [Google Scholar]
- 7. Schmitz N, Admiraal-Behloul F, Arkink EB, Kruit MC, Schoonman GG, et al. (2008) Attack frequency and disease duration as indicators for brain damage in migraine. Headache: The Journal of Head and Face Pain 48: 1044–1055. [DOI] [PubMed] [Google Scholar]
- 8. Valfr W, Rainero I, Bergui M, Pinessi L (2008) Voxel-Based Morphometry Reveals Gray Matter Abnormalities in Migraine. Headache: The Journal of Head and Face Pain 48: 109–117. [DOI] [PubMed] [Google Scholar]
- 9. Rocca MA, Ceccarelli A, Falini A, Colombo B, Tortorella P, et al. (2006) Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 37: 1765–1770. [DOI] [PubMed] [Google Scholar]
- 10. Rocca M, Ceccarelli A, Falini A, Tortorella P, Colombo B, et al. (2006) Diffusion tensor magnetic resonance imaging at 3.0 tesla shows subtle cerebral grey matter abnormalities in patients with migraine. Journal of Neurology, Neurosurgery & Psychiatry 77: 686–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J, Suh S, Seol H, Oh K, Seo W, et al. (2008) Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 28: 598–604. [DOI] [PubMed] [Google Scholar]
- 12. Moulton E, Becerra L, Maleki N, Pendse G, Tully S, et al. (2011) Painful Heat Reveals Hyperexcitability of the Temporal Pole in Interictal and Ictal Migraine States. Cerebral Cortex 21: 435–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eck J, Richter M, Straube T, Miltner WHR, Weiss T (2011) Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain 152: 1104–1113. [DOI] [PubMed] [Google Scholar]
- 14.Yu D, Yuan K, Zhao L, Dong M, Liu P, et al. (2011) Regional homogeneity abnormalities in patients with interictal migraine without aura: a resting state study. NMR in Biomedicine: DOI: 10.1002/nbm.1796. [DOI] [PubMed]
- 15. May A (2009) New insights into headache: an update on functional and structural imaging findings. Nature Reviews Neurology 5: 199–209. [DOI] [PubMed] [Google Scholar]
- 16. May A (2009) Morphing voxels: the hype around structural imaging of headache patients. Brain 132: 1419–1425. [DOI] [PubMed] [Google Scholar]
- 17. Li XL, Fang YN, Gao QC, Lin EJ, Hu SH, et al. (2010) A Diffusion Tensor Magnetic Resonance Imaging Study of Corpus Callosum From Adult Patients With Migraine Complicated With Depressive/Anxious Disorder. Headache: The Journal of Head and Face Pain 51: 237–245. [DOI] [PubMed] [Google Scholar]
- 18. Caminiti R, Ghaziri H, Galuske R, Hof PR, Innocenti GM (2009) Evolution amplified processing with temporally dispersed slow neuronal connectivity in primates. Proceedings of the National Academy of Sciences 106: 19551–19556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hofer S, Frahm J (2006) Topography of the human corpus callosum revisited–comprehensive fiber tractography using diffusion tensor magnetic resonance imaging. Neuroimage 32: 989–994. [DOI] [PubMed] [Google Scholar]
- 20. Gazzaniga MS (2000) Cerebral specialization and interhemispheric communication. Brain 123: 1293–1326. [DOI] [PubMed] [Google Scholar]
- 21. Hoptman MJ, Davidson RJ (1994) How and why do the two cerebral hemispheres interact? Psychological bulletin 116: 195–219. [DOI] [PubMed] [Google Scholar]
- 22. Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews Neuroscience 8: 700–711. [DOI] [PubMed] [Google Scholar]
- 23. Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD (2009) Neurodegenerative diseases target large-scale human brain networks. Neuron 62: 42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, et al. (2009) Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences 106: 13040–13045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Toro R, Fox PT, Paus T (2008) Functional coactivation map of the human brain. Cerebral Cortex 18: 2553–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vincent J, Patel G, Fox M, Snyder AZ, Baker J, et al. (2007) Intrinsic functional architecture in the anaesthetized monkey brain. NATURE-LONDON- 447: 83–86. [DOI] [PubMed] [Google Scholar]
- 27. Roy AK, Shehzad Z, Margulies DS, Kelly A, Uddin LQ, et al. (2009) Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45: 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Di Martino A, Scheres A, Margulies D, Kelly A, Uddin L, et al. (2008) Functional connectivity of human striatum: a resting state FMRI study. Cerebral Cortex 18: 2735–2747. [DOI] [PubMed] [Google Scholar]
- 29. Salvador R, Suckling J, Schwarzbauer C, Bullmore E (2005) Undirected graphs of frequency-dependent functional connectivity in whole brain networks. Philosophical Transactions of the Royal Society B: Biological Sciences 360: 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stark DE, Margulies DS, Shehzad ZE, Reiss P, Kelly A, et al. (2008) Regional variation in interhemispheric coordination of intrinsic hemodynamic fluctuations. The Journal of neuroscience 28: 13754–13764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zuo XN, Kelly C, Di Martino A, Mennes M, Margulies DS, et al. (2010) Growing together and growing apart: regional and sex differences in the lifespan developmental trajectories of functional homotopy. The Journal of neuroscience 30: 15034–15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Anderson JS, Druzgal TJ, Froehlich A, DuBray MB, Lange N, et al. (2011) Decreased interhemispheric functional connectivity in autism. Cerebral Cortex 21: 1134–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, et al. (2011) Reduced Interhemispheric Resting State Functional Connectivity in Cocaine Addiction. Biological Psychiatry 69: 684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Olesen J (2004) The International classification of headache disorders, 2nd ed. Cephalalgia. 24: 9–160. [DOI] [PubMed] [Google Scholar]
- 35. Smith S (2002) Fast robust automated brain extraction. Human Brain Mapping 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Smith S, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols T, et al. (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31: 1487–1505. [DOI] [PubMed] [Google Scholar]
- 37. Smith S, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols T, et al. (2007) Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nature Protocols 2: 499–503. [DOI] [PubMed] [Google Scholar]
- 38.Andersson J, Jenkinson M, Smith S (2007) Non-linear optimisation. FMRIB Analysis Group Technical Reports: TR07JA02 from www.fmrib.ox.ac.uk/analysis/techrep.
- 39.Andersson J, Jenkinson M, Smith S (2007) Non-linear registration, aka Spatial normalisation. FMRIB Analysis Group Technical Reports: TR07JA02 from www.fmrib.ox.ac.uk/analysis/techrep.
- 40. Rueckert D, Sonoda L, Hayes C, Hill D, Leach M, et al. (2002) Nonrigid registration using free-form deformations: application to breast MR images. IEEE Transactions on Medical Imaging 18: 712–721. [DOI] [PubMed] [Google Scholar]
- 41. Nichols T, Holmes A (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Human Brain Mapping 15: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage 44: 83–98. [DOI] [PubMed] [Google Scholar]
- 43. Raichle ME (2010) Two views of brain function. Trends in cognitive sciences 14: 180–190. [DOI] [PubMed] [Google Scholar]
- 44. Mennes M, Kelly C, Zuo XN, Di Martino A, Biswal BB, et al. (2010) Inter-individual differences in resting-state functional connectivity predict task-induced BOLD activity. Neuroimage 50: 1690–1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park DC, Reuter-Lorenz P (2009) The adaptive brain: aging and neurocognitive scaffolding. Annual review of psychology 60: 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Li Z, Moore AB, Tyner C, Hu X (2009) Asymmetric connectivity reduction and its relationship to “HAROLD” in aging brain. Brain research 1295: 149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Peyron R, Laurent B, Garcia-Larrea L (2000) Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiologie Clinique/Clinical Neurophysiology 30: 263–288. [DOI] [PubMed] [Google Scholar]
- 48. Drabant EM, Kuo JR, Ramel W, Blechert J, Edge MD, et al. (2011) Experiential, autonomic, and neural responses during threat anticipation vary as a function of threat intensity and neuroticism. Neuroimage 55: 401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55: 377–391. [DOI] [PubMed] [Google Scholar]
- 50. Vogt BA (2005) Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews Neuroscience 6: 533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, et al. (2011) The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience 12: 154–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Petrovic P, Kalso E, Petersson KM, Ingvar M (2002) Placebo and opioid analgesia–imaging a shared neuronal network. Science 295: 1737–1740. [DOI] [PubMed] [Google Scholar]
- 53. Wager TD, Rilling JK, Smith EE, Sokolik A, Casey KL, et al. (2004) Placebo-induced changes in FMRI in the anticipation and experience of pain. Science 303: 1162–1167. [DOI] [PubMed] [Google Scholar]
- 54. Peyron R, Garcia-Larrea L, Gregoire MC, Costes N, Convers P, et al. (1999) Haemodynamic brain responses to acute pain in humans. Brain 122: 1765–1780. [DOI] [PubMed] [Google Scholar]
- 55. Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience 6: 691–702. [DOI] [PubMed] [Google Scholar]
- 56. Vincent M, Pedra E, Mourão-Miranda J, Bramati I, Henrique A, et al. (2003) Enhanced interictal responsiveness of the migraineous visual cortex to incongruent bar stimulation: a functional MRI visual activation study. Cephalalgia 23: 860–868. [DOI] [PubMed] [Google Scholar]
- 57. Dalgleish T (2004) The emotional brain. Nature Reviews Neuroscience 5: 583–589. [DOI] [PubMed] [Google Scholar]
- 58. Kringelbach ML, Rolls ET (2004) The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in neurobiology 72: 341–372. [DOI] [PubMed] [Google Scholar]
- 59. Leknes S, Tracey I (2008) A common neurobiology for pain and pleasure. Nature Reviews Neuroscience 9: 314–320. [DOI] [PubMed] [Google Scholar]
- 60. May A (2006) A review of diagnostic and functional imaging in headache. The journal of headache and pain 7: 174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Greicius MD, Supekar K, Menon V, Dougherty RF (2009) Resting-state functional connectivity reflects structural connectivity in the default mode network. Cerebral Cortex 19: 72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. van den Heuvel M, Mandl R, Luigjes J, Pol HH (2008) Microstructural organization of the cingulum tract and the level of default mode functional connectivity. The Journal of neuroscience 28: 10844–10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Van Den Heuvel MP, Mandl RCW, Kahn RS, Hulshoff Pol HE (2009) Functionally linked resting state networks reflect the underlying structural connectivity architecture of the human brain. Human brain mapping 30: 3127–3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Schmidt-Wilcke T, Leinisch E, Gänssbauer S, Draganski B, Bogdahn U, et al. (2006) Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain 125: 89–97. [DOI] [PubMed] [Google Scholar]
- 65. Draganski B, Moser T, Lummel N, Ganssbauer S, Bogdahn U, et al. (2006) Decrease of thalamic gray matter following limb amputation. Neuroimage 31: 951–957. [DOI] [PubMed] [Google Scholar]