Abstract
Purpose
The purpose of the present study was to examine the effects of cognitive-linguistic deficits and respiratory physiologic changes on respiratory support for speech in PD, using two speech tasks, reading and extemporaneous speech.
Methods
Five women with PD, 9 men with PD, and 14 age- and sex-matched control participants read a passage and spoke extemporaneously on a topic of their choice at comfortable loudness. Sound pressure level, syllables per breath group, speech rate, and lung volume parameters were measured. Number of formulation errors, disfluencies, and filled pauses were counted.
Results
Individuals with PD produced shorter utterances as compared to control participants. The relationships between utterance length and lung volume initiation and inspiratory duration were weaker in individuals with PD than for control participants, particularly for the extemporaneous speech task. These results suggest less consistent planning for utterance length by individuals with PD in extemporaneous speech. Individuals with PD produced more formulation errors in both tasks and significantly fewer filled pauses in extemporaneous speech.
Conclusions
Both respiratory physiologic and cognitive-linguistic issues affected speech production by individuals with PD. Overall, individuals with PD had difficulty planning or coordinating language formulation and respiratory support, in particular during extemporaneous speech.
INTRODUCTION
Speech production involves not only motor plans and movements of the respiratory, laryngeal, and supralaryngeal systems, but also language planning and cognitive resources. Individuals with Parkinson's disease (PD) are an important population to consider when examining the interaction between cognitive-linguistic factors and physiologic issues. PD results in deficits in the respiratory, laryngeal, and supralaryngeal subsystems during speech (Bunton, 2005; Forrest, Weismer, & Turner, 1989; Sadagopan & Huber, 2007; M. E. Smith, Ramig, Dromey, Perez, & Samandari, 1995; Solomon & Hixon, 1993). Individuals with PD also have cognitive deficits that affect language processing and production (Brown & Marsden, 1990; Hayes, Davidson, Keele, & Rafal, 1998; Oliveira, Gurd, Nixon, Marshall, & Passingham, 1997; Zgaljardic, Borod, Foldi, & Mattis, 2003; Zgaljardic et al., 2006). While most of the previous research on the interaction between motor control and cognitive-linguistic issues has focused on the articulatory system (Goffman, 2004; Kleinow & Smith, 2006; A. Smith, 2006; A. Smith & Goffman, 2004), the present study shows that the respiratory system can also provide a window to the link between these factors.
Respiratory Physiologic Issues
Respiratory support has been reported to be reduced in both non-speech and speech domains as a result of PD. Within the non-speech domain, studies of pulmonary function in individuals with PD have demonstrated disease-related reductions in forced vital capacity and forced expiratory volume in one second (De Pandis et al., 2002; Weiner et al., 2002). Within the speech domain, studies have reported a large number of changes to respiratory support associated with PD, including lower lung volume initiations and terminations, larger abdominal initiations, smaller rib cage volume initiations, larger rib cage excursions, and more variability of respiratory movements than typical older adults (Bunton, 2005; Huber, Stathopoulos, Ramig, & Lancaster, 2003; Lethlean, Chenery, & Murdoch, 1990; Murdoch, Chenery, Bowler, & Ingram, 1989; Sadagopan & Huber, 2007; Solomon & Hixon, 1993). However, respiratory kinematic findings related to PD have not been consistent across studies, and a large amount of variability across subjects has been reported (Bunton, 2005; Huber et al., 2003). For example, while one study of individuals with PD reported that they produced shorter utterances than age-matched control subjects in both reading and extemporaneous speech (Solomon & Hixon, 1993), Bunton (2005) reported a large amount of variability in utterance length across speakers with PD.
In general, these findings suggest that, for individuals with PD, respiratory support for speech may reflect stiffer respiratory muscles (resulting in lower lung volume initiations and terminations) or weaker expiratory muscles (resulting in shorter utterances and higher lung volume initiations and terminations). Importantly, much of the variability across studies may be due to different speech tasks. Data from young adults supports the conclusion that speech task matters when examining respiratory support. During extemporaneous speech, young adults produce longer utterances, larger lung volume excursions, and slower speech rate than during reading (Huber, 2007). Therefore, it is crucial that we take speech task differences into account in assessing the effects of PD on respiratory function for speech.
Cognitive-Linguistic Issues
Individuals with PD have been reported to have cognitive deficits in set switching, inhibition, memory, attention, and self-monitoring behavior (Brown & Marsden, 1990; Hayes et al., 1998; Oliveira et al., 1997; Zgaljardic et al., 2003; Zgaljardic et al., 2006). Previous studies have not focused on how these cognitive deficits affect speech production. However, studies of language processing and comprehension have suggested that individuals with PD may suffer from subtle changes to language skills. This is most likely a result of difficulties with executive function, integration, and inhibition rather than language-specific skill deficits (Bodis-Wollner & Jo, 2006; Grossman, Lee, Morris, Stern, & Hurtig, 2002; Longworth, Keenan, Barker, Marslen-Wilson, & Tyler, 2005). Individuals with PD have impairments in integrating information, as evidenced by difficulties in late-stage syntactic processing (Friederici, Kotz, Werheid, Hein, & von Cramon, 2003) and comprehension of complex sentences (Grossman et al., 2002). Previous research has suggested that while automatic processes related to sentence comprehension are intact, impairments in selective attention or resource limitations create problems for syntactic processing (Friederici et al., 2003; Grossman et al., 1993; Grossman et al., 2000; Grossman et al., 2002).
Although there have been fewer studies of language production in PD, the available evidence suggests that cognitive deficits also affect language production. In one study, individuals with PD produced more silent pauses than control participants during extemporaneous speech, and individuals with more severe PD produced language with lower syntactic complexity and more filled pauses (for example, “um” and “uh”) than individuals with less severe PD (Illes, Metter, Hanson, & Iritani, 1988).
Interaction between Respiratory Physiologic and Cognitive-Linguistic Issues
As noted above, previous studies of respiratory support during speech production in individuals with PD have not systematically examined the interaction between physiologic and cognitive-linguistic factors. Specifically, we used different speech tasks as a method of separating respiratory physiologic support from cognitive-linguistic issues. Because demands for online formulation of language are likely to be much higher in extemporaneous speech than in reading, the allocation of cognitive resources are likely to differ between reading and extemporaneous speech, resulting in differences in both language production and respiratory support during speech. This hypothesis has been supported by data from young adults. Discussing a topic without a prepared outline results in more pauses, fewer syllables per breath group, slower speech rate, and larger volume excursion compared to discussing a topic after preparing an outline (Mitchell, Hoit, & Watson, 1996).
Although such differences have not yet been shown for individuals with PD, we hypothesize that the differences in cognitive resource demands will, similarly, result in difficulty coordinating respiratory control with language formulation. Such difficulty may be quantified in terms of the relationship between utterance length and lung volume. Although traditionally shorter utterances and lower lung volumes during speech have been interpreted physiologically, they may also reflect difficulties with planning motor control with language formulation. For example, Bunton (2005) reported that for 3 of 7 individuals with PD, there was no consistent relationship between lung volume initiation and utterance length in an extemporaneous speech task. This contrasts with findings for healthy adults, for whom lung volumes are consistently higher at speech initiation and lower at speech termination for longer utterances (Bunton, 2005; Huber, 2008; Sperry & Klich, 1992; Winkworth, Davis, Ellis, & Adams, 1994). However, speech task is important for this index as well. The correlation between higher lung volume and longer utterances is weaker during extemporaneous speech than during reading for young adults (Huber, 2007; Winkworth, Davis, Adams, & Ellis, 1995; Winkworth et al., 1994). While Bunton's results are suggestive, it is hard to determine if this finding is task-dependent and to address whether this finding relates only to respiratory physiology or to a combination of respiratory and cognitive-linguistic issues since Bunton only examined extemporaneous speech. The present study directly addresses this issue using two different speech tasks. If individuals with PD cannot use higher lung volumes due to physiologic constraints, different speech tasks should not result in different relationships between utterance length and lung volume. However, if cognitive-linguistic mechanisms are involved, we would expect differences across speech tasks of varying cognitive load (Huber, 2007; Mitchell, Hoit, & Watson, 1996).
In addition to the relationship between utterance length and lung volume, inspiratory duration may also be a useful index for the influence of cognitive-linguistic factors on respiratory support for speech. Speakers plan upcoming language before the onset of an utterance, potentially during inspiration. Inspiratory duration may be increased when the cognitive-linguistic load is higher. Mitchell and colleagues (1996) reported differences in inspiratory duration between two conditions (speaking with an outline and without an outline) in young adults which nearly reached significance (p=0.02). Solomon and Hixon (1993) reported a higher mean inspiratory duration for both individuals with PD and control subjects in extemporaneous speech as compared with reading. Based on these previous findings, inspiratory duration was included as a preliminary measure in the current study.
In summary, the purpose of the present study was to examine the effects of cognitive-linguistic deficits and respiratory physiologic changes on respiratory support for speech in PD, using two speech tasks, reading and extemporaneous speech. We hypothesized that individuals with PD would demonstrate greater difficulty in the extemporaneous speech task. We expected individuals with PD to have slower speech rate, less preplanning for utterance length, longer inspiratory duration, and more formulation errors and filled pauses during extemporaneous speech than age-matched control participants.
METHODS
Participants
Five women with PD, 9 men with PD, and 14 age- and sex-matched control participants served as subjects. The mean ages of the participants were as follows: women with PD: 69 years, age-matched women: 71 years, men with PD: 76 years, and age-matched men: 72 years.
Information about time since diagnosis, medications, and overall speech severity for individuals with PD is presented in Table 1. Speech severity ratings were made independently by three certified speech-language pathologists who were experienced in assessment and treatment of individuals with motor speech disorders. Overall, the patients’ speech severity ranged from mild to moderate, with hypophonia and imprecise articulation most commonly rated as impaired. Patients were Hoehen & Yahr stage II-III. Two of the participants with PD had received speech therapy as a result of their PD symptoms previously (see Table 1), but neither indicated they had received LSVT LOUD® therapy. No patients were undergoing therapy at the time of the study. All patients were seen within 1-3 hours of taking their PD-related medications.
Table 1.
Detailed Information about Participants with Parkinson's disease
| Participant | Age (years) | Time since diagnosis (years) | History of speech treatment | Drugs | Perceptual speech severity |
|---|---|---|---|---|---|
| F01PD | 73 | 1 | No | Mirapex | Mild |
| F02PD | 70 | 9 | No | Eldepryl, Wellbutrin, Zoloft | Normal- Mild |
| F03PD | 51 | 2 | No | Comtan, Elevail, Sinemet | Normal- Mild |
| F04PD | 74 | 5 | No | Bromocriptine, Eldepryl, Sinemet | Normal |
| F05PD | 80 | 6 | No | Eldepryl, Requip, Stalevo | Mild |
| M01PD | 83 | 5 | No | None | Moderate |
| M02PD | 76 | 5 | No | Comtan, Requip, Sinemet | Moderate |
| M03PD | 69 | 3-4 | No | Permax, Stalevo | Normal- Mild |
| M04PD | 90 | 3 | No | Amantadine, Carbidopa | Moderate |
| M05PD | 70 | 4 | No | Stalevo Sinemet Mirapex | Normal- Mild |
| M06PD | 75 | 3 | Yes – increase loudness using SPL meter for feedback | Carbidopa, Comtan, Permax | Moderate |
| M07PD | 73 | 10 | Yes – 2 years of group therapy | Lipitor, Metoprolol, Prozac, Sinemet | Mild |
| M08PD | 70 | 4 | No | Sinemet | Mild |
| M09PD | 82 | 4 | No | Amantadine, Flomax, Sinemet | Mild |
At the time of the experiment, all participants reported being free from colds, infections, and allergy symptoms, having been non-smoking for at least the past five years, no history of respiratory problems, no history of neurological disease (except PD), no head or neck cancer or surgery, and no formal training in singing or speaking. All participants were required to be living independently in the community and ambulatory and had to pass the Mini-Mental State Exam (MMSE) (Folstein, Folstein, & McHugh, 1975). Control participants demonstrated normal hearing (Ventry & Weinstein, 1983), were determined by the first author to have normal speech, language, and voice, and demonstrated normal lung function by producing vital capacity (VC), forced vital capacity (FVC), and forced expiratory volume in one second (FEV1.0) at greater than or equal to 80% of expected values based on age, sex, height, weight, and ethnicity (VacuMed Discovery Handheld Spirometer). Individuals with PD had lower VC, FVC, and FEV1.0, on average, than the control participants. However, normal lung function was not a requirement for inclusion for patients with PD because changes to lung spirometry are a documented effect of PD (De Pandis et al., 2002; Weiner et al., 2002).
Equipment
The acoustic signal was transduced by a microphone (model: Bruel & Kjaer (B&K), type 4936), held at a constant 6-inch mouth-to-microphone distance. The microphone signal was recorded to a digital audiotape (DAT) (model: Tascam DA-P1) and later digitized using a computer software program, Praat (Boersma & Weenink, 2003). A sound level meter, coupled to the microphone, amplified the microphone signal during the study. The gain provided by the sound level meter to the microphone signal varied depending on the vocal intensity of the participants, and was factored in when calibrating the acoustic signal.
Respiratory kinematic data were transduced using respiratory inductive plethysmography (RIP) via the Respitrace (Ambulatory Monitoring, Inc.). An elastic band placed around the rib cage (RC), just under the axilla, transduced RC movement. A second band placed around the abdomen (AB), below the last rib at the level of the participant's belly button, transduced AB movement. Signals from the Respitrace bands were digitized through the analog-to-digital converter in the Optotrak system (Northern Digital Inc.). A second microphone picked up sound from the experimental chamber and was digitized simultaneously with the Respitrace signal.
Procedures and Speech Stimuli
Respiratory Calibrations
Based on the two-compartment model of the respiratory system, the sum of the volume changes of the RC and AB compartments can be used to estimate lung volume (LV) (Konno & Mead, 1967). Volume changes of the RC and AB are estimated from dimensional measurements provided by RIP. Correction factors are needed to “weight” the relative contributions of the RC and AB to provide accurate estimates of LV. This is due to the fact that the RC contributes relatively more (and the AB relatively less) to LV change for equivalent dimensional changes of the respiratory system. To obtain data from which correction factors can be calculated, RIP signals from the RC and AB were recorded simultaneously with LV, provided by a digital spirometer (Vacumed Universal Ventilation Meter (UVM)), during two calibration tasks. The two calibration tasks were rest breathing (one and a half minutes of quiet breathing) and a covert speech task (one and a half minutes reading “You buy Bobby a puppy now if he wants one” silently to themselves). The covert speech task was used because normal speech articulation was not possible given that subjects were breathing into the spirometer's mouth piece. Published literature and our previous experience tells us that this covert task results in similar respiratory kinematics and lung volume changes as overt speech (Reich & McHenry, 1990) and thus is appropriate to the current experimental objectives.
To determine the correction factors for the RC and AB signals to estimate LV, the sum of RC and AB movement signals was compared to the known volume from the digital spirometer (SP). A least squares analysis (Moore-Penrose pseudoinverse function) was calculated offline in a custom-written Matlab code to determine the best calibration factors. These calibration factors were calculated across all of the samples from both calibration tasks. Thus, the combined RC and AB signal approached the SP signal with the least error optimized for all the contributing data points (Huber, 2007, 2008; Huber, Chandrasekaran, & Wolstencroft, 2005). During the speech tasks, estimated LV at any given sample point was computed by summing RC and AB volumes after the correction factors were applied.
Participants also performed at least three trials of the VC task, with the Respitrace bands in place to estimate maximum VC for each participant. LV was estimated during the VC task using the correction factors. Speech measurements were converted to percent VC based on this task by dividing the LV by the VC and multiplying by 100.
Speech Tasks
Two speech tasks were completed by each participant, a reading task and an extemporaneous speech task. These tasks were part of a larger protocol with several different loudness conditions (Sadagopan & Huber, 2007). Two productions of the reading passage were collected at four loudness conditions, comfortable first. The “Papa passage” (Sapienza & Stathopoulos, 1995) was displayed on a computer screen in front of the participants. After the last reading passage condition, comfortable loudness was re-established. Then the participants were asked to talk about a topic of their choice for two minutes (extemporaneous speech task) at two loudness conditions, comfortable first (Huber, 2007). Only the data from the comfortable loudness conditions are presented in this paper. Data from the reading passage for the participants in this study and for the control participants during the extemporaneous speech task have been published previously (Huber, 2008; Sadagopan & Huber, 2007). However, the analyses of utterance length in the current paper were not conducted previously for any of the data from the individuals with PD.
For both tasks, participants were instructed to be sure they were clear and audible to an experimenter sitting about four feet away. The participants could not see the experimenters without turning their head since the researchers were to the side of the participants, at about a 90 degree angle, and participants were instructed to keep their head and trunk facing straight ahead. Experimenters did not interact with the participants in any way during the reading task. During the extemporaneous speech task, experimenters had very little, if any, interaction with participants. Experimenters prompted the participants to continue speaking or change topics if two minutes had not elapsed. However, this was the extent of the interaction between experimenters and participants during the extemporaneous speech task.
Measurements
The extemporaneous speech task was transcribed word for word. Transcriptions were checked by a second transciber to ensure accuracy. The reading passages were checked by two transcribers, and any deviations from the known text were noted on the transcription. Where discrepancies existed between the two transcribers, the transcribers came to consensus together on what was said. The number of disfluencies (repetitions of sounds, syllables, or single words), number of filled pauses (“um” “uh” etc.), and the number of formulation errors (repeated phrases, revised utterances, and abandoned utterances) were counted for each task and each trial. These have been shown to increase with the cognitive demands of a speaking task in young adults (Lay & Paivio, 1969).
A breath group was defined as all of the syllables produced on one breath. The average SPL, in decibels (dB), for each breath group was measured using Praat (Boersma & Weenink, 2003). To obtain the utterance length, the number of syllables produced on each breath group was counted. The duration of each breath group was measured in seconds. The speech rate was calculated by dividing the number of syllables by the duration for each breath group.
Respiratory kinematic measurements were made from each breath group using custom-written algorithms in Matlab (Mathworks, Inc.). The respiratory signals were displayed along with the time-locked acoustic signal. Lung volume initiation was measured at the point where voicing started, based on the acoustic signal. Lung volume termination was measured at the point that voicing ended, based on the acoustic signal. Accuracy of selection of speech initiation and termination points was verified by listening to the audio signal. Initiations and terminations were expressed as a percentage of VC (%VC) and relative to the end expiratory level (EEL). EEL was defined as the volume at the end of a tidal expiration and was computed as an average of at least three steady troughs of rest breathing preceding the production of each speech task trial. Lung volume excursion, expressed as a %VC, was calculated by subtracting the volume at termination from the volume at initiation. The duration of inspiration before each breath was measured in seconds. The %VC expended per syllable was calculated by dividing lung volume excursion by the number of syllables in a breath group.
Statistical Analysis
A general linear mixed model was used to analyze all data. Factors were group (PD vs. control) and task (reading vs. extemporaneous speech). Utterance length (in syllables) was modeled as a covariate. Interactions between group, task, and utterance length were examined in the model as well. Reported means are the means adjusted for utterance length. For analyses of utterance length and the linguistic measures (number of filled pauses, number of disfluencies, and number of formulation errors), the same analysis was used; however, utterance length was not a covariate. Summary statistics for the general linear model are presented in Appendix 1.
For significant categorical factors (group, task, and group by task), tukey HSD post-hoc tests were completed to determine statistically significant pairwise comparisons. When the covariate, utterance length, was significant, means were computed for the dependent variable for each utterance length (in syllables), so there was one data point for each length value. Linear regressions were completed to assess the nature of the relationship between utterance length and the dependent variables. If there was a significant interaction between utterance length and group and/or task, indicating that the slopes of the lines differed across the groups or tasks, the means for each length and the regressions were completed for each level of the categorical variable. The level of significance was set as p < 0.05 for all statistical tests.
To establish inter-measurer reliability, two men and two women from each group (PD and control) were randomly chosen for remeasurement. ANOVAs and Pearson Correlations were used to examine differences across the two sets of data for the measured (not computed) variables. Mean and median differences across the two measurement sets were small, indicating good measurement reliability (see Appendix 2). The largest differences across the two measurements occurred when an utterance boundary was chosen differently by the two measurers. For the control participants, 6 out of 422 utterance boundaries were chosen differently across the two measurements. For the participants with PD, 5 out of 400 utterance boundaries were chosen differently across the two measurements.
No reliability measures were completed on the transcriptions since they were completed by two transcribers and differences in transcriptions were resolved by consensus agreement between the two transcribers. No reliability was assessed for number of filled pauses, disfluencies, and formulation errors in extemporaneous speech since these analyses were completed by the two authors for all subjects, and consensus agreement was used when differences in interpretation occurred.
RESULTS
Utterance Length
There were main effects of group and task, but no interaction. Relative to the group effect, control participants produced significantly longer utterances (M=13.2 syllables, SE=0.27) than individuals with PD (M=12.4 syllables, SE=0.28). Relative to the task effect, utterances were significantly longer in extemporaneous speech (M=13.6 syllables, SE=0.28) than in reading (M=12.0 syllables, SE=0.26).
Sound Pressure Level (SPL)
There were significant main effects of group, task, and utterance length and significant group by task and task by utterance length interaction effects. Relative to the group by task interaction, individuals with PD had significantly lower SPL in reading than in extemporaneous speech and significantly lower SPL than control participants in reading (see Table 2). There was no significant difference in SPL between tasks for control participants, and no difference between the groups in extemporaneous speech.
Table 2.
Means and standard errors (in parentheses) for significant group by task interaction effects.
| Variable | Task | Controls | PD |
|---|---|---|---|
| Sound Pressure Level (dB SPL) | Reading | 77.4 (0.20) | 75.6 (0.21) |
| Extemp. | 78.0 (0.22) | 77.6 (0.24) | |
| Speech Rate (syll/sec) | Reading | 4.6 (0.05) | 4.7 (0.05) |
| Extemp. | 3.9 (0.05) | 4.3 (0.05) | |
| Inspiratory Duration (seconds) | Reading | 0.53 (0.016) | 0.58 (0.016) |
| Extemp. | 0.56 (0.017) | 0.75 (0.018) | |
| Number of Filled Pauses | Reading | 0.04 (0.94) | 0.04 (0.97) |
| Extemp. | 11.36 (1.33) | 4.08 (1.38) |
Extemp. = extemporaneous speech task; dB = decibels; syll/sec = syllables per second; PD = individuals with Parkinson's disease
The slopes of the regression lines between SPL and utterance length differed according to task (see Figure 1). For reading, SPL was significantly negatively correlated with utterance length (r= -0.69; R2=0.14), but there was no significant relationship in extemporaneous speech (r= -0.21; R2=0.04).
Figure 1.
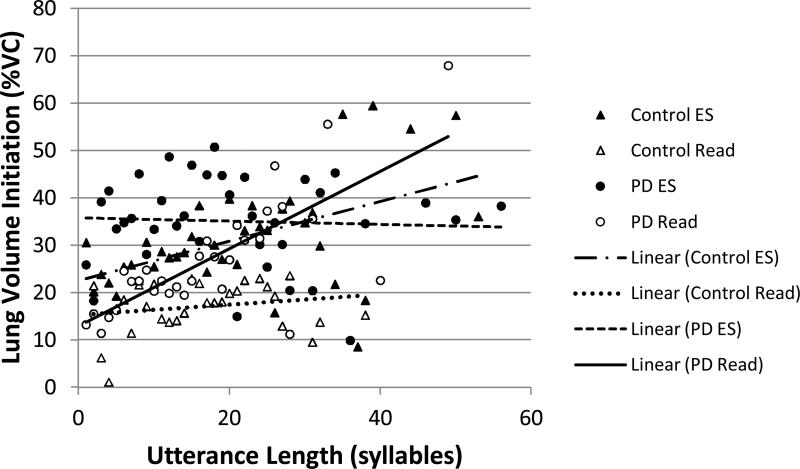
Lung volume initiation by utterance length (in syllables): Open symbols represent the means at each utterance length for reading (Read). Filled symbols represent the means at each utterance length for extemporaneous speech (ES). Triangles represent data from control subjects. Circles represent data from individuals with PD. Lines demonstrate linear relationships between the two variables for each group and task.
Speech Rate
There were significant main effects of group, task, and utterance length and a significant group by task interaction effect. Both control participants and individuals with PD spoke significantly slower in extemporaneous speech than in reading (see Table 2). Individuals with PD spoke significantly more quickly than control participants in extemporaneous speech, but there was no difference across groups in reading. Speech rate was significantly positively correlated with utterance length (r=51; R2=0.26).
Lung Volume Initiation
There were significant main effects of group, task, and utterance length and significant task by utterance length and group by task by utterance length interaction effects. Relative to the group by task interaction, individuals with PD (M=30.2 %VC, SE=0.67) used significantly higher lung volume initiations than control participants (M=22.6 %VC, SE=0.64). For both groups, lung volume initiation was significantly higher during extemporaneous speech (M=32.1 %VC, SE=0.69) than reading (M=20.7, SE=0.62).
The slopes of the regression lines between lung volume initiation and utterance length differed according to group and task (see Figure 2). Lung volume initiation was significantly positively correlated with utterance length for individuals with PD in reading (r=0.75; R2=0.56) and control participants in extemporaneous speech (r=0.50; R2=0.25). There was no significant relationship between these variables for individuals with PD in extemporaneous speech (r= -0.05; R2=0.002) or control participants in reading (r=0.20; R2=0.04).
Figure 2.
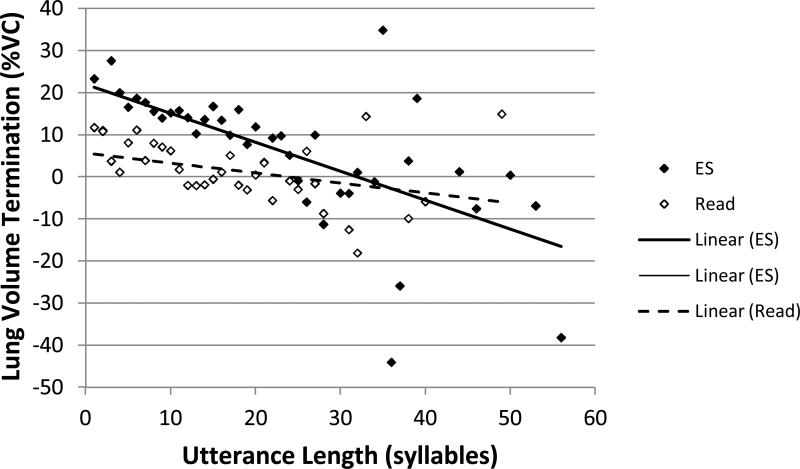
Lung volume termination by utterance length (in syllables): Open symbols represent the means at each utterance length for reading (Read). Filled symbols represent the means at each utterance length for extemporaneous speech (ES). Lines demonstrate linear relationships between the two variables for each task.
Lung Volume Termination
There were significant main effects of group, task, and utterance length and a significant task by utterance length interaction effect. Relative to the group effect, individuals with PD (M=9.5 %VC, SE=0.62) had significantly higher lung volume terminations than control participants (M=7.0 %VC, SE=0.60). Relative to the task effect, lung volume termination was significantly higher for extemporaneous speech (M=13.5 %VC, SE=0.64) than reading (M=3.1, SE=0.58).
The slopes of the regression lines between lung volume termination and utterance length differed according to task (see Figure 3). In extemporaneous speech, lung volume termination was significantly negatively correlated with utterance length (r= -0.65; R2=0.42), but the relationship was not significant in reading (r= -0.37; R2=0.14).
Figure 3.
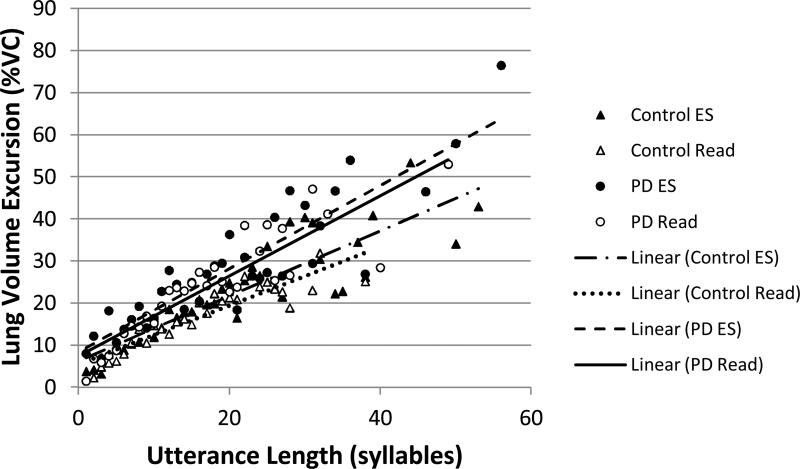
Lung volume excursion by utterance length (in syllables): Open symbols represent the means at each utterance length for reading (Read). Filled symbols represent the means at each utterance length for extemporaneous speech (ES). Triangles represent data from control subjects. Circles represent data from individuals with PD. Lines demonstrate linear relationships between the two variables for each group and task.
Lung Volume Excursion
There were significant main effects of group and utterance length and a significant group by task by utterance length interaction effect. Relative to the group effect, individuals with PD (M=20.9 %VC, SE=0.37) had significantly larger lung volume excursions than control participants (M=15.7 %VC, SE=0.35).
The slopes of the regression lines between lung volume excursion and utterance length differed according to group and task (see Figure 4). In all cases, lung volume excursion was significantly positively correlated with utterance length. The relationship was approximately the same strength for all groups and tasks, individuals with PD (reading: r=0.80 R2=0.89; extemporaneous speech: r=0.81; R2=0.90) and control participants (reading: r=0.83; R2=0.91; extemporaneous speech: r=0.77; R2=0.88).
Figure 4.
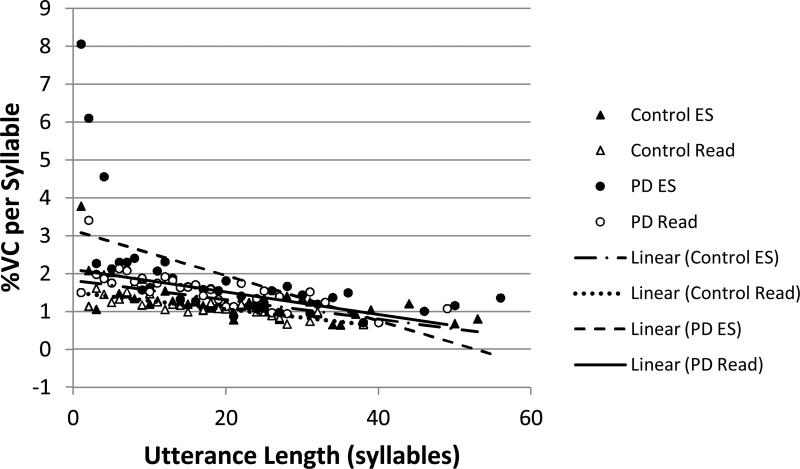
Percent vital capacity (%VC) expended per syllable by utterance length (in syllables): Open symbols represent the means at each utterance length for reading (Read). Filled symbols represent the means at each utterance length for extemporaneous speech (ES). Triangles represent data from control subjects. Circles represent data from individuals with PD. Lines demonstrate linear relationships between the two variables for each group and task.
Inspiratory Duration
There were significant main effects of group, task, and utterance length, and a significant group by task interaction effect. Individuals with PD had a significantly longer inspiratory duration in extemporaneous speech than control participants, but there was no difference between groups in the reading task (see Table 2). Individuals with PD had a significantly longer inspiratory duration in extemporaneous speech than in reading, but there was no significant difference in inspiratory duration for control participants between speech tasks. Inspiratory duration was positively, but not significantly, related to utterance length (r=0.23; R2=0.05).
Percent Vital Capacity Expended per Syllable (%VC/syll)
There were significant main effects of group, task, and utterance length, and significant task by utterance length, group by utterance length, and group by task by utterance length interaction effects. Relative to the group effect, individuals with PD (M=1.94 %VC, SE=0.074) had significantly higher %VC/syll than control participants (M=1.37 %VC, SE=0.045). Relative to the task effect, %VC/syll was significantly higher for extemporaneous speech (M=1.81 %VC, SE=0.048) than reading (M=1.50, SE=0.044).
The slopes of the regression lines between %VC/syll and utterance length differed according to group and task (see Figure 5). In all cases, %VC/syll was significantly negatively correlated with utterance length. The relationship was weakest for individuals with PD in extemporaneous speech (r= -0.56; R2=0.32). The relationship was approximately the same for individuals with PD in the reading task (r= -0.71; R2=0.50) and control participants in both tasks (reading: r= -0.82; R2=0.67; extemporaneous speech: r= -0.63; R2=0.40).
Number of Formulation Errors
There were significant main effects of task and group, but no significant interaction effect. Relative to the group effect, individuals with PD (M=3.4, SE=0.42) produced more formulation errors than control participants (M=2.1, SE=0.40). Relative to the task effect, there were significantly more formulation errors in extemporaneous speech (M=5.0, SE=0.47) than in reading (M=0.53, SE=0.34).
Number of Filled Pauses
There were significant main effects of task and group and a significant group by task interaction effect. Individuals with PD produced significantly fewer filled pauses in extemporaneous speech than the control participants, but there was no significant difference between the two groups during reading. Both groups produced significantly more filled pauses in extemporaneous speech than in reading (see Table 2). Only two subjects (one control and one individual with PD) produced a filled pause (one each) during reading.
Number of Disfluencies
There was a significant main effect of task, but no significant main effect of group and no significant interaction effect. There were significantly more disfluencies in extemporaneous speech (M=2.5, SE=0.26) than in reading (M=0.4, SE=0.18). There were no significant differences between the two groups, control participants (M=1.5, SE=0.22) and individuals with PD (M=1.4, SE=0.23).
DISCUSSION
The purpose of the present study was to examine the effects of cognitive-linguistic deficits and respiratory physiologic changes on respiratory support for speech in PD, using two speech tasks, reading and extemporaneous speech. Results suggest that both respiratory physiologic and cognitive-linguistic impairments affect respiratory support for speech in individuals with PD. Our results strongly supported the hypothesis that individuals with PD would have greater difficulty coordinating language formulation with respiratory support for speech as compared with age-matched control participants, particularly in the extemporaneous speech task.
Task-related differences in respiratory support for speech were found for both individuals with PD and control participants, although the relationships among task, utterance length, and lung volume were not the same for both groups. Both groups used higher lung volume initiations and terminations in extemporaneous speech than in reading. This difference across speech tasks was expected since participants from both groups used longer utterances in extemporaneous speech than in reading (see Figure 1). Both groups demonstrated a strong relationship between lung volume termination and utterance length for both extemporaneous speech and reading. As expected, weaker relationships between lung volume initiation and utterance length were observed during extemporaneous speech (but not during reading) for individuals with PD. This indicates that rather than breathing to a higher lung volume to support longer utterances, as control participants did, individuals with PD used lower lung volume terminations. Also of interest is the finding that individuals with PD showed the weakest relationship between %VC expended per syllable and utterance length in the extemporaneous speech task This finding suggests that individuals with PD were not able to control %VC expended per syllable relative to utterance length as well as control participants did. Taken together, these findings demonstrate that there was less consistent planning for utterance length by the individuals with PD in extemporaneous speech. Because the same difficulties were not found for reading, these data provide strong evidence that cognitive-linguistic demands account for some disease-related changes in respiratory support for speech.
The inspiratory duration results corroborate the hypothesis that individuals with PD had difficulty planning in extemporaneous speech. Similar to findings from Solomon and Hixon (1993), individuals with PD took longer to inspire before utterances than the control participants in extemporaneous speech, but not reading. One explanation for this finding could be that the individuals with PD increased lung volume initiations in extemporaneous speech, leading to a longer inspiratory duration. Both individuals with PD and control subjects increased lung volume initiation in the extemporaneous speech task, as compared to reading, but only the individuals with PD showed a significant effect on inspiratory duration. If this was just a product of the volume inspired, we would have expected to see a significant difference in inspiratory duration across tasks for the control subjects as well. Another explanation could be that individuals with PD increased SPL in the extemporaneous speech task, leading to increased inspiratory duration. However, it is unlikely that a 2 dB increase in SPL, could account for a 120 ms increase in inspiratory duration. Further, SPL did not differ between individuals with PD and control subjects in extemporaneous speech. If increased inspiratory duration was an effect of SPL, inspiratory duration should be larger for the control subjects too.
Increased inspiratory duration is likely to be related to cognitive-linguistic factors, specifically formulation difficulties. Individuals with PD inspired more slowly, perhaps allowing for more time for language formulation in extemporaneous speech. Since language was not controlled in the extemporaneous speech task, it is difficult to discern the exact nature of the difficulty. However, the difference between reading and extemporaneous speech strongly suggests that cognitive-linguistic mechanisms are involved, as opposed to a strictly physiologic explanation. Further support for subtle changes in cognitive-linguistic mechanisms in individuals with PD comes from the linguistic data.
It is clear that language formulation demands were higher in extemporaneous speech for both individuals with PD and older adults. Both groups produced more formulations errors, filled pauses, and disfluencies in extemporaneous speech than in reading. These data agree with Mitchell and colleagues (1996) who reported more pauses when young adults discussed a topic without an outline, as compared to discussing a topic after preparing an outline. Earlier literature on task difficulty reported revisions, repetitions, and filled pauses increased as language complexity increased, although the findings for filled pauses were the most consistent (Lay & Paivio, 1969; Reynolds & Paivio, 1968). In addition, both groups produced shorter utterances and slower speech rate during extemporaneous speech than during reading, consistent with previous research (Hoit & Hixon, 1987; Hoit, Hixon, Altman, & Morgan, 1989; Mitchell et al., 1996). In general, the current findings suggest that language formulation demands are greater in extemporaneous speech than in reading for both typical older adults and individuals with PD. However, individuals with PD demonstrated greater effects of the language formulation demands than the control participants.
Individuals with PD produced more formulation errors than the control participants in both tasks suggesting greater difficulty in integrating, producing, and formulating language. Individuals with PD, like the control participants, produced almost no filled pauses in reading. In extemporaneous speech, individuals with PD produced significantly fewer filled pauses than the control participants. This was a surprising finding. Examination of unpublished data collected in our laboratory from young adults indicated that the older control participants produced about the same number of filled pauses as the young adults did in the extemporaneous speech task. Therefore, the explanation of the findings in the current study is not that the control participants produced an atypical number of filled pauses. The literature on filled pauses demonstrates that as language complexity increases, the number of filled pauses increases (Lay & Paivio, 1969; Reynolds & Paivio, 1968). This suggests that individuals with PD may have used less complex language in the extemporaneous speech task, reducing language formulation demands and therefore, reducing filled pauses. This interpretation fits with the findings from the Illes et al. (1988) study. However, further study is needed to elucidate this issue.
There are some overall differences, regardless of task, between the individuals with PD and the control participants demonstrating that physiologic issues play a role in changes to respiratory support for speech in PD as well. Individuals with PD had higher lung volume initiations and terminations in both tasks as compared to control participants. The values for lung volume initiation and termination are within the range of data reported in Bunton (2005), but this finding does not fit with all previous studies. Across studies, reported lung volume values in individuals with PD are variable and potentially dependent upon disease severity for the subjects studied. Individuals with PD produced shorter utterances than the control participants, regardless of task, similar to findings from Solomon and Hixon (1993). Individuals with PD used larger lung volume excursions as compared to control participants in both tasks. The excursion data can be explained by the findings from %VC expended per syllable. Individuals with PD used more %VC per syllable, most likely due to reduced laryngeal valving. The excursion data then reflect laryngeal physiologic issues, rather than respiratory physiology. Overall, these data confirm that physiologic changes as a result of PD lead to reduced respiratory support for speech as well as cognitive-linguistic issues.
This study has several limitations. The major limitation is that the tasks were not counterbalanced for order. Because of this, the differences between the tasks may be the result of increasing fatigue, rather than an inherent difference across the tasks. However, some of the task differences are not what we would expect with fatigue. For example, in extemporaneous speech, we found longer utterances, slower rate, and higher lung volume initiations and termination. Another limitation is that reading is fairly different from extemporaneous speech in the amount of language formulation required. Future work should examine tasks which are more real-world or closer in language formulation demands to extemporaneous speech to further examine the role of language formulation. Finally, this study did not test cognition, beyond the MMSE. It would be of interest to examine the results of a complete cognitive battery with respect to respiratory differences across tasks.
In summary, the results show both changes in respiratory physiologic and cognitive-linguistic status affect respiratory support for speech in individuals with PD. While, differences in respiratory support for speech across tasks were found for both individuals with PD and control participants, the individuals with PD demonstrated more difficulty integrating language and respiratory support for speech in the extemporaneous speech task than the control participants. The data support the hypothesis that individuals with PD have difficulty planning or coordinating language formulation and respiratory support for speech, especially during extemporaneous speech.
Acknowledgements
This research was supported by Grant Number 1R03DC05731 from the National Institutes of Health, National Institute on Deafness and Other Communication Disorders, and a Research Support Incentive Grant from the Center on Aging and the Life Course at Purdue University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Deafness and Other Communication Disorders, the National Institutes of Health, or the Center on Aging and the Life Course at Purdue University.
Appendix 1
Appendix 1.
Summary Statistics from ANOVA. Grey-shaded cells are significant at p<0.05, format: F (p), numerator degrees of freedom = 1.
| Variable | Group | Task | Group*Task | Utterance Length (UL) | Task*UL | Group*UL | Group*Task*UL |
|---|---|---|---|---|---|---|---|
| Utterance Length | 4.21 (0.040) | 18.04 (<0.001) | 0.01 (0.7456) | n/a | n/a | n/a | n/a |
| Sound Pressure Level | 24.21 (<0.001) | 37.82 (<0.001) | 12.37 (<0.001) | 24.52 (<0.001) | 4.61 (0.032) | 3.47 (0.063) | 0.421 (0.517) |
| Speech Rate | 27.23 (<0.001) | 130.24 (<0.001) | 6.39 (0.011) | 82.13 (<0.001) | 2.04 (0.153) | 0.003 (0.956) | 3.22 (0.0.073) |
| Lung Volume Initiation | 66.18 (<0.001) | 150.66 (<0.001) | 0.11 (0.740) | 35.24 (<0.001) | 4.35 (0.037) | 0.23 (0.653) | 9.82 (0.002) |
| Lung Volume Termination | 9.74 (0.002) | 142.96 (<0.001) | 0.42 (0.515) | 102.29 (<0.001) | 4.37 (0.037) | 2.55 (0.110) | 3.72 (0.054) |
| Lung Volume Excursion | 89.80 (<0.001) | 3.85 (0.050) | 0.26 (0.613) | 784.06 (<0.001) | 0.06 (0.814) | 3.45 (0.064) | 5.85 (0.016) |
| Inspiratory Duration | 51.83 (<0.001) | 30.20 (<0.001) | 15.86 (0.002) | 26.11 (<0.001) | 2.34 (0.126) | 1.00 (0.317) | 0.69 (0.407) |
| %VC Expended per Syllable | 77.21 (<0.001) | 23.09 (<0.001) | 3.06 (0.080) | 110.73 (<0.001) | 9.43 (0.002) | 14.61 (<0.001) | 5.41 (0.020) |
| Formulation Errors | 5.13 (0.026) | 59.01 (<0.001) | 0.85 (0.359) | n/a | n/a | n/a | n/a |
| Filled Pauses | 9.66 (0.003) | 43.01 (<0.001) | 9.67 (0.003) | n/a | n/a | n/a | n/a |
| Disfluencies | 0.13 (0.717) | 42.17 (<0.001) | 1.35 (0.249) | n/a | n/a | n/a | n/a |
UL = utterance length
Appendix 2
Appendix 2.
Summary statistics from the inter-rater reliability measurements.
| Measurement | Mean Difference | Median Difference | Correlation (r) | p value from F-test |
|---|---|---|---|---|
| Sound Pressure Level | 0.09 dB | 0.01 dB | 0.97 | 0.65 |
| Utterance Length | 0.02 syllables | 0.00 syllables | 0.87 | 0.86 |
| Duration | -0.07 seconds | -0.09 syllables | 0.87 | 0.59 |
| Lung Volume Initiation | -0.21 %VC | -0.03 %VC | 0.97 | 0.91 |
| Lung Volume Termination | -0.77 %VC | -0.08 %VC | 0.95 | 0.61 |
| Inspiratory Duration | -0.04 seconds | -0.03 seconds | 0.48 | 0.12 |
| Number of Disfluencies (reading) | 0.00 | 0.00 | 1.00 | 1.00 |
| Number of Filled Pauses (reading) | 0.00 | 0.00 | 1.00 | 1.00 |
| Number of Formulation Errors (reading) | 0.00 | 0.00 | 1.00 | 1.00 |
Mean and median differences were computed by subtracting the reliability measurement from the original measurement for each utterance.
REFERENCES
- Bodis-Wollner I, Jo M-Y. Getting around and communicating with the environment: visual cognition and language in Parkinson's disease. Journal of Neural Transmission. 2006;70(Suppl):333–338. doi: 10.1007/978-3-211-45295-0_50. [DOI] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat (Version 4.1) Institute of Phonetic Sciences; Amsterdam: 2003. [Google Scholar]
- Brown RG, Marsden CD. Cognitive function in Parkinson's disease: From description to theory. Trends in Neuroscience. 1990;13:21–29. doi: 10.1016/0166-2236(90)90058-i. [DOI] [PubMed] [Google Scholar]
- Bunton K. Patterns of lung volume use during an extemporaneous speech task in persons with Parkinson's disease. Journal of Communication Disorders. 2005;38:331–348. doi: 10.1016/j.jcomdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- De Pandis M, Staracce A, Stefanelli F, Marruzzo P, Meoli I, De Simone G, et al. Modification of respiratory function parameters in patients with severe Parkinson's disease. Neurological Sciences. 2002;23(Suppl 2):S69–70. doi: 10.1007/s100720200074. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini Mental State” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forrest K, Weismer G, Turner GS. Kinematic, acoustic, and perceptual analyses of connected speech produced by Parkinsonian and normal geriatric speakers. The Journal of the Acoustical Society of America. 1989;85(6):2608–2622. doi: 10.1121/1.397755. [DOI] [PubMed] [Google Scholar]
- Friederici AD, Kotz SA, Werheid K, Hein G, von Cramon DY. Syntactic comprehension in Parkinson's disease: Investigating early automatic and late integrational processes using event-related brain potentials. Neuropsychology. 2003;17:133–142. [PubMed] [Google Scholar]
- Goffman L. Kinematic differentiation of prosodic categories in normal and disordered language development. Journal of Speech, Language, and Hearing Research. 2004;47:1088–1102. doi: 10.1044/1092-4388(2004/081). [DOI] [PubMed] [Google Scholar]
- Grossman M, Carvell S, Gollomp S, Stern MB, Reivich M, Morrison D, et al. Cognitive and physiological substrates of impaired sentence processing in Parkinson's disease. Journal of Cognitive Neuroscience. 1993;5:480–498. doi: 10.1162/jocn.1993.5.4.480. [DOI] [PubMed] [Google Scholar]
- Grossman M, Kalmanson J, Bernhardt N, Morris J, Stern MB, Hurtig HI. Cognitive resource limitations during sentence comprehension in Parkinson's disease. Brain and Language. 2000;73:1–16. doi: 10.1006/brln.2000.2290. [DOI] [PubMed] [Google Scholar]
- Grossman M, Lee C, Morris J, Stern MB, Hurtig HI. Assessing resource demands during sentence processing in Parkinson's disease. Brain and Language. 2002;80:603–616. doi: 10.1006/brln.2001.2630. [DOI] [PubMed] [Google Scholar]
- Hayes AE, Davidson MC, Keele SW, Rafal RD. Toward a functional analysis of the basal ganglia. Journal of Cognitive Neuroscience. 1998;10:178–198. doi: 10.1162/089892998562645. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ. Age and speech breathing. Journal of Speech and Hearing Research. 1987;30:351–366. doi: 10.1044/jshr.3003.351. [DOI] [PubMed] [Google Scholar]
- Hoit JD, Hixon TJ, Altman ME, Morgan WJ. Speech breathing in women. Journal of Speech and Hearing Research. 1989;32:353–365. doi: 10.1044/jshr.3202.353. [DOI] [PubMed] [Google Scholar]
- Huber JE. Effects of cues to increase sound pressure level on respiratory kinematic patterns during connected speech. Journal of Speech, Language, and Hearing Research. 2007;50:621–634. doi: 10.1044/1092-4388(2007/044). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE. Effects of utterance length and vocal loudness on speech breathing in older adults. Respiratory Physiology and Neurobiology. 2008;164:323–330. doi: 10.1016/j.resp.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Chandrasekaran B, Wolstencroft JJ. Changes to respiratory mechanisms during speech as a result of different cues to increase loudness. Journal of Applied Physiology. 2005;98:2177–2184. doi: 10.1152/japplphysiol.01239.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber JE, Stathopoulos ET, Ramig LO, Lancaster SL. Respiratory function and variability in individuals with Parkinson disease: Pre- and post-Lee Silverman Voice Treatment. Journal of Medical Speech-Language Pathology. 2003;11:185–201. [Google Scholar]
- Illes J, Metter EJ, Hanson WR, Iritani S. Language production in Parkinson's disease: Acoustic and linguistic considerations. Brain and Language. 1988;33:146–160. doi: 10.1016/0093-934x(88)90059-4. [DOI] [PubMed] [Google Scholar]
- Kleinow J, Smith A. Potential interactions among, linguistic, autonomic, and motor factors in speech. Developmental Psychobiology. 2006;48:275–287. doi: 10.1002/dev.20141. [DOI] [PubMed] [Google Scholar]
- Konno K, Mead J. Measurement of the separate volume changes of the rib cage and the abdomen during breathing. Journal of Applied Physiology. 1967;22(3):407–422. doi: 10.1152/jappl.1967.22.3.407. [DOI] [PubMed] [Google Scholar]
- Lay CH, Paivio A. The effects of task difficulty and anxiety on hesitations in speech. Canadian Journal of Behavioral Science. 1969;1(1):25–27. [Google Scholar]
- Lethlean J,B, Chenery HJ, Murdoch BE. Disturbed respiratory and prosodic function in Parkinson's disease: A perceptual and instrumental analysis. Australian Journal of Human Communication Disorders. 1990;18(2):83–97. [Google Scholar]
- Longworth CE, Keenan SE, Barker RA, Marslen-Wilson WD, Tyler LK. The basal ganglia and rule-governed language use: evidence from vascular and degenerative conditions. Brain. 2005;128:584–596. doi: 10.1093/brain/awh387. [DOI] [PubMed] [Google Scholar]
- Mitchell HL, Hoit JD, Watson PJ. Cognitive-linguistic demands and speech breathing. Journal of Speech, Language, and Hearing Research. 1996;39:93–104. doi: 10.1044/jshr.3901.93. [DOI] [PubMed] [Google Scholar]
- Murdoch BE, Chenery HJ, Bowler S, Ingram JCL. Respiratory function in Parkinson's subjects exhibiting a perceptible speech deficit: A kinematic and spirometric analysis. Journal of Speech and Hearing Disorders. 1989;54:610–626. doi: 10.1044/jshd.5404.610. [DOI] [PubMed] [Google Scholar]
- Oliveira RM, Gurd JM, Nixon P, Marshall JC, Passingham RE. Micrographia in Parkinson's disease: the effect of providing external cues. Journal of Neurology, Neurosurgery, and Psychiatry. 1997;63:429–433. doi: 10.1136/jnnp.63.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich AR, McHenry MA. Estimating respiratory volumes from rib cage and abdominal displacements during ventilatory and speech activities. Journal of Speech and Hearing Research. 1990;33:467–475. doi: 10.1044/jshr.3303.467. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Paivio A. Cognitive and emotional determinants of speech. Canadian Journal of Psychology. 1968;22(3):164–175. doi: 10.1037/h0082757. [DOI] [PubMed] [Google Scholar]
- Sadagopan N, Huber JE. Effects of loudness cues on respiration in individuals with Parkinson's disease. Movement Disorders. 2007;22:651–659. doi: 10.1002/mds.21375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapienza CM, Stathopoulos ET. Speech task effects on acoustic and aerodynamic measures of women with vocal nodules. Journal of Voice. 1995;9(4):413–418. doi: 10.1016/s0892-1997(05)80203-6. [DOI] [PubMed] [Google Scholar]
- Smith A. Speech motor development: integrating muscles, movements, and linguistic units. Journal of Communication Disorders. 2006;39:331–349. doi: 10.1016/j.jcomdis.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Smith A, Goffman L. Interaction of language and motor factors in speech production. In: Maasen B, Kent RD, Peters HFM, Peters H, Lieshout P. v., Hulstijn W, editors. Speech Motor Control in Normal and Disordered Speech. Oxford University Press; 2004. pp. 225–252. [Google Scholar]
- Smith ME, Ramig LO, Dromey C, Perez KS, Samandari R. Intensive voice treatment in Parkinson disease: Laryngostroboscopic findings. Journal of Voice. 1995;9(4):453–459. doi: 10.1016/s0892-1997(05)80210-3. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Hixon TJ. Speech breathing in Parkinson's disease. Journal of Speech and Hearing Research. 1993;36:294–310. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- Sperry EE, Klich RJ. Speech breathing in senescent and younger women during oral reading. Journal of Speech and Hearing Research. 1992;35:1246–1255. doi: 10.1044/jshr.3506.1246. [DOI] [PubMed] [Google Scholar]
- Ventry IM, Weinstein BE. Identification of elderly people with hearing problems. ASHA. 1983;25:37–42. [PubMed] [Google Scholar]
- Weiner P, Inselberg R, Davidovich A, Nisipeanu P, Magadle R, Berar-Yanay N, et al. Respiratory muscle performance and the perception of dyspnea in Parkinson's disease. Canadian Journal of Neurological Sciences. 2002;29:68–72. doi: 10.1017/s031716710000175x. [DOI] [PubMed] [Google Scholar]
- Winkworth AL, Davis PJ, Adams RD, Ellis E. Breathing patterns during spontaneous speech. Journal of Speech and Hearing Research. 1995;38:124–144. doi: 10.1044/jshr.3801.124. [DOI] [PubMed] [Google Scholar]
- Winkworth AL, Davis PJ, Ellis E, Adams RD. Variability and consistency in speech breathing during reading: Lung volumes, speech intensity, and linguistic factors. Journal of Speech and Hearing Research. 1994;37:535–556. doi: 10.1044/jshr.3703.535. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis P. A review of the cognitive and behavioral sequelae of Parkinson's disease: Relationship to frontostriatal circuitry. Cognitive and Behavioral Neurology. 2003;16:193–210. doi: 10.1097/00146965-200312000-00001. [DOI] [PubMed] [Google Scholar]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Geigin A, et al. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson's disease. Journal of Clinical and Experimental Neuropsychology. 2006;28:1127–1144. doi: 10.1080/13803390500246910. [DOI] [PMC free article] [PubMed] [Google Scholar]