Abstract
Background
Binge eating predisposes children to excessive weight gain. However, it is unknown if pediatric binge eating predicts other obesity-associated adverse health outcomes.
Objective
The objective of this study was to investigate the relationship between binge eating and metabolic syndrome (MetS) in children.
Method
Children 5–12 y at high risk for adult obesity, either because they were overweight/obese when first examined or because their parents were overweight/obese, were recruited from Washington, DC and its suburbs. Children completed a questionnaire assessment of binge eating at baseline and underwent measurements of MetS components at baseline and at a follow-up visit approximately 5 years later. Magnetic resonance imaging was used to measure visceral adipose tissue (VAT) in a subset.
Results
One hundred eighty children were studied between July, 1996 and August, 2010. Baseline self-reported binge eating presence was associated with a 5.33 greater odds of having MetS at follow-up (95% CI: 1.47, 19.27, p = 0.01). The association between binge eating and BMI only partially explained changes in MetS components: baseline binge eating predicted higher follow-up triglycerides, even after accounting for baseline triglycerides, baseline BMI, BMI change, sex, race, baseline age, and time in study (p = 0.05). Also, adjusting for baseline VAT and demographics, baseline binge eating predicted greater follow-up L2-3 VAT (p = 0.01).
Discussion
Children’s reports of binge eating predicted development of MetS, worsening triglycerides, and increased VAT. The excessive weight gain associated with children’s binge eating partly explained its adverse metabolic health outcomes. Reported binge eating may represent an early behavioral marker upon which to focus interventions for obesity and MetS.
Keywords: binge eating, metabolic syndrome, children, adolescents, longitudinal studies
Binge eating, defined as the consumption of a large amount of food while experiencing a sense of loss of control over eating, is the hallmark behavior of binge eating disorder (BED) [1]. Although few children meet full-syndrome criteria for BED, a substantial proportion report engaging in infrequent episodes of binge eating [2]. Studies indicate that the presence of even infrequent binge eating in children is a prospective risk factor for excessive gain in weight [3] and body fat [4] over time. However, there are limited longitudinal data regarding other potential adverse health outcomes of binge eating in youth.
The metabolic syndrome (MetS) is a cluster of metabolic abnormalities associated with obesity. Although there are no definitive criteria for MetS in youth, there is consensus that, as in adults, the syndrome consist of abdominal obesity (≥90th percentile for age and sex), impaired fasting glucose (≥100 mg/dL), high triglycerides (≥90th percentile for age and sex), low HDL-cholesterol (≤10th percentile for age and sex), and high blood pressure (≥90th percentile for age, sex, and height) [5–8]. Despite moderate instability of MetS in youth [9], pediatric MetS may place children at elevated risk for the presence of adult MetS and the development of cardiovascular disease and type 2 diabetes [10–13].
There is a strong link between children’s binge eating and excessive body weight and adiposity [2]. Yet, there are virtually no data examining the relationship of binge eating to MetS and its components in youth. One cross-sectional study of obese children seeking weight-loss treatment found no significant differences in metabolic characteristics between those with and without binge eating [14]. Studies of adults with and without BED suggest that irregular eating patterns such as skipping meals are cross-sectionally, positively associated with MetS presence [15, 16]. Furthermore, in a prospective study of adults with and without BED, matched for age, sex, and baseline body mass index (BMI, kg/m2), those with BED were significantly more likely than those without BED to develop components of MetS, specifically, dyslipidemia, hypertension, and dysglycemia, five years later [17].
No study has prospectively studied the impact of binge eating on the development of MetS or its components in children. We, therefore, longitudinally assessed the relationship of children’s self-reported binge eating with the development of MetS and changes in its components among non-treatment-seeking children. We hypothesized that binge eating at baseline would significantly predict greater odds of developing MetS and worsening of MetS components over time. Moreover, given the strong association between children’s binge eating and excessive body weight gain in youth [3, 4], we tested whether any observed relationships between binge eating and development of MetS or its components were independent of, or largely explained by, BMI change.
Subjects and Methods
Participants
A non-treatment seeking sample of overweight or obese and non-overweight children (age 5–12 years) was studied between July, 1996 and August, 2010 (ClinicalTrials.gov IDs: NCT00001522 and NCT00001195). Children were believed to be at increased risk (beyond shared environmental risk) for adult obesity by virtue of their own overweight or obesity (BMI for age and sex ≥85th percentile [18] or their parents’ overweight or obesity (BMI ≥25 kg/m2). Participants were recruited through two waves of notices mailed to 1st through 5th grade children in the Montgomery County and Prince Georges County, Maryland school districts, advertisements in local newspapers, and two mailings to local family physicians and pediatricians. Mailings to families and physicians requested participation of children willing to undergo phlebotomy and imaging assessments for studies investigating hormones and metabolic functioning in children. All understood that they would not receive treatment as part of the study, but would be financially compensated for their participation. Children were healthy and medication-free for at least two weeks prior to baseline evaluation. By design, the sample was enriched for overweight youth. Children provided written assent and parents gave written consent for participation in the protocol. Study protocols were approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development Institutional Review Board.
Procedure
Participants were seen for a baseline assessment and then again a minimum of 2 years later for a follow-up assessment.
Binge eating
At baseline, children completed the Questionnaire on Eating and Weight Patterns – Adolescent Version [19], a self-report measure based upon the adult Questionnaire on Eating and Weight Patterns – Revised [20], to assess their frequency of reported binge eating. This questionnaire is a screening tool designed to identify children with possible DSM-IV-Text Revision [1] bulimia nervosa and BED. Questions were read to very young (<8 years of age) children and explanations offered by trained research assistants as previously described [21]. For purposes of the current study, children were categorized as those never reporting binge eating and those who reported at least 1 episode of binge eating in the 6 months prior to the visit.
MetS
At baseline and follow-up visits, components of MetS were assessed following an overnight fast. Weight, height, and waist circumference were obtained with the use of standardized techniques [22]. Weight was measured to the nearest 0.1 kg by using a calibrated digital scale (Scale-Tronix, Wheaton, IL). Height was measured in triplicate to the nearest 1 mm by using a stadiometer calibrated before each set of height measurements (Holtain Ltd, Crymych, Wales). BMI was calculated as weight in kg divided by height in m2, and BMI standard deviation (z) scores for sex and age were calculated according to the Centers for Disease Control and Prevention 2000 standards [18]. Abdominal waist circumference was measured with a flexible non-elastic tape measure just above the lateral border of the iliac crest. Blood pressure (Dynamap, GE Heathcare) was measured via automated oscillometric device (Dinamap, GE Heathcare) at the right brachial artery while participants were seated. Venous blood sampling was performed in children for serum glucose and lipids. Glucose, total cholesterol, and triglycerides were measured on a Hitachi 917 analyzer using reagents from Roche Diagnostics (Indianapolis, IN). Direct determinations of HDL-cholesterol were performed on a Cobas FARA analyzer (Roche Diagnostics, Indianapolis, IN) using reagents from Sigma Chemical (St. Louis, MO).
MetS was defined using an age- and sex-specific percentile-based cut-off definition commonly used in previous reports [9, 23–26]. The MetS definition used values of at least 90th percentile for waist circumference[6], systolic or diastolic blood pressure [8], and triglycerides [27] and no higher than 10th percentile for HDL cholesterol[7]. A fasting glucose value of at least 100 mg/dL was used to indicate impaired fasting glucose. MetS was considered present when a child met the cut-off points for at least three of these factors.
Intra-abdominal Adipose Tissue by Magnetic Resonance Imaging
A subset of youth in the larger study opted to participate in abdominal magnetic resonance imaging (MRI), utilized for a more precise assessment of abdominal adiposity at baseline and follow-up. The MRI scan was obtained through the abdomen of each participant with the plane of section parallel to the lumbar disc spaces. Contiguous, 1-cm transaxial, T1-weighted spin-echo magnetic resonance images (0.5 T, relaxation time = 400 ms, time of excitation = 10 ms, number of repetitions of excitations = 10) were obtained from sections encompassing L2-3 and L4-5. On each image, the body circumference and cross-sectional areas of the subcutaneous and total fat were measured by one observer. The visceral adipose tissue (VAT) measurement was determined by subtracting the subcutaneous fat from the total body fat in each section.
Statistical Analyses
Analyses were conducted with SPSS 18.0 and SAS 9.2. Data were screened for problems of outliers, skew, and kurtosis [28]. According to our a priori analytic plan, to prepare data for analyses, outliers (<3% of all data points) were adjusted to fall 1.5 times the interquartile range below the 25th percentile or above the 75th percentile (e.g., to the whiskers in Tukey’s boxplot [29]). We routinely utilize this strategy because it minimizes outliers’ influence on the characteristics of the distribution, minimally changes the distribution overall, and avoids potential bias associated with eliminating outliers altogether. Skew and kurtosis were satisfactory on all variables. Missing data patterns were characterized to test baseline differences in study variables between children who did and did not complete a follow-up assessment. Independent samples t-tests and chi-square analyses were used to describe baseline differences in demographic, anthropometric and metabolic characteristics between youth with and without binge eating (presence versus absence) at baseline. Chi-square and logistic regression analyses were used to describe the relationship between binge eating presence at baseline and presence of MetS at follow-up. Baseline BMI (kg/m2) was considered as a covariate. A series of analyses of covariance (ANCOVAs) were conducted with the follow-up MetS components as the dependent variable (follow-up waist circumference, blood pressure, triglycerides, HDL cholesterol, or glucose), and binge eating (presence versus absence) as the independent variable. Covariates included the baseline MetS component that corresponded to the dependent variable (baseline waist circumference, blood pressure, triglycerides, HDL cholesterol, or glucose), baseline age (years), sex, race (Caucasian versus Other), time in study (years between baseline and follow-up), and baseline BMI (kg/m2). Baseline BMI was included so that any observed effects of binge eating on the development of components of a MetS would be determined after controlling for body weight. We also calculated the effects of baseline binge eating on MetS components when including BMI change from baseline to follow-up as an additional covariate in the models. Pubertal stage was considered as a covariate, but it was removed because of its multi-collinearity with age. Its inclusion did not alter the significance or direction of any effects for binge eating.
We conducted secondary analyses examining binge eating as a predictor of MetS and its components using multiple imputation to handle missing data. The missing data model included demographic characteristics (sex, race, and baseline age), body measurements (baseline BMI and BMI change from baseline to follow-up), MetS components (baseline and follow-up waist circumference, blood pressure, triglycerides, HDL cholesterol, and glucose), binge eating, and time from baseline to follow-up. Ten imputed data sets were produced due to the large missing data fraction [30, 31]. Following standard multiple imputation procedures, each data set was analyzed separately, and then, the effects were combined using the SAS MIANALYZE procedure.
In the sub-group of youth who completed MRIs at baseline and follow-up, ANCOVAS were conducted with follow-up abdominal fat (L2-3 or L4-5) as the dependent variable and binge eating status as the independent variable. Covariates included baseline abdominal fat (L2-3 or L4-5), age, sex, race, and time in study. In a second model, we included total subcutaneous abdominal fat (L2-3 or L4-5) in addition to the aforementioned covariates. We did not conduct multiple imputation with MRI data given that only a small subset of youth in the larger study completed these procedures (33.5%).
For all analyses, effects were considered significant when P < 0.05. All tests were two-tailed.
Results
Participant Characteristics
One hundred eighty youth completed a baseline screening appointment. Table 1 presents baseline differences in demographic, anthropometric, and metabolic characteristics among youth with and without binge eating. No child met BED criteria [1]. A smaller percentage of children who reported baseline binge eating were Caucasian (vs. Other race/ethnicity) compared to children without binge eating (P < 0.01). Children with binge eating had a higher baseline BMI, BMI-z score, and greater baseline waist circumference than children without binge eating (Ps ≤ 0.001; Table 1). At baseline, a total of 11 children met criteria for MetS. All met the threshold for waist circumference and HDL cholesterol. Nine met the threshold for triglycerides, two for blood pressure, and one for impaired fasting glucose.
Table 1.
Baseline demographic, anthropometric, and metabolic characteristics of children with and without binge eating reported at baseline
| Variable | Binge Eating1 | No Binge Eating | |
|---|---|---|---|
| n = 24 | n = 156 | P | |
| Sex (% female) | 50.0 | 51.9 | 0.52 |
| Race (% Caucasian) | 33.3 | 64.1 | 0.004 |
| Baseline age (y)† | 8.5 ± 1.8 | 8.7 ± 1.6 | 0.66 |
| Baseline BMI (kg/m2)† | 27.8 ± 6.1 | 22.3 ± 5.8 | < 0.001 |
| Baseline BMI-z | 2.3 ± 0.7 | 1.4 ± 1.0 | < 0.001 |
| Baseline MetS diagnosis (% presence)2a | 14.3 | 5.6 | 0.15 |
| Baseline waist circumference (cm)†b | 80.3 ± 14.8 | 70.1 ± 13.8 | 0.001 |
| Baseline systolic blood pressure (mmHg)† | 110.6 ± 12.5 | 108.0 ± 11.2 | 0.29 |
| Baseline diastolic blood pressure (mmHg)† | 62.2 ± 9.0 | 62.8 ± 6.8 | 0.70 |
| Baseline triglycerides (mg/dL)†a | 77.1 ± 35.7 | 70.4 ± 31.9 | 0.38 |
| Baseline HDL cholesterol (mg/dL)†c | 46.7 ± 13.3 | 47.5 ± 10.7 | 0.76 |
| Baseline glucose (mg/dL)†d | 89.8 ± 5.9 | 88.4 ± 6.2 | 0.34 |
Mean±SD;
Binge eating defined as the presence of at least 1 episode in the 6 months prior to assessment on the Questionnaire on Eating and Weight Patterns – Adolescent Version (QEWP-A);
Metabolic syndrome (MetS) defined as meeting at least 3 of the proposed cut-off criteria for waist circumference (≥90th percentile), blood pressure (≥90th percentile), triglycerides (≥90th percentile), HDL cholesterol (≤10th percentile), or glucose (≥100 mg/dL);
Binge eating n = 21, No binge eating n = 143;
Binge eating n = 24, No binge eating n = 150;
Binge eating n = 21, No binge eating n = 142;
Binge eating n = 21, No binge eating n = 144
Of those who attended a baseline assessment, 148 (82.2%) returned for a follow-up appointment an average of 5.4±1.9 years later (range = 2.1–8.6 years) to re-assess MetS components. Baseline and follow-up MetS data were available in 11–16 binge eating and 99–126 no binge eating participants. There were no significant differences on any study variable between youth who did and did not return for a follow-up visit. At follow-up, a total of 18 participants met criteria for MetS. Of the 11 children who had MetS at baseline, 3 were lost to follow-up, 6 continued to have MetS, and 2 no longer met MetS criteria. Twelve youth who did not have MetS at baseline developed MetS at follow-up.
Baseline Binge Eating as a Predictor of Follow-up MetS
A significantly greater percentage (41.7%; n = 5 of 12) of children with baseline binge eating had MetS at follow-up compared to children without baseline binge eating (11.8%, n = 13 of 110, P = 0.006). Baseline binge eating presence was associated with a 5.33 greater odds of having MetS at follow-up (95% CI: 1.47, 19.27, P = 0.01). Accounting for baseline BMI, baseline binge eating presence was no longer significantly associated with having MetS at follow-up (Odds Ratio: 2.26, 95% CI: 0.53, 9.72, P = 0.27). When selecting only children who did not meet criteria for MetS at baseline, findings were similar. Children’s baseline binge eating remained significantly associated with MetS at follow-up, such that 33.3% of children with baseline binge eating developed MetS at follow-up compared to 9.1% of children without baseline binge eating (P = 0.027). However, accounting for baseline BMI, the association between baseline binge eating and development of MetS was not statistically significant (Odds Ratio: 2.49, 95% CI: 0.43, 14.25, P = 0.31). Findings were similar when employing multiple imputation; there was a trend association between children’s binge eating at baseline and MetS at follow-up (F = 3.59, P = 0.065) and the association between binge eating at baseline and MetS at follow-up was not significant after controlling for baseline BMI (Odds Ratio: 1.67, 95% CI: −0.73, 2.06, P = 0.34).
Baseline Binge Eating as a Predictor of Follow-up Components of MetS
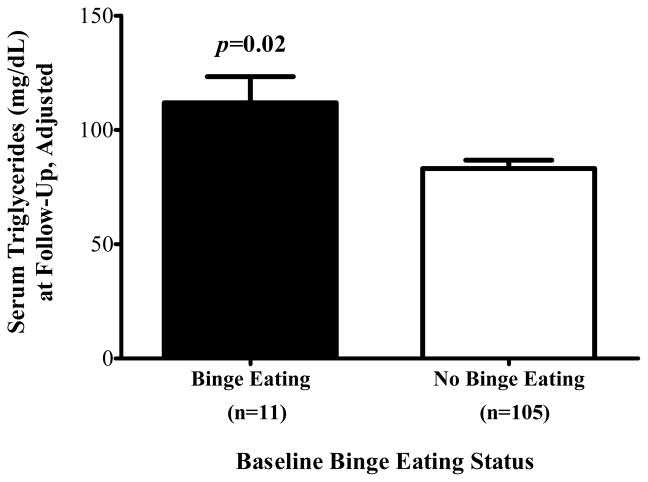
Table 2 displays the analyses testing binge eating as a predictor of follow-up MetS components. Accounting for covariates, children’s baseline binge eating predicted follow-up triglycerides such that those with baseline binge eating had higher triglycerides at follow-up than children without binge eating at baseline (P = 0.02; Figure 1). By contrast, baseline binge eating did not significantly predict follow-up waist circumference (P = 0.15), systolic blood pressure (P = 0.07), diastolic blood pressure (P = 0.50), HDL cholesterol (P = 0.13), or glucose (P = 0.50). The association between children’s baseline binge eating and follow-up triglycerides remained significant (P = 0.05) when BMI change was added to the model. In analyses utilizing imputed data to example baseline binge eating as a predictor of follow-up MetS components, all findings were similar in direction and significance (see Table 2).
Table 2.
Analyses of covariance examining baseline binge eating as a predictor of follow-up metabolic syndrome (MetS) components
| Follow-up MetS Component | Binge Eating M±SE1,2 | No Binge Eating M±SE2 | Model I2 Difference (95% CI) | P | Model I + ΔBMI3 Difference (95% CI) | P | Imputation Model4 Difference (95% CI) | P |
|---|---|---|---|---|---|---|---|---|
| Waist Circumference | 90.1±2.2 | 86.7±0.8 | −3.5 (−8.2, 1.3) | 0.15 | −0.2 (−2.5, 2.1) | 0.85 | 0.03 (−2.0, 2.0) | 0.98 |
| Systolic Blood Pressure (mmHg) | 124.1±2.8 | 118.6±1.0 | −5.5 (−11.6, 0.5) | 0.07 | −4.9 (−11.0, 1.2) | 0.11 | −3.7 (−10.0, 2.6) | 0.25 |
| Diastolic Blood Pressure (mmHg) | 67.4±1.9 | 66.0±0.7 | −1.4 (−5.4, 2.6) | 0.50 | −0.8 (−4.8, 3.3) | 0.71 | −0.11 (−4.9, 4.7) | 0.96 |
| Triglycerides (mg/dL) | 111.8±11.4 | 83.1±3.7 | −28.7 (−52.9, −4.5) | 0.02 | −24.2 (−48.7, 0.3) | 0.05 | −30.7 (−56.4, −5.1) | 0.02 |
| HDL Cholesterol (mg/dL) | 41.8±2.8 | 46.3±0.9 | 4.5 (−1.4, 10.3) | 0.13 | 3.3 (−2.7, 9.2) | 0.28 | 3.9 (−1.8, 9.6) | 0.18 |
| Glucose (mg/dL) | 89.3±3.6 | 86.6±1.2 | −2.6 (−10.3, 5.0) | 0.50 | −3.4 (−11.3, 4.4) | 0.39 | −1.2 (−6.7, 4.2) | 0.65 |
Binge eating presence (vs. absence) defined as ≥1 episode in the 6 months prior to assessment on the Questionnaire on Eating and Weight Patterns – Adolescent Version (QEWP-A).
Non-imputed data (binge eating n = 11–16, no binge eating n = 99–126) adjusted for sex, race, baseline age, baseline BMI, respective baseline MetS component, and time in study.
Non-imputed data (binge eating n = 11–16, no binge eating n = 99–126) adjusted for sex, race, baseline age, baseline BMI, BMI change from baseline to follow-up, respective baseline MetS component, and time in study.
Imputed data (binge eating n = 24, no binge eating n = 156) adjusted for sex, race, baseline age, baseline BMI, BMI change from baseline to follow-up, respective baseline MetS component, and time in study.
Figure 1.
On average (±SE), children with binge eating at baseline had higher follow-up triglycerides than children without binge eating at baseline, adjusting for sex, race, baseline age, body mass index (kg/m2), baseline triglycerides, and time in study (P = 0.02).
Binge Eating and Intra-Abdominal Adipose Tissue via MRI
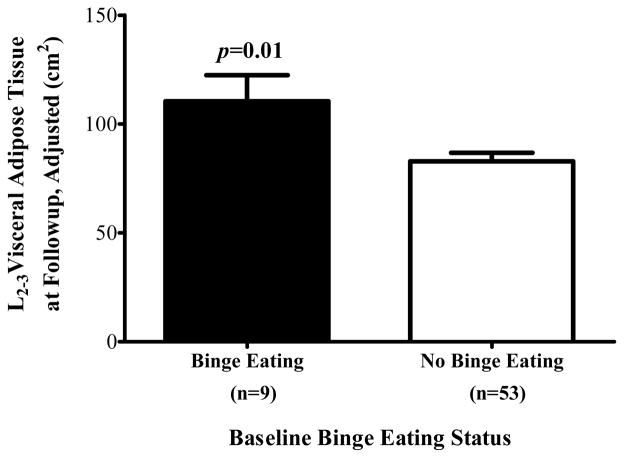
One hundred nineteen children completed an MRI at baseline. There was no difference between children who did and did not complete an MRI at baseline with regard to sex, race, binge presence, age, BMI-z, waist circumference, systolic or diastolic blood pressure, triglycerides, HDL cholesterol or glucose. However, a greater percentage of those who completed an MRI had MetS at baseline (9.6%) compared to those who did not have an MRI (0%, P = 0.013). Of those, 62 (52.1%) completed a second MRI at follow-up (binge eating n = 9, no binge eating n = 53). Longitudinal analyses examining binge eating as a predictor of follow-up adipose tissue are displayed in Table 3. Children’s binge eating at baseline significantly predicted follow-up visceral adipose tissue at L2-3, accounting for baseline visceral adipose tissue at L2-3, baseline age, sex, race, and time in study (P = 0.01; Figure 2). In secondary analyses including the same covariates as well as baseline subcutaneous fat at L2-3, the finding was attenuated (P = 0.06). Binge eating did not predict follow-up VAT at L4-5 with all covariates in the model including baseline VAT at L4-5 (P = 0.20) or when baseline subcutaneous fat at L4-5 was added to the model (P = 0.55).
Table 3.
Analyses of covariance examining baseline binge eating as a predictor of follow-up visceral adipose tissue assessed with magnetic resonance imaging
| Predictor variables | Binge Eating M±SE1,2 | No Binge Eating M±SE2 | Model I2 Difference (95% CI) | P | Model II3 Difference (95% CI) | P |
|---|---|---|---|---|---|---|
| Visceral Adipose Tissue L2-3 (kg) | 85.1±11.6 | 51.5±5.2 | −33.6 (−59.7, −7.6) | 0.01 | −21.9 (−45.0, 1.2) | 0.06 |
| Visceral Adipose Tissue L4-5 (kg) | 63.8±9.4 | 50.6±4.1 | −13.2 (−34.2, 7.8) | 0.21 | −6.1 (−26.4, 14.2) | 0.55 |
Binge eating presence (vs. absence) defined as at least 1 episode in the 6 months prior to assessment on the Questionnaire on Eating and Weight Patterns – Adolescent Version (QEWP-A).
Non-imputed data (binge eating n = 9, no binge eating n = 53) adjusted for sex, race, baseline age, baseline visceral adipose tissue, and time in study.
Non-imputed data (binge eating n = 9, no binge eating n = 53) adjusted for sex, race, baseline age, baseline visceral adipose tissue, baseline total subcutaneous abdominal fat, and time in study.
Figure 2.
On average (±SE), children with binge eating at baseline had greater visceral adipose tissue at L2-3 at follow-up than children without binge eating at baseline, adjusting for sex, race, baseline age, baseline visceral adipose tissue at L2-3, and time in study (P = 0.01).
Post-Hoc Binge Eating Interactions with Pubertal Stage and Sex
In post-hoc analyses, we explored whether there were any significant interactional effects of binge eating with baseline pubertal stage or with sex in the prediction of follow-up waist circumference, blood pressure, triglycerides, HDL cholesterol, glucose, or VAT at L2-3 or L4-5. In the analyses examining binge eating by pubertal stage interactions, the main effect of pubertal stage and the binge eating by pubertal stage interaction were added to models. The binge eating by pubertal stage interaction was not significant in any model (Ps = 0.10–0.94). The binge eating by sex interaction was significant for systolic blood pressure (P = 0.02). Accounting for baseline systolic blood pressure, baseline age, race, time in study, baseline BMI, and BMI change, boys with baseline binge eating had higher follow-up systolic blood pressure than boys without baseline binge eating (M±SE 130.3±4.3 vs. 117.5±1.5, P < 0.01). In contrast, girls with and without baseline binge eating did not differ in their follow-up systolic blood pressure (116.7±3.9 vs. 119.9±1.3, P = 0.45). No other binge eating by sex interaction reached significance (Ps = 0.09–0.75).
Discussion
In a prospective examination of non-treatment seeking children at high-risk for adult obesity, those who reported binge eating at baseline were at greater risk for having metabolic syndrome (MetS) at baseline and developing MetS approximately 5 years later than children without baseline binge eating in unadjusted analyses. The association between children’s infrequent episodes of binge eating and subsequent MetS is potentially important because the cluster of metabolic abnormalities that comprise pediatric MetS is related to heightened risk for cardiovascular disease and type 2 diabetes onset in adulthood [32–35]. The relationship between baseline binge eating and follow-up MetS was attenuated after adjustment for baseline BMI and BMI change. These observations suggest that children with binge eating may be, in large measure, at increased odds of acquiring MetS because they are heavier and gaining excessive weight over time [3, 4].
Children’s reported binge eating also predicted greater increases in several components of MetS independent of body mass or abdominal fat. Baseline binge eating predicted worsening triglycerides independent of both baseline BMI and BMI change over time. This pattern is consistent with Hudson and colleagues’ result that adults with full-syndrome BED had greater odds of developing dyslipidemia after accounting for BMI gain [17]. The eating patterns that characterize youth who report binge eating partially may contribute to significantly greater increases in triglycerides relative to youth without binge eating. Reported [36] and observational laboratory [37] data suggest that children with binge eating patterns consume more energy from carbohydrate and refined sugar in desserts and snacks compared to youth without such behaviors. Consumption of simple carbohydrates has been shown to impact serum triglycerides adversely [38, 39]. We therefore hypothesize that altered macronutrient selection may be one mechanism by which children’s binge eating promotes worsening triglycerides.
In line with the robust effect of binge eating on excessive weight gain observed in prior reports [3, 4], children’s baseline binge eating also predicted greater follow-up visceral fat, accounting for baseline fat and other relevant covariates. Notably, this relationship was found using MRI, which generates a more precise measurement of visceral adiposity than simple waist circumference. The link between children’s binge eating and greater gains in central adiposity is troublesome in light of some [40], but not all [41], data indicating that central fat may be a significant risk factor for cardiovascular disease. The mechanisms by which binge eating may promote visceral fat gain are poorly understood. Children’s binge eating often occurs in response to emotional triggers (e.g., interpersonal conflict) or stress [42], which in turn, has been linked to abdominal fat deposition in adults [43]. Therefore, one possibility is that binge eating reflects increased stress among children who report the behavior, which in turn, may induce behavioral and physiological consequences that promote central fat gain[44].
Accounting for all covariates including BMI change over time, binge eating also predicted increased systolic blood pressure. However, this effect only was observed among boys and requires replication with a larger sample size. Furthermore, despite non-significant trends for HDL cholesterol and waist circumference, binge eating did not significantly predict prospective changes in the other components of MetS. These findings somewhat differ from one prior adult study that found full-syndrome BED to be predictive of hypertension and type 2 diabetes [17]. Unlike adults, children are still physically developing, such that weight gain may exert a relatively stronger effect on metabolic characteristics and consequently, the unique contributions of binge eating and excessive weight gain on cholesterol and glucose may be more difficult to disentangle. An alternative, but not mutually exclusive, possibility is that full-syndrome BED may have a greater impact on metabolic characteristics compared to the infrequent, subthreshold binge eating behaviors that are often reported by children. It is also possible that sample size limitations in the present study account for these differences. Further investigation of metabolic changes in children and adolescents with more frequent binge eating behaviors is warranted.
Strengths of this investigation include the prospective design, the young age of participants at their initial visits, and the use of objective measures for the assessment of all MetS components. Limitations include the small number of children with MetS and with binge eating, and consequently, the possibility of failing to detect scientifically important differences between groups. These data require replication with larger samples either enriched for children with MetS or with a longer follow-up interval into young and middle adulthood. Tracking youth with and without binge eating into adulthood, when metabolic problems such as hypertension and dysglycemia become more prevalent, may be particularly informative. Although nearly 40% of the sample was comprised of African American children, only three participants were Hispanic, limiting the generalizability of the results. Although statistical models included race as a covariate, further exploration of binge eating and MetS in racial/ethnic minority samples such as Hispanic or Asian youth is an important next research step, especially due to established differences in MetS components among youths from varying racial/ethnic backgrounds [45, 46]. In addition, our findings may only be generalizable to children who are at similar increased risk for adult obesity, given that the majority of our sample was overweight or obese at their first assessment. However, elucidating factors that promote adverse health outcome in this population that experiences rapid and excess weight gain is urgently required. Furthermore, the reliance on questionnaire, rather than clinical interview, for assessment of binge eating lacks specificity in the identification of this behavior [47]. Also of note, although a non-treatment-seeking sample was recruited, there was significant attrition over time, and families willing to participate in studies at the NIH may differ from those in the general population.
Children’s self-reported binge eating may place youth at heightened risk for MetS, primarily consistent with their excessive weight or fat gain, with independent impact on triglycerides and visceral adipose tissue. The indirect – rather than direct – impact of children’s binge eating on MetS does not diminish the importance of pediatric binge eating as a potentially modifiable, behavioral risk factor for obesity. Obesity is a risk factor for MetS and its associated health consequences [48]. Therefore, interventions aimed at reducing binge eating to decrease excessive weight gain [49] may have positive consequences for metabolic health trajectories. This hypothesis warrants investigation given the critical need for novel behavioral interventions for obesity and MetS in youth.
Acknowledgments
Research support: National Research Service Award 1F32HD056762 from the NICHD (to LBS) and Intramural Research Program grant 1ZIAHD000641 from the NICHD with supplemental funding from NIMHD (to JAY).
JAY is a Commissioned Officer in the U.S. Public Health Service, DHHS.
The authors’ responsibilities were as follows: MTK, LBS, SZY, and JAY designed the study. MTK, LBS, EAS, RM, NS, DD, SZY, VSH, and JAY analyzed and interpreted the data and drafted of the manuscript. All authors contributed to the collection and assembly of data, provided critical revision of the article for content, and approved the final version of the manuscript. We thank the volunteers who participated for their help in completing these studies.
The funding organization played no role in design and conduct of the study; collection, management, analysis, and interpretation of the data; nor preparation or review of the manuscript.
Footnotes
ClinicalTrials.gov IDs: NCT00001522, NCT00001195
None of the authors had any conflict of interest.
Disclaimer: The opinions and assertions expressed herein are those of the authors and are not to be construed as reflecting the views of the PHS, USUHS or the U.S. Department of Defense.
References
- 1.Anonymous. Diagnostic and statistical manual of mental disorders DSM-IV-TR. 4. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 2.Tanofsky-Kraff M. Binge eating among children and adolescents. In: Jelalian E, Steele R, editors. Handbook of Child and Adolescent Obesity. Springer; 2008. pp. 41–57. [Google Scholar]
- 3.Field AE, Austin SB, Taylor CB, Malspeis S, Rosner B, Rockett HR, Gillman MW, Colditz GA. Relation between dieting and weight change among preadolescents and adolescents. Pediatrics. 2003;112(4):900–906. doi: 10.1542/peds.112.4.900. [DOI] [PubMed] [Google Scholar]
- 4.Tanofsky-Kraff M, Cohen ML, Yanovski SZ, Cox C, Theim KR, Keil M, Reynolds JC, Yanovski JA. A prospective study of psychological predictors of body fat gain among children at high risk for adult obesity. Pediatrics. 2006;117(4):1203–1209. doi: 10.1542/peds.2005-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 6.Fernández JR, Redden DT, Pietrobelli A, Allison DB. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. The Journal of Pediatrics. 2004;145(4):439–444. doi: 10.1016/j.jpeds.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 7.Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, Johnson CL. Distributions and Trends of Serum Lipid Levels among United States Children and Adolescents Ages 4–19 Years: Data from the Third National Health and Nutrition Examination Survey. Preventive Medicine. 1998;27(6):879–890. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 8.Anonymous. Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program. National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents [see comments] Pediatrics. 1996;98(4 Pt 1):649–658. [PubMed] [Google Scholar]
- 9.Gustafson JK, Yanoff LB, Easter BD, Brady SM, Keil MF, Roberts MD, Sebring NG, Han JC, Yanovski SZ, Hubbard VS, et al. The stability of metabolic syndrome in children and adolescents. J Clin Endocrinol Metab. 2009;94(12):4828–4834. doi: 10.1210/jc.2008-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood cardiovascular risk factors and carotid vascular changes in adulthood: the Bogalusa Heart Study. JAMA. 2003;290(17):2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 11.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: the Princeton Lipid Research Clinics Follow-up Study. Pediatrics. 2007;120(2):340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 12.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Huang TT, Nansel TR, Belsheim AR, Morrison JA. Sensitivity, specificity, and predictive values of pediatric metabolic syndrome components in relation to adult metabolic syndrome: the Princeton LRC follow-up study. J Pediatr. 2008;152(2):185–190. doi: 10.1016/j.jpeds.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lourenco BH, Arthur T, Rodrigues MD, Guazzelli I, Frazzatto E, Deram S, Nicolau CY, Halpern A, Villares SM. Binge eating symptoms, diet composition and metabolic characteristics of obese children and adolescents. Appetite. 2008;50(2–3):223–230. doi: 10.1016/j.appet.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Roehrig M, Masheb RM, White MA, Grilo CM. The metabolic syndrome and behavioral correlates in obese patients with binge eating disorder. Obesity (Silver Spring) 2009;17(3):481–486. doi: 10.1038/oby.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierra-Johnson J, Unden AL, Linestrand M, Rosell M, Sjogren P, Kolak M, De Faire U, Fisher RM, Hellenius ML. Eating meals irregularly: a novel environmental risk factor for the metabolic syndrome. Obesity (Silver Spring) 2008;16(6):1302–1307. doi: 10.1038/oby.2008.203. [DOI] [PubMed] [Google Scholar]
- 17.Hudson JI, Lalonde JK, Coit CE, Tsuang MT, McElroy SL, Crow SJ, Bulik CM, Hudson MS, Yanovski JA, Rosenthal NR, et al. Longitudinal study of the diagnosis of components of the metabolic syndrome in individuals with binge-eating disorder. Am J Clin Nutr. 2010;91(6):1568–1573. doi: 10.3945/ajcn.2010.29203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: methods and development. Vital Health Stat. 2002;11(246):1–190. [PubMed] [Google Scholar]
- 19.Johnson WG, Grieve FG, Adams CD, Sandy J. Measuring binge eating in adolescents: adolescent and parent versions of the questionnaire of eating and weight patterns. Int J Eat Disord. 1999;26(3):301–314. doi: 10.1002/(sici)1098-108x(199911)26:3<301::aid-eat8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 20.Yanovski S. Binge eating disorder: Current knowledge and future directions. Obes Res. 1993;1:306–324. doi: 10.1002/j.1550-8528.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 21.Morgan CM, Yanovski SZ, Nguyen TT, McDuffie J, Sebring NG, Jorge MR, Keil M, Yanovski JA. Loss of control over eating, adiposity, and psychopathology in overweight children. Int J Eat Disord. 2002;31(4):430–441. doi: 10.1002/eat.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Center for Health Statistics. NHANES III Anthropometric Procedures Video. Washington, DC: US Government Printing Office; 1996. Stock Number 017-022-01335-5. [Google Scholar]
- 23.Butte NF, Comuzzie AG, Cole SA, Mehta NR, Cai G, Tejero M, Bastarrachea R, Smith EOB. Quantitative genetic analysis of the metabolic syndrome in hispanic children. Pediatric Research. 2005;58(6):1243–1248. doi: 10.1203/01.pdr.0000185272.46705.18. [DOI] [PubMed] [Google Scholar]
- 24.Cruz ML, Weigensberg MJ, Huang TT, Ball G, Shaibi GQ, Goran MI. The metabolic syndrome in overweight Hispanic youth and the role of insulin sensitivity. J Clin Endocrinol Metab. 2004;89(1):108–113. doi: 10.1210/jc.2003-031188. [DOI] [PubMed] [Google Scholar]
- 25.Vikram NK, Misra A, Pandey RM, Luthra K, Wasir JS, Dhingra V. Heterogeneous phenotypes of insulin resistance and its implications for defining metabolic syndrome in Asian Indian adolescents. Atherosclerosis. 2006;186(1):193–199. doi: 10.1016/j.atherosclerosis.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 26.Whincup PH, Gilg JA, Donald AE, Katterhorn M, Oliver C, Cook DG, Deanfield JE. Arterial distensibility in adolescents: the influence of adiposity, the metabolic syndrome, and classic risk factors. Circulation. 2005;112(12):1789–1797. doi: 10.1161/CIRCULATIONAHA.104.532663. [DOI] [PubMed] [Google Scholar]
- 27.Hickman TB, Briefel RR, Carroll MD, Rifkind BM, Cleeman JI, Maurer KR, Johnson CL. Distributions and trends of serum lipid levels among United States children and adolescents ages 4–19 years: data from the Third National Health and Nutrition Examination Survey. Prev Med. 1998;27(6):879–890. doi: 10.1006/pmed.1998.0376. [DOI] [PubMed] [Google Scholar]
- 28.Behrens JT. Principles and procedures of exploratory data analysis. Psychol Methods. 1997;2(2):131–160. [Google Scholar]
- 29.Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- 30.Schafer JL, Graham JW. Missing data: our view of the state of the art. Psychol Methods. 2002;7(2):147–177. [PubMed] [Google Scholar]
- 31.Allison PD. Missing data. In: Millsap RE, Maydeu-Olivares A, editors. The SAGE Handbook of Quantitative Methods in Psychology. Thousand Oaks, CA: Sage Publications; 2009. pp. 72–89. [Google Scholar]
- 32.Li S, Chen W, Srinivasan SR, Bond MG, Tang R, Urbina EM, Berenson GS. Childhood Cardiovascular Risk Factors and Carotid Vascular Changes in Adulthood: The Bogalusa Heart Study. Journal of the American Medical Association. 2003;290(17):2271–2276. doi: 10.1001/jama.290.17.2271. [DOI] [PubMed] [Google Scholar]
- 33.Morrison JA, Friedman LA, Gray-McGuire C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton lipid research clinics follow-up study. Pediatrics. 2007;120(2):340–345. doi: 10.1542/peds.2006-1699. [DOI] [PubMed] [Google Scholar]
- 34.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic Syndrome in Childhood Predicts Adult Metabolic Syndrome and Type 2 Diabetes Mellitus 25 to 30 Years Later. Journal of Pediatrics. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Huang TTK, Nansel TR, Belsheim AR, Morrison JA. Sensitivity, Specificity, and Predictive Values of Pediatric Metabolic Syndrome Components in Relation to Adult Metabolic Syndrome: The Princeton LRC Follow-up Study. The Journal of Pediatrics. 2008;152(2):185–190.e185. doi: 10.1016/j.jpeds.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Theim KR, Tanofsky-Kraff M, Salaita CG, Haynos AF, Mirch MC, Ranzenhofer LM, Yanovski SZ, Wilfley DE, Yanovski JA. Children’s descriptions of the foods consumed during loss of control eating episodes. Eat Behav. 2007;8(2):258–265. doi: 10.1016/j.eatbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanofsky-Kraff M, McDuffie JR, Yanovski SZ, Kozlosky M, Schvey NA, Shomaker LB, Salaita C, Yanovski JA. Laboratory assessment of the food intake of children and adolescents with loss of control eating. Am J Clin Nutr. 2009;89(3):738–745. doi: 10.3945/ajcn.2008.26886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Farquhar JW, Frank A, Gross RC, Reaven GM. Glucose, insulin, and triglyceride responses to high and low carbohydrate diets in man. J Clin Invest. 1966;45(10):1648–1656. doi: 10.1172/JCI105472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Segall MM, Tamir I, Fosbrooke AS, Lloyd JK, Wolff OH. Effects of short-term high-carbohydrate feeding on serum triglyceride of children with familial hypercholesterolaemia. Arch Dis Child. 1970;45(241):393–398. doi: 10.1136/adc.45.241.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yusuf S, Hawken S, Ounpuu S, Bautista L, Franzosi MG, Commerford P, Lang CC, Rumboldt Z, Onen CL, Lisheng L, et al. Obesity and the risk of myocardial infarction in 27,000 participants from 52 countries: a case-control study. Lancet. 2005;366(9497):1640–1649. doi: 10.1016/S0140-6736(05)67663-5. [DOI] [PubMed] [Google Scholar]
- 41.Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–1095. doi: 10.1016/S0140-6736(11)60105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tanofsky-Kraff M, Goossens L, Eddy KT, Ringham R, Goldschmidt A, Yanovski SZ, Braet C, Marcus MD, Wilfley DE, Olsen C, et al. A multisite investigation of binge eating behaviors in children and adolescents. J Consult Clin Psychol. 2007;75(6):901–913. doi: 10.1037/0022-006X.75.6.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom Med. 2000;62(5):623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Kuo LE, Czarnecka M, Kitlinska JB, Tilan JU, Kvetnansky R, Zukowska Z. Chronic stress, combined with a high-fat/high-sugar diet, shifts sympathetic signaling toward neuropeptide Y and leads to obesity and the metabolic syndrome. Ann N Y Acad Sci. 2008;1148:232–237. doi: 10.1196/annals.1410.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen B, Glueck C, Kwiterovich P, Degroot I, Chase G, Heiss G, Mowery R, Tamir I, Rifkind B. Plasma cholesterol and triglyceride distributions in 13,665 children and adolescents: the Prevalence Study of the Lipid Research Clinics Program. Pediatr Res. 1980;14(3):194–202. doi: 10.1203/00006450-198003000-00004. [DOI] [PubMed] [Google Scholar]
- 46.Casazza K, Dulin-Keita A, Gower BA, Fernandez JR. Intrabdominal fat is related to metabolic risk factors in Hispanic Americans, African Americans and in girls. Acta Paediatr. 2009;98(12):1965–1971. doi: 10.1111/j.1651-2227.2009.01474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanofsky-Kraff M, Morgan CM, Yanovski SZ, Marmarosh C, Wilfley DE, Yanovski JA. Comparison of assessments of children’s eating-disordered behaviors by interview and questionnaire. Int J Eat Disord. 2003;33(2):213–224. doi: 10.1002/eat.10128. [DOI] [PubMed] [Google Scholar]
- 48.Vanhala M. Childhood weight and metabolic syndrome in adults. Ann Med. 1999;31(4):236–239. doi: 10.3109/07853899908995885. [DOI] [PubMed] [Google Scholar]
- 49.Tanofsky-Kraff M, Wilfley DE, Young JF, Mufson L, Yanovski SZ, Glasofer DR, Salaita CG. Preventing excessive weight gain in adolescents: interpersonal psychotherapy for binge eating. Obesity (Silver Spring) 2007;15(6):1345–1355. doi: 10.1038/oby.2007.162. [DOI] [PMC free article] [PubMed] [Google Scholar]