Abstract
Recent findings from our lab have shown that peripheral infection of adult mice with influenza A/PR/8/34 (H1N1) virus induces a neuroinflammatory response that is paralleled by loss of neurotrophic and glial regulatory factors in the hippocampus, and deficits in cognitive function. Environmental enrichment has been shown to exert beneficial effects on the brain and behavior in many central nervous system (CNS) disorders, but its therapeutic potential during peripheral viral infection remains unknown. Therefore, the objective of the present study was to determine if long-term continuous exposure to environmental enrichment could prevent and/or attenuate the negative effects of influenza infection on the hippocampus and spatial cognition. Mice were housed in enriched or standard conditions for 4 months, and continued to live in their respective environments throughout influenza infection. Cognitive function was assessed in a reversal learning version of the Morris water maze, and changes in hippocampal expression of proinflammatory cytokines (IL-1β, IL-6, TNF-α, IFN-α), neurotrophic (BDNF, NGF), and immunomodulatory (CD200, CX3CL1) factors were determined. We found that environmental enrichment reduced neuroinflammation and helped prevent the influenza-induced reduction in hippocampal CD200. These changes were paralleled by improved cognitive performance of enriched mice infected with influenza when compared to infected mice in standard housing conditions. Collectively, these data are the first to demonstrate the positive impact of environmental enrichment on the brain and cognition during peripheral viral infection, and suggest that enhanced modulation of the neuroimmune response may underlie these beneficial effects.
Keywords: influenza, hippocampus, cognition, neuroinflammation, cytokines, microglia, neurotrophins, fractalkine, CD200
Introduction
Influenza is a common and highly contagious viral pathogen that remains a significant health concern worldwide. Neurological symptoms have been reported following respiratory infection with pandemic strains of influenza A (CDC, 2009; Ravenholt and Foege, 1982; Studahl, 2003; Wang et al., 2010), suggesting that peripheral infection can impact central nervous system (CNS) function. While the mechanisms underlying the effects of non-neurotropic viral strains on the brain remain unclear (Schlesinger et al., 1998; Studahl, 2003), it has been hypothesized that glia-mediated neuroinflammation in association with common respiratory viruses may lead to cumulative neuronal damage across the lifespan (Majde, 2010).
Using a mouse model of influenza, we have recently shown that peripheral infection with influenza A/PR8/34 (H1N1) induces a neuroimmune response, and impacts hippocampal structure and function (Jurgens et al., 2012). Infected mice demonstrated cognitive deficits in hippocampal-dependent tasks that were paralleled by significant alterations in hippocampal neuron morphology in the CA1 region and dentate gyrus. Concurrent with cognitive impairment, influenza-infected mice had increased hippocampal expression of inflammatory cytokines (interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α and interferon (IFN)-α), and decreased expression of brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF). Previous work has shown that elevated levels of proinflammatory cytokines in the hippocampus can inhibit neurotrophic factors (Barrientos et al., 2004; Tong et al., 2008) and impair hippocampal synaptic plasticity (Lynch, 2002; Mendoza-Fernandez et al., 2000; Pickering and O'Connor, 2007), providing a potential mechanism by which hippocampal neuroinflammation can induce cognitive dysfunction. In addition, influenza significantly reduced the levels of immunomodulatory factors CD200 and CX3CL1 (fractalkine), which play an important role in the neuronal control of microglia activation (Cardona et al., 2006; Hoek et al., 2000), and have recently been found to impact synaptic development and integrity (Costello et al., 2011; Paolicelli et al., 2011; Ransohoff and Stevens, 2011). Overall, the loss of neurotrophic support along with dysregulated neuron-microglia crosstalk, could negatively impact cognitive function and leave the brain vulnerable to inflammatory damage during influenza infection.
A potential strategy for increasing brain plasticity and resiliency is environmental enrichment. Environmental enrichment (EE) is classically defined as a “complex combination of social and inanimate stimulation” (Rosenzweig and Bennett, 1996), in comparison to standard housing conditions. While there is variation in experimental protocols (Simpson and Kelly, 2011), in general EE refers to conditions that provide increased social, cognitive, and physical stimulation. An enriched environment for rodents typically includes group housing in a large cage with nesting materials, various toys, tunnels and ladders for exploration, and running wheels for voluntary exercise (Nithianantharajah and Hannan, 2006; van Praag et al., 2000). Items of enrichment are routinely changed during the experimental period to maintain novelty and provide continuous opportunity for exploration and learning.
Environmental enrichment has been shown to exert positive effects on cognitive and emotional behaviors including enhanced learning and memory (Bruel-Jungerman et al., 2005; Leggio et al., 2005; Nilsson et al., 1999) and improved ability to cope with fear, anxiety and stress (Fox et al., 2006; Larsson et al., 2002; Schloesser et al., 2010). Early work demonstrated that exposure to a complex and stimulating environment could produce changes in neuron morphology, myelination and synaptogenesis during development, adulthood and aging (Bennett et al., 1969; Diamond et al., 1976; Green et al., 1983; Greenough et al., 1986; Juraska and Kopcik, 1988). This suggests that neural plasticity in response to environmental experience lasts throughout the lifespan. In addition to morphological changes, EE has also been shown to elevate levels of neurotrophic factors (Ickes et al., 2000; Pham et al., 1999) and synaptic proteins (Nithianantharajah et al., 2004; Rampon et al., 2000), as well as enhance hippocampal synaptic plasticity (Artola et al., 2006; Green and Greenough, 1986) and increase neurogenesis (Kempermann et al., 1997; Rossi et al., 2006)
While the beneficial effects of EE have been explored during aging, as well as in animal models of neurodegenerative disease, psychiatric disorders and brain injury (Laviola et al., 2008; Mora et al., 2007; Nithianantharajah and Hannan, 2006; Will et al., 2004), the impact of EE on the central immune response and cognitive behavior during peripheral viral infection remains unknown. Since influenza infection has been shown to negatively impact measures of hippocampal and cognitive function, which are influenced by EE, the present study sought to determine if the deleterious effects of influenza infection on the CNS could be prevented or attenuated by long-term continuous exposure to environmental enrichment. We examined the impact of environmental enrichment on influenza-induced sickness response and cognitive behavior, and assessed changes in inflammatory, neurotrophic, and immunomodulatory factors in the hippocampus. We focused our study on the hippocampus, as this is a brain region that demonstrates a high degree of plasticity, is vulnerable to inflammation, and plays an important role in cognitive function. We hypothesized that continuous exposure to an enriched environment during rearing and throughout influenza infection would help modulate inflammation, preserve levels of neurotrophic and glial regulatory factors, and prevent or reduce cognitive deficits induced by peripheral infection with live influenza virus.
Materials and Methods
Subjects
Young (6 weeks old) male BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME). On arrival, mice were group housed and allowed at least 7 days to acclimate before experimental housing treatments were initiated. Following acclimation, animals were randomly assigned to the enriched environment (EE, n=5–8/cage) or standard environment (SE, n=1/cage) conditions. Briefly, the enriched environment consisted of a large cage (100 × 51 × 37 cm) equipped with toys, tunnels, ladders, housing chambers, nesting material and 2 running wheels (Figure 1). Mice were housed continuously in the enriched environment with rearrangement of toys, tunnels, and wheels 3–4 times per week to maintain novelty. The standard environment consisted of a laboratory polypropylene cage with bedding. All mice were maintained under a reverse 12:12 h light:dark cycle at 24°C and given ad libitum access to food and water. All animal care and experimental procedures are in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and approved by the University of Illinois Institutional Animal Care and Use Committee.
Figure 1.
Enriched environment cage. Environmental enrichment consisted of social interaction (n=5–8 mice in the cage), stimulation of exploratory behavior with objects such as toys and a set of tunnels, shelters, nesting materials, multiple locations of food and water ad libitum, and running wheels for voluntary exercise.
Mouse viral infection
Mouse-adapted human influenza A/Puerto Rico/8/34 (H1N1) virus was a generous gift from Dr. John Sheridan and Dr. David Padgett (The Ohio State University). Mice were lightly anesthetized with isoflurane and intranasally (i.n.) inoculated with 1 HAU (hemagglutinating unit) of influenza A/PR/8/34 virus in 50 µl sterile PBS. Control animals were inoculated (i.n.) with 50 µl of sterile PBS. Use of isoflurane anesthesia allowed for quick recovery (<2min) and accurate delivery of viral dose. Following viral infection, mice were monitored daily for signs of morbidity, including response to handling, food intake, and physical appearance (i.e. ruffled fur, huddled or hunched behavior). In mouse models of influenza, weight loss is considered a reliable indicator of disease progress (Matsuoka et al., 2009), thus body weight was measured at the same time daily (between 09:00–10:00) and percent change from initial body weight (day 0) was calculated. Previous studies (Lowder et al., 2005) have shown that during influenza infection, mice can lose a substantial amount of body weight (30–35%) and still recover, however as an established humane endpoint, mice that reached a 30% loss of their initial body weight were euthanized (day 7 post-inoculation).
Morris water maze
The effects of environmental enrichment and influenza infection on spatial learning and memory were assessed in a reversal learning version of the Morris water maze. In this hippocampal-dependent task, the animal must learn to use distinctive distal visuo-spatial cues surrounding the pool to navigate a direct path to the hidden platform (D'Hooge and De Deyn, 2001; Morris, 1984). The Morris water maze (MWM) consisted of a circular pool (130 cm diameter, 23–25 °C) with a transparent round platform (10 cm diameter) hidden 0.5 cm below the surface of the water. Mice were inoculated (day 0) and 2 days later began a 5-day acquisition phase (days 2–6 post-inoculation). The platform remained in the same location during the acquisition training. Animals were placed on the platform for 30 s preceding the start of each training session. The trials were conducted using a pseudorandom protocol in which mice were placed in the water in one of three preset entry locations. Mice were allowed to swim freely for 60 s or until the platform was reached. If the platform was not located during the 60 s, mice were guided to the platform and allowed to remain for 30 s. After completion of three consecutive trials, mice were placed in their home cage under a heat lamp to dry for 10 min. On day 7 post-inoculation (24 h after the last day of acquisition training), the platform was removed and mice received a 30 s probe trial to assess spatial memory for the platform location. Following the probe trial, mice were subjected to a reversal test in which the hidden platform was moved to the opposite quadrant of the pool, but all distal visual cues remained constant. Animals were placed on the platform for 30 s preceding the start of the reversal test, and given three trials to locate the platform in the new target quadrant. Reversal learning measures how quickly an animal is able to extinguish their initial learning of the platform’s position and acquire a direct path to the new location (Vorhees and Williams, 2006). A video camera in conjunction with a computerized animal tracking system (Ethovision; Noldus Information Technologies) was used to record swim speed, latency to the platform, and distance swam (pathlength to the platform). For acquisition training and reversal testing, distance traveled was averaged across the three consecutive trials administered each day. Performance during the probe trial was measured as percent time in each quadrant location.
Quantitative real-time PCR
After completion of behavioral testing, mice were euthanized by CO2 asphyxiation and blood and tissue samples were quickly collected and stored in RNAlater at −80°C. Total RNA was isolated from homogenized tissue using the Tri Reagent protocol (Sigma, St. Louis, MO). A QuantiTect Reverse Transcription Kit (Qiagen, Valencia, CA) was used for cDNA synthesis with integrated removal of genomic DNA contamination according to the manufacturer protocol. Quantitative real-time PCR was performed using the Applied Biosystems (Foster, CA) Assay-on Demand Gene Expression protocol. In short, cDNA was amplified by PCR where a target cDNA (IL-1β, IL-6, TNF-α, IFN-α, BDNF, NGF, CD200, CX3CL1 and influenza matrix M1) and a reference cDNA (glucose-3 phosphate dehydrogenase) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM) and a 3′ quencher dye (NFQ). PCR reactions were performed at the following conditions: 50 °C for 2 min, 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s, and 60 °C for 1 min. Fluorescence was determined on an ABI PRISM 7900HT-sequence detection system (Perkin Elmer, Forest City, CA). Data were analyzed using the comparative threshold cycle (Ct) method, and results are expressed as fold change (Schmittgen and Livak, 2008). For cytokines, neurotrophic and immunomodulatory factors, results are expressed as fold change from standard-housed control mice. For M1 viral protein expression in the lung, results are expressed as fold change from lung tissue collected at day 1 post-inoculation as MI was non-detectable in control (non-infected) tissue.
Experimental design
Mice remained in their respective housing (SE or EE) continuously for 4 months (7–8 weeks old to 6 months old). At 6 months old, mice were inoculated with influenza A/PR8/34 virus (1 HAU) or sterile PBS. Following inoculation, mice continued to live in their respective environments (SE or EE) throughout the entire post-inoculation period until they were euthanized for tissue collection at day 7 post-inoculation following behavioral testing. For each study, there were 2 enriched environment cages: one EE cage for control mice (n = 5–7/cage) and one EE cage for influenza-infected mice (n = 5–7/cage). For mice housed in the standard environment (SE), there were 6 cages of single-housed control mice, and 6 cages of single-housed influenza-infected mice. Therefore, a 2×2 factorial design was employed in this study: SE, standard environment; EE, enriched environment; CON, control (PBS inoculation); FLU, influenza (1 HAU viral inoculation), thus creating four treatment groups: SE CON, SE FLU, EE CON, and EE FLU. Using identical experimental parameters, two replications were performed, resulting in a final treatment number of n = 10–12 for all treatment groups.
Statistical Analysis
Change in body weight, water maze acquisition, and probe trial data were subjected to repeated measures ANOVA as a two-way design in which housing (standard vs. enriched) and treatment (control vs. influenza) were between-subjects factors. The within-subjects factors were as follows: day post-inoculation (body weight), day of acquisition training (water maze acquisition), and water maze quadrant (probe trial). For expression of influenza M1 in the lungs, Student’s t-test compared the means of SE FLU vs. EE FLU as M1 expression was non-detectable in the lungs of control mice. All other data were analyzed using a 2-way ANOVA in which housing (standard vs. enriched) and treatment (control vs. influenza) were independent variables. When appropriate, differences between treatment means were evaluated with Fisher’s protected least significant difference (PLSD) or Tukey-Kramer’s multiple comparisons tests.
Results
Effects of environmental enrichment on influenza-induced sickness response
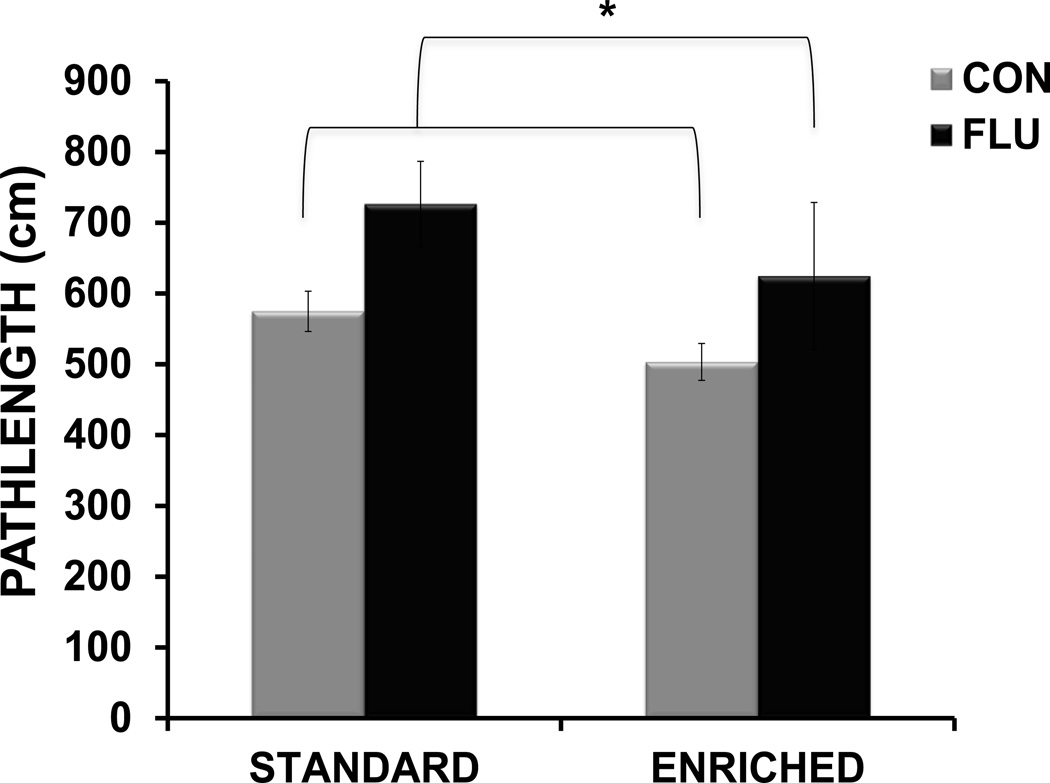
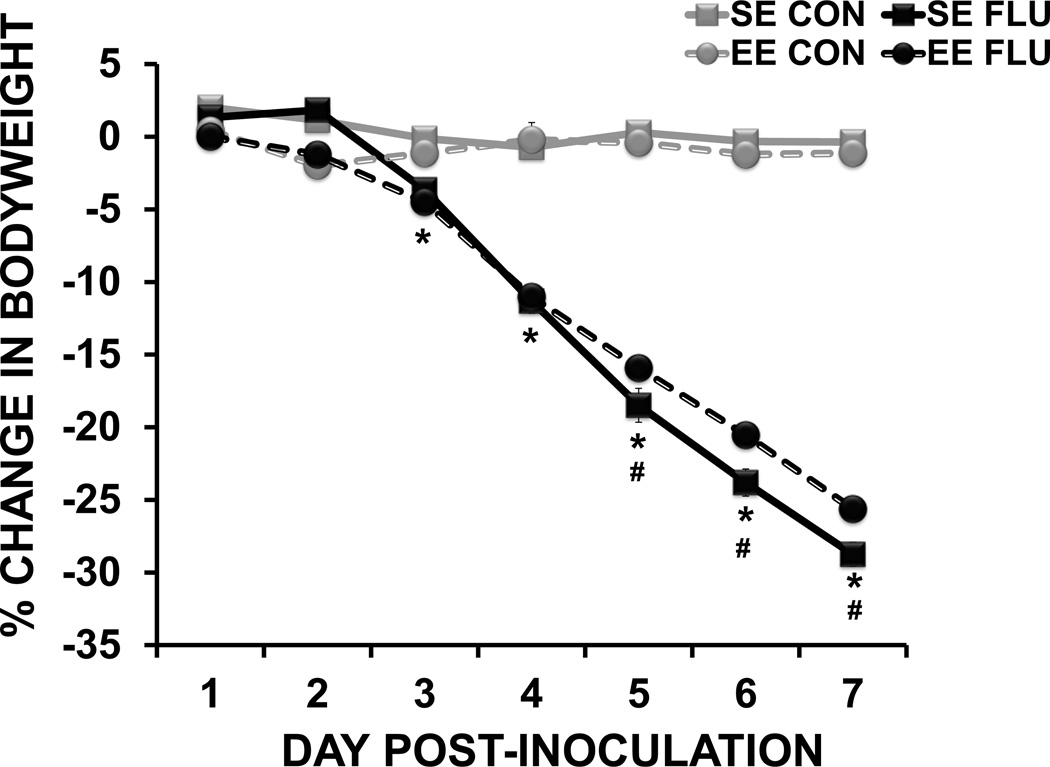
For body weight data, repeated measures ANOVA revealed a day × housing × treatment (F(6,39) = 3.5, p < 0.003) interaction, indicating that influenza differentially affected body weight in SE and EE mice across days post-infection. While all influenza-infected mice lost weight over the course of infection (treatment × day interaction F(6,39) = 457.22, p < 0.0001), post-hoc analysis revealed that SE FLU mice lost more weight than EE FLU mice on days 5–7 (p <0.05 for all), suggesting that environmental enrichment moderately attenuated the sickness response as the infection progressed.
Effects of environmental enrichment on influenza-induced cognitive impairment
In the Morris water maze (MWM), a decrease in time (latency) to find the hidden platform, or a decrease in pathlength (distance) to the platform, indicate an improvement in spatial learning. Since latency to reach the platform can be affected by motor function (swim speed) and motivation, distance swam is considered a more reliable measure of learning and memory performance (Contet et al., 2001; Cunningham and Sanderson, 2008), and therefore was used determine the performance of mice during both acquisition training and reversal testing.
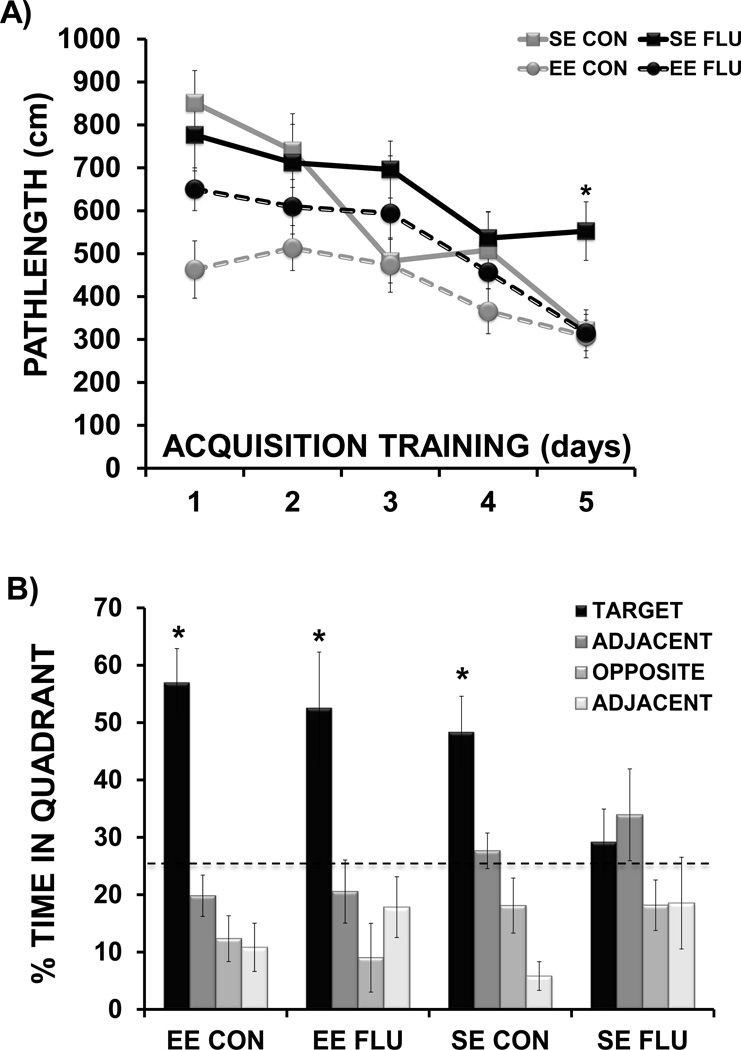
For the water maze acquisition data (Figure 3A), repeated measures ANOVA revealed a significant effect of day (F(4,36) = 15.04, p< 0.0001) indicating that mice in all treatment groups showed improved performance (shorter pathlength) over days of acquisition training. The analysis also showed a main effect of housing (F(1,36) = 14.02, p <0.001), indicating that mice in EE demonstrated superior performance to mice in SE and a main effect of treatment (F(1,36) = 4.26, p <0.05), with influenza infection impairing water maze performance to some extent in both SE and EE mice. The interaction of day × housing × treatment approached significance (F(4,36) = 2.31, p=0.06), suggesting that influenza infection differentially altered the performance of SE versus EE mice over acquisition training. On the last day of training (day 5), there was a significant housing × treatment interaction (F(1,36) = 4.8, p < 0.05), with post-hoc analysis showing that SE FLU mice were impaired compared to all other treatment groups (p<0.01 for all comparisons). Overall the data suggested that enriched mice with influenza infection showed improved acquisition of the task when compared to influenza mice living in standard conditions.
Figure 3.
Morris water maze acquisition and probe trial. (A) Distance to reach the hidden platform over 5 days of acquisition training (* p< 0.01 SE FLU vs. all other groups). (B) Quadrant occupancy during probe trial performed 24 hours after the last day of acquisition training (** p< 0.05 target quadrant vs. non-target quadrants). Dashed line marks chance performance during testing.
For the probe trial (Figure 3B), repeated measures ANOVA was employed to determine the effects of housing and influenza infection across time spent in each quadrant of the water maze. An interaction of housing × quadrant (F(3,32) = 3.38, p<0.05) indicated that quadrant preference during the probe trial differed for enriched and standard housed mice. Within each treatment group, differences in means were evaluated using Tukey-Kramer’s procedure for multiple comparisons. For all treatment groups except SE FLU, there was a clear preference for the target quadrant (p<0.05). Overall these data suggested that environmental enrichment helped prevent the influenza-induced impairment in spatial reference memory as assessed by probe trial performance.
For reversal testing data (Figure 4), a 2-way ANOVA revealed a main effect of treatment (F(1,30) = 6.03, p <0.05), but no effect of housing or housing × treatment interaction, indicating that influenza infection impaired reversal learning (as indicated by increased pathlength to the new platform location) in mice from both standard and enriched environments.
Figure 4.
Morris water maze reversal testing. Distance (pathlength) to reach the platform during reversal testing (average distance for 3 trial), * p< 0.05 influenza vs. control.
Effects of environmental enrichment and influenza on hippocampal cytokines
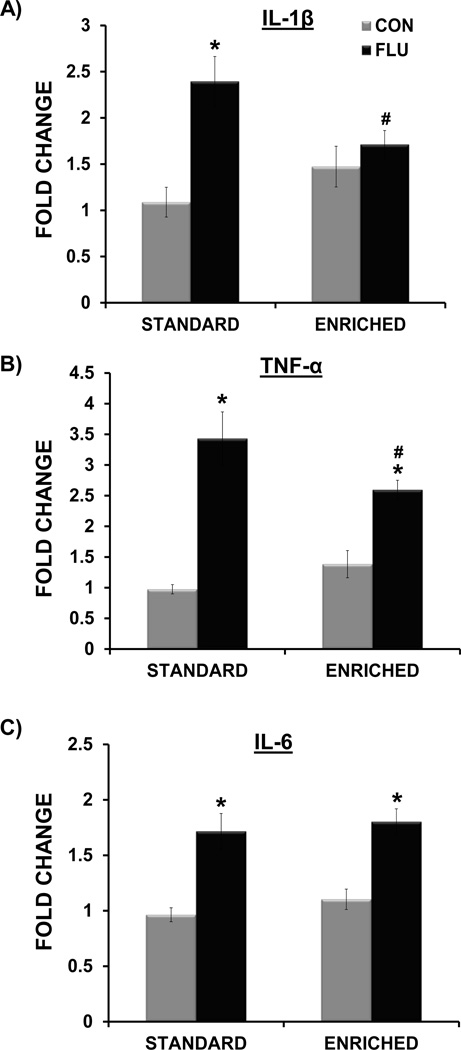
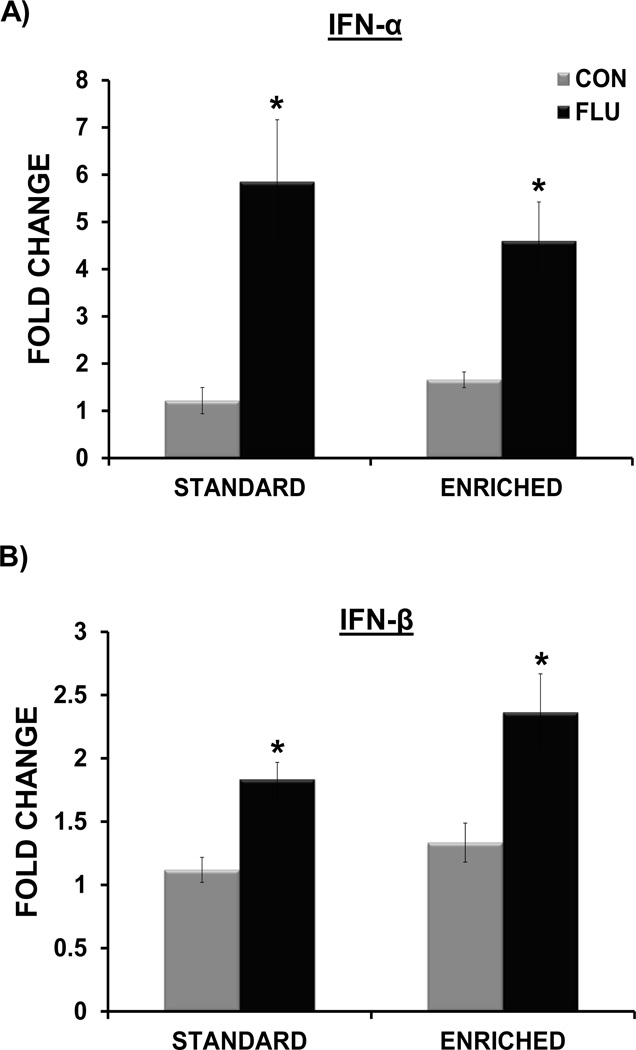
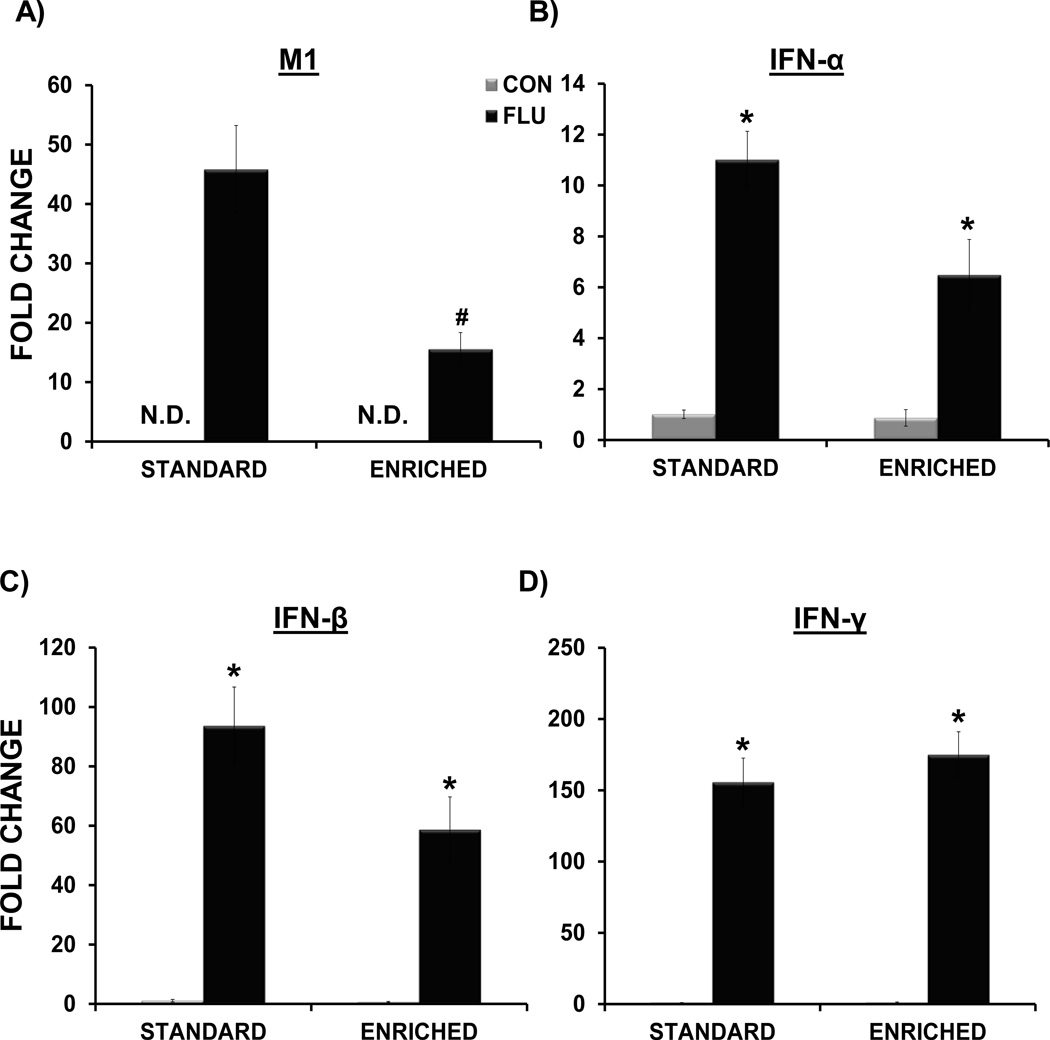
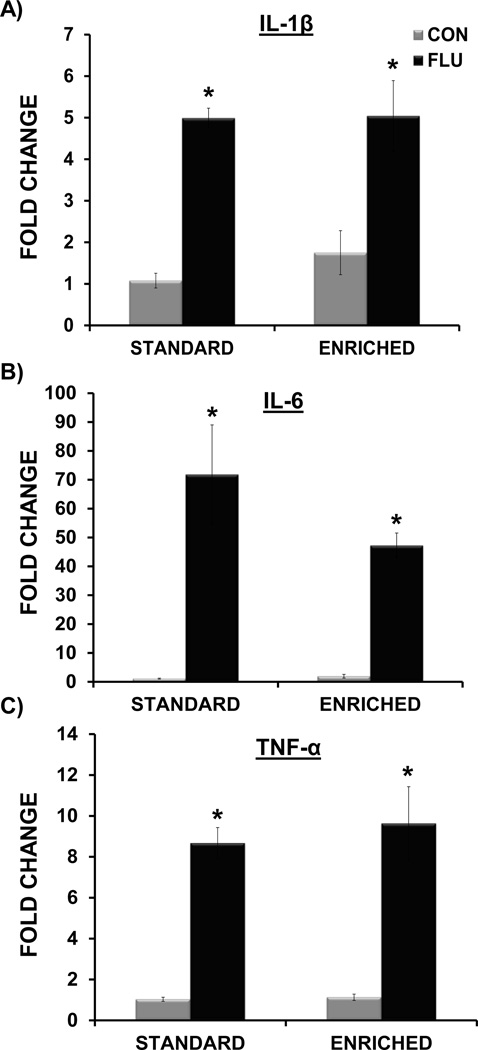
Overall, the analysis showed that there was no main effect of housing (standard vs. enriched) on the expression of IL-1β, IL-6, TNF-α, IFN-α, or IFN-β (p > 0.05 for all), indicating that basal levels of hippocampal cytokines were not affected by living condition (Figures 5 and 6). A housing × treatment interaction for both IL-1β (F(1,35) = 6.88, p <0.02) and TNF-α (F(1,34) = 5.89, p < 0.03) indicated that expression of these proinflammatory cytokines following influenza infection was altered by environmental conditions (Figure 5A,B). Post-hoc tests revealed that influenza-infected mice living in enrichment has significantly attenuated expression of both IL-1β (p<0.05) and TNF-α (p<0.05) compared to standard housed infected mice. A significant main effect of treatment for IL-6 (F(1,34) = 42.95, p < 0.0001), IFN-α (F(1,25) = 24.4, p < 0.0001), and IFN-β (F(1,36) = 25.35, p < 0.0001) but no interaction between housing and treatment, indicated that influenza increased the expression of these cytokines to a similar extent in standard housed and enriched mice (Figure 5C, Figure 6A,B).
Figure 5.
Impact of environment and influenza infection on hippocampal proinflammatory cytokine expression at day 7 post-inoculation. Environmental enrichment attenuated the influenza-induced increased in IL-1β (A) and TNF-α (B). Influenza infection induced IL-6 in both standard and enriched mice (C). * p< 0.05 compared to control, # p< 0.05 SE FLU vs. EE FLU.
Figure 6.
Impact of environmental enrichment and influenza infection on hippocampal interferon cytokine expression at day 7 post-inoculation. Influenza infection induced IFN-α (A) and IFN-β (B) in both standard and enriched mice (* p< 0.05 compared to control).
Effects of environmental enrichment and influenza on hippocampal neurotrophic and immunomodulatory factors
Neurotrophins
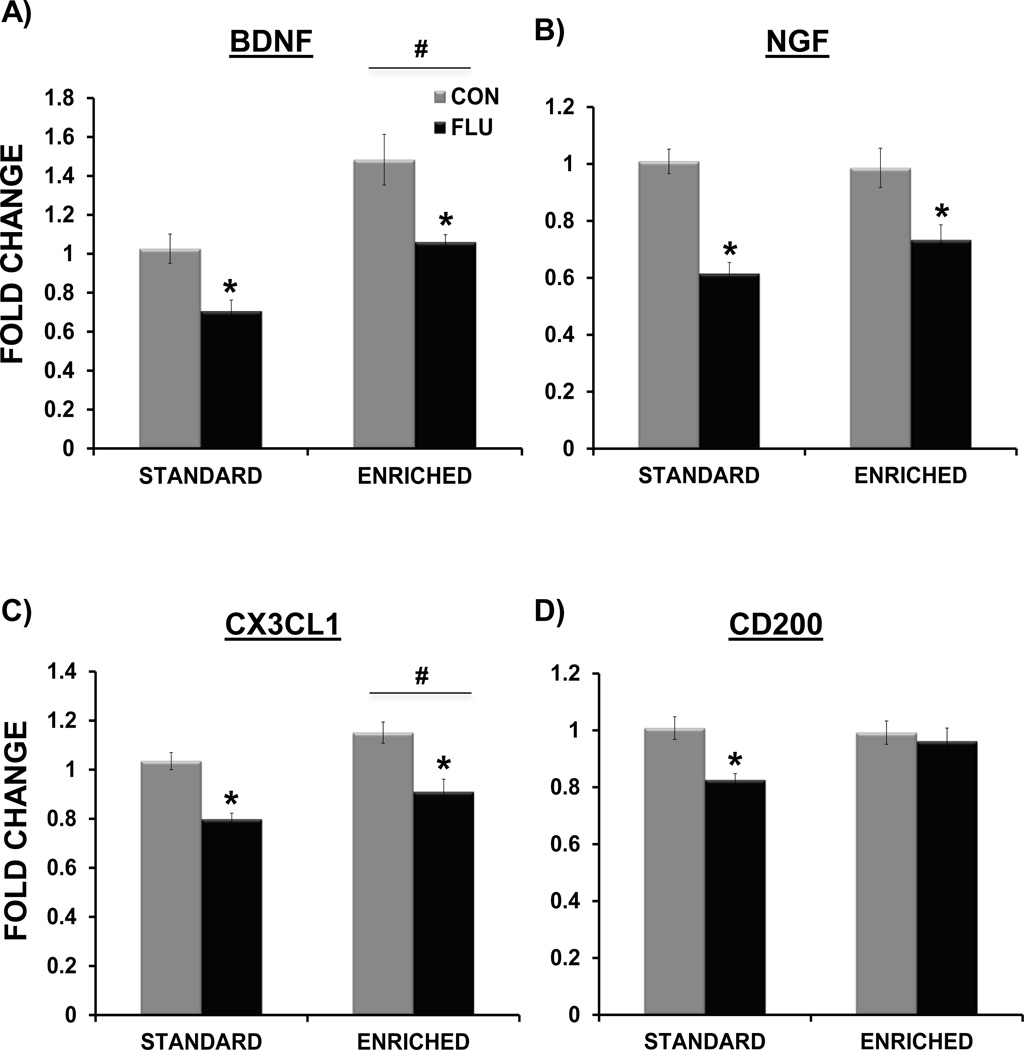
For BDNF expression (Figure 7A), there was a main effect of housing (F(1,35) = 22.16, p<0.0001) with enriched animals having increased hippocampal BDNF expression, and a main effect of treatment (F(1,35) = 18.54, p < 0.0001) indicating that influenza decreased BDNF in both enriched and standard housed mice. For NGF expression (Figure 7B), there was a main effect of treatment (F(1,35) = 38.04, p<0.0001), indicating that influenza decreased NGF in both standard housed and enriched mice. There was no main effect of housing on NGF expression (p>0.05), indicating that unlike BDNF, environmental enrichment did not increase NGF expression in the hippocampus.
Figure 7.
Impact of environmental enrichment and influenza infection on hippocampal neurotrophic and immunomodulatory factor expression at day 7 post-inoculation. Environmental enrichment increased BDNF (A) and CX3CL1 expression (C). Influenza infection decreased the expression of BDNF, NGF and CX3CL1 in both enriched and standard housed mice (A,B,C). Environmental enrichment attenuated the influenza-induced decrease in CD200 (D). * p< 0.05 compared to control, # p< 0.05 standard vs. enriched housing.
Immunomodulatory factors
For CX3CL1 (Figure 7C), a main effect of treatment (F(1,37 = 35.83, p<0.0001), indicated that influenza reduced CX3CL1 expression in mice from both environments. Interestingly, there was a main effect of housing (F(1,37) = 8.19, p<0.05), in which environmental enrichment increased hippocampal CX3CL1 expression. For CD200 (Figure 7D), the interaction of housing × treatment approached significance (F(1,37) = 3.65, p=0.06), with a main effect of treatment (F(1,37) = 7.04, p<0.05) being driven by the decrease in CD200 expression in SE FLU mice. Post-hoc tests using Tukey-Kramer’s procedure for multiple comparisons revealed that while SE FLU mice had significantly decreased CD200 compared to controls (p<0.05), expression of CD200 in the hippocampus of EE FLU mice was maintained at control levels (p>0.05).
Effects of environmental enrichment on influenza viral infection and cytokine levels in the lungs
The data analysis indicated that on day 7 post-inoculation, influenza virus M1 expression was attenuated in the lungs of EE FLU mice when compared to SE FLU mice (p<0.01) (Figure 8A). Influenza M1 gene expression was non-detectable (ND) in the lungs of control mice. In addition, similar to our previous findings (Jurgens et al., 2012), there was no evidence of M1 gene expression in the hippocampus of control or infected mice (data not shown). A main effect of treatment for IL-1β (F(1,16)= 50.45, p<.0.0001), IL-6 (F(1,17) = 45.12, p < 0.0001), TNF-α (F(1,17) = 63.98, p<.0.0001), IFN-α (F(1,11) = 31.64, p < 0.0005), and IFN-β (F(1,16) = 62.59, p<.0.0001) indicated that influenza infection increased the expression antiviral and inflammatory cytokines in the lungs of both EE FLU and SE FLU groups (Figures 8 and 9).
Figure 8.
Impact of environmental enrichment and influenza infection on expression of influenza virus and cytokines in the lung at day 7 post-inoculation. (A) Environmental enrichment attenuated the expression of influenza matrix (MI) protein. M1 expression was non-detectable (N.D.) in control samples. Influenza infection induced IFN-α (B) and IFN-β (C) and IFN-γ (D) in both standard and enriched mice. * p< 0.05 compared to control, # p< 0.05 SE FLU vs. EE FLU.
Figure 9.
Impact of environmental enrichment and influenza on expression of inflammatory cytokines in the lung at day 7 post-inoculation. Influenza infection induced IL-1β (A), IL-6 (B) and TNF-α (C) in both standard and enriched mice. * p< 0.05 compared to control.
Discussion
While the effects of environmental enrichment (EE) on the brain and behavior have been explored in healthy animals and in models of neuropsychiatric disease and neurodegeneration (Laviola et al., 2008; van Praag et al., 2000), its potential therapeutic role during infectious disease remains less understood. In the present study, we determined if long-term exposure to EE would have beneficial effects on the brain and behavior in a mouse model of influenza infection. We found that environmental enrichment helped attenuate the sickness response and cognitive dysfunction associated with influenza infection. In addition, environmental enrichment buffered the influenza-induced alterations in hippocampal expression of inflammatory and immunomodulatory factors. These novel findings suggest that a non-invasive, non-pharmacological intervention such as environmental enrichment may help ameliorate the negative cognitive effects of peripheral infection. In regard to central function, this may be due to increased resiliency of the enriched brain to neuroinflammation, and/or the preservation of neuroimmune communication between neurons and microglia.
Environmental enrichment attenuates influenza-induced cognitive deficits
Overall, the data indicated the EE was moderately protective against influenza-induced deficits in hippocampal-dependent spatial learning and memory. Enriched influenza-infected mice showed improved performance during both acquisition training and the probe trial when compared to infected mice in standard housing conditions. Influenza-infected mice from both housing conditions were impaired during reversal testing, suggesting that when task difficulty is increased, or cognitive flexibility is needed, influenza-induced deficits are revealed. While hippocampal dysfunction can impede spatial reversal learning (Whishaw and Tomie, 1997), the frontal cortex plays an important role in the behavioral flexibility associated with reversal tasks (Kesner and Churchwell, 2011), and additional studies to determine the impact of influenza infection on this region are warranted.
In our previous study (Jurgens et al., 2012), we also found deficits during reversal learning in influenza infected mice, but did not find influenza-induced impairment during acquisition or the probe trial. This difference is likely attributed to the use of a larger maze in the present study. Since it has been shown that enriched mice demonstrate superior performance in the water maze (van Praag et al., 2000), a larger maze was utilized in the present study to prevent a “ceiling effect” from interfering with the detection of environmental or influenza-induced alterations in spatial learning and memory. Previous work has demonstrated that maze diameter significantly affects the acquisition and retention of spatial information in the water maze, and mice that perform well in smaller mazes, can demonstrate cognitive impairment when a larger maze diameter is employed (Van Dam et al., 2006; Vorhees and Williams, 2006). Overall, the results suggest that influenza-induced cognitive impairments are revealed when the difficulty of the task is increased, either by the use of larger maze or during reversal testing. Most importantly, influenza-infected EE mice demonstrated performance comparable to non-infected mice by the end of acquisition training and during the probe trial, suggesting that environmental enrichment may help to buffer the deleterious effects of influenza infection on hippocampal-dependent cognitive behavior.
Environmental enrichment attenuates influenza-induced hippocampal neuroinflammation
A potential contributing factor to cognitive detriment during influenza infection is hippocampal inflammation. While the central cytokine response to peripheral infection is essential for mounting an appropriate immune response and inducing adaptive sickness behaviors (Hart, 1988; Konsman et al., 2002), elevated or prolonged exposure to inflammatory mediators can have detrimental effects on cognitive and emotional function (McAfoose and Baune, 2009; Yirmiya and Goshen, 2011). Similar to our previous findings, influenza infection increased expression of IL-1β, IL-6, TNF-α and IFN-α in the hippocampus of standard housed mice. Most interestingly, environmental enrichment helped attenuate the influenza-induced increase in both IL-1β and TNF-α expression. As IL-1β in particular is known to specifically impact hippocampal-dependent tasks (Goshen et al., 2007; Hein et al., 2010), the EE-induced modulation IL-1β expression could help preserve cognitive function during influenza infection. Additional neuroprotective effects of EE could be afforded by the attenuation of the influenza-induced increase in hippocampal TNF-α, which can impact synaptic plasticity, and at increased levels contribute to neurodegeneration (McCoy and Tansey, 2008; Pickering and O'Connor, 2007). As enrichment only partially attenuated IL-1β and TNF-α, and did not prevent the increase in IL-6, IFN-α or IFN-β, which have also been shown to impact hippocampal function and cognition (Beyer et al., 2009; Sas et al., 2009; Sparkman et al., 2006), this may help explain why enriched mice only showed a partial rescue from cognitive deficits induced by influenza infection.
Effects of environmental enrichment on hippocampal expression of neurotrophic and immunoregulatory factors during influenza infection
As substantial evidence suggests that neurotrophic factors play an important role in learning and memory and hippocampal plasticity (Tyler et al., 2002; Waterhouse and Xu, 2009), the influenza-induced reduction in hippocampal BDNF could have deleterious effects on cognitive function during influenza infection. While the enrichment paradigm increased basal levels of BDNF in the hippocampus, the expression of neurotrophic factors was blunted across housing conditions during influenza infection. This loss of neurotrophic support could help explain why even though enrichment improved cognitive function, deficits were still apparent during reversal testing in infected mice from both housing conditions. As recent work has shown that even low levels of neurotrophic overexpression in the hippocampus can attenuate epileptogenic neuroinflammation and prevent IL-1β expression (Bovolenta et al., 2010), the higher “reserve” levels of BDNF in the hippocampus of enriched mice could provide neuroprotection and modulate inflammation upon immune challenge.
In addition to increasing BDNF, we also found that enrichment increased the expression of CX3CL1 (fractalkine), a chemokine constitutively expressed in the brain, that exerts immunomodulatory and anti-inflammatory effects during acute and chronic neuroinflammation (Cardona et al., 2006; Harrison et al., 1998; Lyons et al., 2009; Zujovic et al., 2001). While well-known for its role in immune function, recent evidence suggests that disruption of CX3CL1 signaling can inhibit neurogenesis (Bachstetter et al., 2011), impair microglia-mediated synaptic pruning in the hippocampus (Paolicelli et al., 2011), and prevent the enhancement of neuronal plasticity and spatial cognition induced by environmental enrichment (Maggi et al., 2011), suggesting it may play an important role in hippocampal function. The loss of CX3CL1 during influenza infection could contribute to changes in both glial regulation and cognitive function. While enrichment was not effective in preventing the influenza-induced decrease in CX3CL1, it is possible that the prior EE-induced increase CX3CL1 could set the stage for constrained induction of neuroinflammation and better maintenance of neuronal structural and synaptic plasticity during viral infection.
The ability of EE to attenuate the influenza-induced decrease in CD200 could be an important factor in maintaining essential neuron-microglia interactions during influenza infection. While it is known that CD200 is important for keeping microglia in a resting state in both the healthy and diseased brain (Chitnis et al., 2007; Hoek et al., 2000), recent evidence suggests that constitutive expression of CD200 is essential for normal hippocampal plasticity and resiliency from inflammatory stimuli (Costello et al., 2011). In addition, it was recently shown that hippocampal dendrites express high levels of CD200, suggesting a role for this immune mediator in glial-synaptic interactions (Ojo et al., 2011). As we have previously shown that influenza infection induces hippocampal dendritic atrophy (Jurgens et al., 2012), this could contribute to decreased expression of CD200 in the hippocampus of standard housed infected mice. While future work is needed to determine the influence of EE on neuronal architecture during influenza infection, it is possible that enrichment could reduce neuronal atrophy and help preserve CD200 levels in the hippocampus of influenza-infected mice. Overall, the maintenance of physiological levels of CD200 in the hippocampus of enriched mice during influenza infection may reduce neuronal vulnerability to inflammation and help maintain neuron-glia interactions important in synaptic function.
Effect of environmental enrichment on viral infection and immune response in the lungs
While environmental enrichment reduced proinflammatory cytokine production in the brain during influenza infection, there was no effect of EE on the expression of inflammatory or anti-viral cytokine production in the lungs of infected mice. However, enrichment did reduce influenza viral load (as measured by influenza M1 expression in the lungs), and attenuate the loss of body weight over the course of infection, suggesting that enriched mice were better able to cope with the viral infection. While not addressed in the current study, it has been shown that EE can alter NK and T cell activity (Benaroya-Milshtein et al., 2004; Marashi et al., 2004) which could influence the immune response during infection, as these cell populations play a vital role in the control of influenza viral infection (Culley, 2009; Kim et al., 2011).
In addition, previous work has demonstrated that moderate exercise following infection with influenza virus reduced lung cellular infiltrate and pathology, and increased survival in adult mice (Lowder et al., 2006). Similar to our findings, these studies found there to be no reduction in lung IL1-β or TNF-α expression, but interferon levels were reduced, and there was tendency toward reduction of influenza M1 expression at day 3 and 5 post-infection. Taken together, these findings suggest it is likely that the effects of increased physical activity afford some portion of the beneficial impact of EE during influenza infection. Indeed, exercise has been shown to modulate both the peripheral and central immune response (Packer et al., 2010; Walsh et al., 2011), as well as exert positive effects on brain morphology, plasticity, and cognition (Cotman et al., 2007; van Praag, 2009). Furthermore, a recent study found exercise to be the main factor mediating increased BDNF levels and hippocampal neurogenesis in the hippocampus of environmentally enriched mice (Kobilo et al., 2011). While additional studies could determine the extent to which individual components of enrichment (i.e. physical activity, social or cognitive stimulation) effect the peripheral versus central immune response to influenza infection, evidence suggests that at least in terms of the brain, the greatest benefits are gained from additive or synergistic effects of the full repertoire of environmental enrichment (Faherty et al., 2003; Leggio et al., 2005; Sozda et al., 2010). It is also important to point out that while the majority of studies investigating the neural effects of EE use single-housed animals as the control group (Simpson and Kelly, 2011), there is evidence that paradigms of “social isolation” can induce adverse immune and behavioral effects (Lu et al., 1999; Voikar et al., 2005). Further studies are warranted to investigate the effects of individual versus social housing on neuroimmune and cognitive parameters during influenza infection. While EE may provide resiliency toward influenza infection, the possibility that isolation could potentiate the effects of peripheral viral infection is an important and interesting question that requires further exploration.
The impact of environmental enrichment on the neuroimmune response
While the beneficial effects of environmental enrichment have primarily focused on changes in neuronal morphology, synaptic plasticity, and neurogenesis, EE also induces changes in non-neuronal components including cerebrovasculature, oligodendrocytes, and astrocytes (Markham and Greenough, 2004; Soffie et al., 1999; Viola et al., 2009). While less is known regarding the effects of EE on microglia, evidence suggests that they are also responsive to physical and cognitive stimulation. Previous work has found that enriched adult rats had increased numbers of microglia in the dentate gyrus that displayed a phenotype associated with neuroprotection and enhanced neurogenesis (Ziv et al., 2006). In a model of neurodegeneration, EE was shown to shift microglia from a neurotoxic to phagocytic phenotype that was associated with reduced amyloid burden, upregulation of anti-inflammatory factors, and improvements in learning and memory (Dong et al., 2007). Overall these data suggest that environmental enrichment can promote a microglia phenotype associated with reduced inflammation and increased neuroprotection in both health and disease.
While these exciting findings suggest that microglia are sensitive to enriched conditions, there is very little data regarding the impact of EE on the central immune response to infectious disease. Previous work has investigated the effects of EE in models of direct CNS infection, and while one study found no benefit on neurogenesis or spatial learning when implemented after infection with bacterial meningitis (Tauber et al., 2009), another study found mice reared in EE to have less viral neuroinvasion, reduced microglial activation, and improved behavioral outcome in a model of neurotropic viral encephalitis (de Sousa et al., 2011). In addition, it was recently shown that exposure to EE altered glial phenotype and attenuated the proinflammatory response in the hippocampus of rats injected peripherally LPS (Williamson et al., 2012). Overall these data provide evidence that the effects of immune activation on the brain and behavior are shaped by environmental conditions.
To our knowledge the present study is the first to implement EE during peripheral viral infection thus extending the beneficial effects of environmental enrichment on CNS function to a highly pathogenic and clinically relevant respiratory disease. The ability of enriched mice to better modulate the brain’s response to peripheral infection could contribute to more efficient maintenance of CNS homeostasis during influenza infection, thus allowing for improved preservation of cognitive function. The protective effects of EE during influenza infection may be related to the development of brain and cognitive reserve, in which a physically and mentally active lifestyle promotes brain resiliency through more efficient and flexible networks that are less susceptible to disruption induced by injury or neurodegeneration (Petrosini et al., 2009). While environmental enrichment itself is a simple, non-invasive therapeutic that can provide resiliency and reserve during brain insult, injury or inflammation, the elucidation of the mechanisms underlying the positive effects of environmental enrichment could help in the implementation of more specific interventions aimed at protecting neural function during peripheral infection.
Figure 2.
Change in body weight following influenza infection. Influenza infection induced loss of body weight in both standard environment (SE) and enriched environment (EE) influenza mice. Influenza-infected mice in SE lost more body weight at day 5–7 post-inoculation than infected EE mice. Data are represented as means ± SEM. (*p< 0.05 compared to control, # p< 0.05 SE FLU vs. EE FLU mice).
Research Highlight.
Environmental enrichment reduces hippocampal neuroinflammation and helps to preserve cognitive function during peripheral influenza viral infection.
Acknowledgements
This research was supported by National Institutes of Health grant AG016710 (R.W.J.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Artola A, von Frijtag JC, Fermont PCJ, Gispen WH, Schrama LH, Kamal A, Spruijt BM. Long-lasting modulation of the induction of LTD and LTP in rat hippocampal CA1 by behavioural stress and environmental enrichment. European Journal of Neuroscience. 2006;23:261–272. doi: 10.1111/j.1460-9568.2005.04552.x. [DOI] [PubMed] [Google Scholar]
- Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiology of aging. 2011;32:2030–2044. doi: 10.1016/j.neurobiolaging.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Sprunger DB, Campeau S, Watkins LR, Rudy JW, Maier SF. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Benaroya-Milshtein N, Hollander N, Apter A, Kukulansky T, Raz N, Wilf A, Yaniv I, Pick CG. Environmental enrichment in mice decreases anxiety, attenuates stress responses and enhances natural killer cell activity. The European journal of neuroscience. 2004;20:1341–1347. doi: 10.1111/j.1460-9568.2004.03587.x. [DOI] [PubMed] [Google Scholar]
- Bennett EL, Rosenzwe Mr, Diamond MC. Rat Brain - Effects of Environmental Enrichment on Wet and Dry Weights. Science. 1969;163 doi: 10.1126/science.163.3869.825. 825&. [DOI] [PubMed] [Google Scholar]
- Beyer S, Raether G, Stadler K, Hoffrogge R, Scharf C, Rolfs A, Mix E, Strauss U. Interferon-beta modulates protein synthesis in the central nervous system. J Neuroimmunol. 2009;213:31–38. doi: 10.1016/j.jneuroim.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Bovolenta R, Zucchini S, Paradiso B, Rodi D, Merigo F, Navarro Mora G, Osculati F, Berto E, Marconi P, Marzola A, Fabene PF, Simonato M. Hippocampal FGF-2 and BDNF overexpression attenuates epileptogenesis-associated neuroinflammation and reduces spontaneous recurrent seizures. J Neuroinflammation. 2010;7:81. doi: 10.1186/1742-2094-7-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruel-Jungerman E, Laroche S, Rampon C. New neurons in the dentate gyrus are involved in the expression of enhanced long-term memory following environmental enrichment. European Journal of Neuroscience. 2005;21:513–521. doi: 10.1111/j.1460-9568.2005.03875.x. [DOI] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- CDC. Neurologic complications associated with novel influenza A (H1N1) virus infection in children - Dallas, Texas, May 2009. MMWR Morb Mortal Wkly Rep. 2009;58:773–778. [PubMed] [Google Scholar]
- Chitnis T, Imitola J, Wang Y, Elyaman W, Chawla P, Sharuk M, Raddassi K, Bronson RT, Khoury SJ. Elevated neuronal expression of CD200 protects Wlds mice from inflammation-mediated neurodegeneration. Am J Pathol. 2007;170:1695–1712. doi: 10.2353/ajpath.2007.060677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contet C, Rawlins JN, Bannerman DM. Faster is not surer--a comparison of C57BL/6J and 129S2/Sv mouse strains in the watermaze. Behavioural brain research. 2001;125:261–267. doi: 10.1016/s0166-4328(01)00295-9. [DOI] [PubMed] [Google Scholar]
- Costello DA, Lyons A, Browne T, Denieffe S, Cox FF, Lynch MA. Long-term potentiation is impaired in CD200-deficient mice: a role for Toll-like receptor activation. The Journal of biological chemistry. 2011 doi: 10.1074/jbc.M111.280826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in neurosciences. 2007;30:464–472. doi: 10.1016/j.tins.2007.06.011. [DOI] [PubMed] [Google Scholar]
- Culley FJ. Natural killer cells in infection and inflammation of the lung. Immunology. 2009;128:151–163. doi: 10.1111/j.1365-2567.2009.03167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham C, Sanderson DJ. Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory. Brain, behavior, and immunity. 2008;22:1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res Brain Res Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- de Sousa AA, Reis R, Bento-Torres J, Trevia N, Lins NA, Passos A, Santos Z, Diniz JA, Vasconcelos PF, Cunningham C, Perry VH, Diniz CW. Influence of enriched environment on viral encephalitis outcomes: behavioral and neuropathological changes in albino Swiss mice. PLoS One. 2011;6:e15597. doi: 10.1371/journal.pone.0015597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond MC, Ingham CA, Johnson RE, Bennett EL, Rosenzweig MR. Effects of Environment on Morphology of Rat Cerebral-Cortex and Hippocampus. J Neurobiol. 1976;7:75–85. doi: 10.1002/neu.480070108. [DOI] [PubMed] [Google Scholar]
- Dong S, Li C, Wu P, Tsien JZ, Hu Y. Environment enrichment rescues the neurodegenerative phenotypes in presenilins-deficient mice. The European journal of neuroscience. 2007;26:101–112. doi: 10.1111/j.1460-9568.2007.05641.x. [DOI] [PubMed] [Google Scholar]
- Faherty CJ, Kerley D, Smeyne RJ. A Golgi-Cox morphological analysis of neuronal changes induced by environmental enrichment. Brain Res Dev Brain Res. 2003;141:55–61. doi: 10.1016/s0165-3806(02)00642-9. [DOI] [PubMed] [Google Scholar]
- Fox C, Merali Z, Harrison C. Therapeutic and protective effect of environmental enrichment against psychogenic and neurogenic stress. Behavioural brain research. 2006;175:1–8. doi: 10.1016/j.bbr.2006.08.016. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, Levy-Lahad E, Yirmiya R. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32:1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Green EJ, Greenough WT. Altered Synaptic Transmission in Dentate Gyrus of Rats Reared in Complex Environments - Evidence from Hippocampal Slices Maintained Invitro. J Neurophysiol. 1986;55:739–750. doi: 10.1152/jn.1986.55.4.739. [DOI] [PubMed] [Google Scholar]
- Green EJ, Greenough WT, Schlumpf BE. Effects of Complex or Isolated Environments on Cortical Dendrites of Middle-Aged Rats. Brain Research. 1983;264:233–240. doi: 10.1016/0006-8993(83)90821-1. [DOI] [PubMed] [Google Scholar]
- Greenough WT, Mcdonald JW, Parnisari RM, Camel JE. Environmental-Conditions Modulate Degeneration and New Dendrite Growth in Cerebellum of Senescent Rats. Brain Research. 1986;380:136–143. doi: 10.1016/0006-8993(86)91437-x. [DOI] [PubMed] [Google Scholar]
- Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, Costa AC, O'Banion MK. Sustained hippocampal IL-1beta overexpression impairs contextual and spatial memory in transgenic mice. Brain, behavior, and immunity. 2010;24:243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- Ickes BR, Pham TM, Sanders LA, Albeck DS, Mohammed AH, Granholm AC. Long-term environmental enrichment leads to regional increases in neurotrophin levels in rat brain. Experimental Neurology. 2000;164:45–52. doi: 10.1006/exnr.2000.7415. [DOI] [PubMed] [Google Scholar]
- Juraska JM, Kopcik JR. Sex and Environmental-Influences on the Size and Ultrastructure of the Rat Corpus-Callosum. Brain Research. 1988;450:1–8. doi: 10.1016/0006-8993(88)91538-7. [DOI] [PubMed] [Google Scholar]
- Jurgens HA, Amancherla K, Johnson RW. Influenza infection induces neuroinflammation, alters hippocampal neuron morphology, and impairs cognition in adult mice. J Neurosci. 2012;32:3958–3968. doi: 10.1523/JNEUROSCI.6389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiology of learning and memory. 2011 doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Kim TS, Sun J, Braciale TJ. T cell responses during influenza infection: getting and keeping control. Trends in immunology. 2011;32:225–231. doi: 10.1016/j.it.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learn Mem. 2011;18:605–609. doi: 10.1101/lm.2283011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konsman JP, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends in neurosciences. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Larsson F, Winblad B, Mohammed AH. Psychological stress and environmental adaptation in enriched vs. impoverished housed rats. Pharmacol Biochem Be. 2002;73:193–207. doi: 10.1016/s0091-3057(02)00782-7. [DOI] [PubMed] [Google Scholar]
- Laviola G, Hannan AJ, Macri S, Solinas M, Jaber M. Effects of enriched environment on animal models of neurodegenerative diseases and psychiatric disorders. Neurobiology of disease. 2008;31:159–168. doi: 10.1016/j.nbd.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Mandolesi L, Federico F, Spirito F, Ricci B, Gelfo F, Petrosini L. Environmental enrichment promotes improved spatial abilities and enhanced dendritic growth in the rat. Behavioural Brain Research. 2005;163:78–90. doi: 10.1016/j.bbr.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Lowder T, Padgett DA, Woods JA. Moderate exercise protects mice from death due to influenza virus. Brain Behav Immun. 2005;19:377–380. doi: 10.1016/j.bbi.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Lowder T, Padgett DA, Woods JA. Moderate exercise early after influenza virus infection reduces the Th1 inflammatory response in lungs of mice. Exerc Immunol Rev. 2006;12:97–111. [PubMed] [Google Scholar]
- Lu ZW, Hayley S, Ravindran AV, Merali Z, Anisman H. Influence of psychosocial, psychogenic and neurogenic stressors on several aspects of immune functioning in mice. Stress. 1999;3:55–70. doi: 10.3109/10253899909001112. [DOI] [PubMed] [Google Scholar]
- Lynch MA. Interleukin-1 beta exerts a myriad of effects in the brain and in particular in the hippocampus: analysis of some of these actions. Vitam Horm. 2002;64:185–219. doi: 10.1016/s0083-6729(02)64006-3. [DOI] [PubMed] [Google Scholar]
- Lyons A, Lynch AM, Downer EJ, Hanley R, O'Sullivan JB, Smith A, Lynch MA. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J Neurochem. 2009;110:1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- Maggi L, Scianni M, Branchi I, D'Andrea I, Lauro C, Limatola C. CX(3)CR1 deficiency alters hippocampal-dependent plasticity phenomena blunting the effects of enriched environment. Front Cell Neurosci. 2011;5:22. doi: 10.3389/fncel.2011.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majde JA. Neuroinflammation resulting from covert brain invasion by common viruses - a potential role in local and global neurodegeneration. Medical hypotheses. 2010;75:204–213. doi: 10.1016/j.mehy.2010.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marashi V, Barnekow A, Sachser N. Effects of environmental enrichment on males of a docile inbred strain of mice. Physiology & behavior. 2004;82:765–776. doi: 10.1016/j.physbeh.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron glia biology. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka Y, Lamirande EW, Subbarao K. The mouse model for influenza. Chapter 15, Unit 15G 13. Curr Protoc Microbiol. 2009 doi: 10.1002/9780471729259.mc15g03s13. [DOI] [PubMed] [Google Scholar]
- McAfoose J, Baune BT. Evidence for a cytokine model of cognitive function. Neuroscience and biobehavioral reviews. 2009;33:355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C. Interferon-alpha inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain research. 2000;885:14–24. doi: 10.1016/s0006-8993(00)02877-8. [DOI] [PubMed] [Google Scholar]
- Mora F, Segovia G, del Arco A. Aging, plasticity and environmental enrichment: structural changes and neurotransmitter dynamics in several areas of the brain. Brain Res Rev. 2007;55:78–88. doi: 10.1016/j.brainresrev.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Morris RG. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Nilsson M, Perfilieva E, Johansson U, Orwar O, Eriksson PS. Enriched environment increases neurogenesis in the adult rat dentate gyrus and improves spatial memory. J Neurobiol. 1999;39:569–578. doi: 10.1002/(sici)1097-4695(19990615)39:4<569::aid-neu10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Hannan AJ. Enriched environments, experience- dependent plasticity and disorders of the nervous system. Nature reviews. Neuroscience. 2006;7:697–709. doi: 10.1038/nrn1970. [DOI] [PubMed] [Google Scholar]
- Nithianantharajah J, Levis H, Murphy M. Environmental enrichment results in cortical and subcortical changes in levels of synaptophysin and PSD-95 proteins. Neurobiology of Learning and Memory. 2004;81:200–210. doi: 10.1016/j.nlm.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Ojo B, Rezaie P, Gabbott PL, Davies H, Colyer F, Cowley TR, Lynch M, Stewart MG. Age-related changes in the hippocampus (loss of synaptophysin and glial-synaptic interaction) are modified by systemic treatment with an NCAM- derived peptide, FGL. Brain, behavior, and immunity. 2011 doi: 10.1016/j.bbi.2011.09.013. [DOI] [PubMed] [Google Scholar]
- Packer N, Pervaiz N, Hoffman-Goetz L. Does exercise protect from cognitive decline by altering brain cytokine and apoptotic protein levels? A systematic review of the literature. Exercise immunology review. 2010;16:138–162. [PubMed] [Google Scholar]
- Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, Giustetto M, Ferreira TA, Guiducci E, Dumas L, Ragozzino D, Gross CT. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- Petrosini L, De Bartolo P, Foti F, Gelfo F, Cutuli D, Leggio MG, Mandolesi L. On whether the environmental enrichment may provide cognitive and brain reserves. Brain research reviews. 2009;61:221–239. doi: 10.1016/j.brainresrev.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Pham TM, Ickes B, Albeck D, Soderstrom S, Granholm AC, Mohammed AH. Changes in brain nerve growth factor levels and nerve growth factor receptors in rats exposed to environmental enrichment for one year. Neuroscience. 1999;94:279–286. doi: 10.1016/s0306-4522(99)00316-4. [DOI] [PubMed] [Google Scholar]
- Pickering M, O'Connor JJ. Pro-inflammatory cytokines and their effects in the dentate gyrus. Prog Brain Res. 2007;163:339–354. doi: 10.1016/S0079-6123(07)63020-9. [DOI] [PubMed] [Google Scholar]
- Rampon C, Jiang CH, Dong H, Tang YP, Lockhart DJ, Schultz PG, Tsien JZ, Hu YH. Effects of environmental enrichment on gene expression in the brain. P Natl Acad Sci USA. 2000;97:12880–12884. doi: 10.1073/pnas.97.23.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransohoff RM, Stevens B. Neuroscience. How many cell types does it take to wire a brain? Science. 2011;333:1391–1392. doi: 10.1126/science.1212112. [DOI] [PubMed] [Google Scholar]
- Ravenholt RT, Foege WH. 1918 influenza, encephalitis lethargica, parkinsonism. Lancet. 1982;2:860–864. doi: 10.1016/s0140-6736(82)90820-0. [DOI] [PubMed] [Google Scholar]
- Rosenzweig MR, Bennett EL. Psychobiology of plasticity: effects of training and experience on brain and behavior. Behavioural brain research. 1996;78:57–65. doi: 10.1016/0166-4328(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Rossi C, Angelucci A, Costantin L, Braschi C, Mazzantini M, Babbini F, Fabbri ME, Tessarollo L, Maffei L, Berardi N, Caleo M. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. European Journal of Neuroscience. 2006;24:1850–1856. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- Sas AR, Bimonte-Nelson H, Smothers CT, Woodward J, Tyor WR. Interferon-alpha causes neuronal dysfunction in encephalitis. J Neurosci. 2009;29:3948–3955. doi: 10.1523/JNEUROSCI.5595-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger RW, Husak PJ, Bradshaw GL, Panayotov PP. Mechanisms involved in natural and experimental neuropathogenicity of influenza viruses: evidence and speculation. Adv Virus Res. 1998;50:289–379. doi: 10.1016/s0065-3527(08)60811-8. [DOI] [PubMed] [Google Scholar]
- Schloesser RJ, Lehmann M, Martinowich K, Manji HK, Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Molecular psychiatry. 2010;15:1152–1163. doi: 10.1038/mp.2010.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nature protocols. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Simpson J, Kelly JP. The impact of environmental enrichment in laboratory rats--behavioural and neurochemical aspects. Behavioural brain research. 2011;222:246–264. doi: 10.1016/j.bbr.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Soffie M, Hahn K, Terao E, Eclancher F. Behavioural and glial changes in old rats following environmental enrichment. Behavioural brain research. 1999;101:37–49. doi: 10.1016/s0166-4328(98)00139-9. [DOI] [PubMed] [Google Scholar]
- Sozda CN, Hoffman AN, Olsen AS, Cheng JP, Zafonte RD, Kline AE. Empirical comparison of typical and atypical environmental enrichment paradigms on functional and histological outcome after experimental traumatic brain injury. J Neurotrauma. 2010;27:1047–1057. doi: 10.1089/neu.2010.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studahl M. Influenza virus and CNS manifestations. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2003;28:225–232. doi: 10.1016/s1386-6532(03)00119-7. [DOI] [PubMed] [Google Scholar]
- Tauber SC, Bunkowski S, Ebert S, Schulz D, Kellert B, Nau R, Gerber J. Enriched environment fails to increase meningitis-induced neurogenesis and spatial memory in a mouse model of pneumococcal meningitis. Journal of neuroscience research. 2009;87:1877–1883. doi: 10.1002/jnr.22010. [DOI] [PubMed] [Google Scholar]
- Tong L, Balazs R, Soiampornkul R, Thangnipon W, Cotman CW. Interleukin-1 beta impairs brain derived neurotrophic factor-induced signal transduction. Neurobiol Aging. 2008;29:1380–1393. doi: 10.1016/j.neurobiolaging.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler WJ, Alonso M, Bramham CR, Pozzo-Miller LD. From acquisition to consolidation: on the role of brain-derived neurotrophic factor signaling in hippocampal- dependent learning. Learn Mem. 2002;9:224–237. doi: 10.1101/lm.51202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dam D, Lenders G, De Deyn PP. Effect of Morris water maze diameter on visual-spatial learning in different mouse strains. Neurobiology of learning and memory. 2006;85:164–172. doi: 10.1016/j.nlm.2005.09.006. [DOI] [PubMed] [Google Scholar]
- van Praag H. Exercise and the brain: something to chew on. Trends in neurosciences. 2009;32:283–290. doi: 10.1016/j.tins.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nature reviews. Neuroscience. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Viola GG, Rodrigues L, Americo JC, Hansel G, Vargas RS, Biasibetti R, Swarowsky A, Goncalves CA, Xavier LL, Achaval M, Souza DO, Amaral OB. Morphological changes in hippocampal astrocytes induced by environmental enrichment in mice. Brain research. 2009;1274:47–54. doi: 10.1016/j.brainres.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes, brain, and behavior. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh NP, Gleeson M, Shephard RJ, Woods JA, Bishop NC, Fleshner M, Green C, Pedersen BK, Hoffman-Goetz L, Rogers CJ, Northoff H, Abbasi A, Simon P. Position statement. Part one: Immune function and exercise. Exercise immunology review. 2011;17:6–63. [PubMed] [Google Scholar]
- Wang GF, Li W, Li K. Acute encephalopathy and encephalitis caused by influenza virus infection. Curr Opin Neurol. 2010;23:305–311. doi: 10.1097/wco.0b013e328338f6c9. [DOI] [PubMed] [Google Scholar]
- Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Molecular and cellular neurosciences. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie J. Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7:361–370. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Will B, Galani R, Kelche C, Rosenzweig MR. Recovery from brain injury in animals: relative efficacy of environmental enrichment, physical exercise or formal training (1990–2002) Progress in neurobiology. 2004;72:167–182. doi: 10.1016/j.pneurobio.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Williamson LL, Chao A, Bilbo SD. Environmental enrichment alters glial antigen expression and neuroimmune function in the adult rat hippocampus. Brain Behav Immun. 2012;26:500–510. doi: 10.1016/j.bbi.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, behavior, and immunity. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFalpha and 8- isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol. 2001;115:135–143. doi: 10.1016/s0165-5728(01)00259-4. [DOI] [PubMed] [Google Scholar]