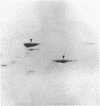
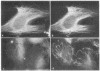
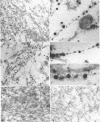
Abstract
We have used a human glioma cell line (U-251MG) to study the expression and cytoplasmic organization of vimentin (decamin) and the glial filament protein (GFP). Four clones of the parental U-251 cultures were isolated and found to express GFP from 1-2% to 99% of the cells in the population. Double immunofluorescence microscopy with antibodies to vimentin and GFP has shown that, in all four clonal cell lines, vimentin-containing filaments are expressed in most cells as an organized network and, in GFP-positive cells, GFP and vimentin are associated with the same filament network. Immunoelectron microscopy with specific antibodies labeled with colloidal gold particles of various sizes shows that GFP and vimentin are localized in the same filaments. These findings confirm in vitro studies of the copolymerization of subunits of different biochemical nature into the same intermediate filament and suggest the in vivo probability of the coassembly of GFP and vimentin from a possible soluble pool of monomers.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blikstad I., Lazarides E. Vimentin filaments are assembled from a soluble precursor in avian erythroid cells. J Cell Biol. 1983 Jun;96(6):1803–1808. doi: 10.1083/jcb.96.6.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burr J. G., Dreyfuss G., Penman S., Buchanan J. M. Association of the src gene product of Rous sarcoma virus with cytoskeletal structures of chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3484–3488. doi: 10.1073/pnas.77.6.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairncross J. G., Mattes M. J., Beresford H. R., Albino A. P., Houghton A. N., Lloyd K. O., Old L. J. Cell surface antigens of human astrocytoma defined by mouse monoclonal antibodies: identification of astrocytoma subsets. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5641–5645. doi: 10.1073/pnas.79.18.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnek H., Soifer D. Comparison of the proteins of 10 nm filaments from rabbit sciatic nerve and spinal cord by electrophoresis in two dimensions. FEBS Lett. 1980 Aug 11;117(1):175–178. doi: 10.1016/0014-5793(80)80939-2. [DOI] [PubMed] [Google Scholar]
- Eng L. F., Lasek R. J., Bigbee J. W., Eng D. L. Immunocytologic analyses of 10 nm intermediate filaments in the nervous system of Myxicola. J Histochem Cytochem. 1980 Dec;28(12):1312–1318. doi: 10.1177/28.12.7014711. [DOI] [PubMed] [Google Scholar]
- Faulk W. P., Taylor G. M. An immunocolloid method for the electron microscope. Immunochemistry. 1971 Nov;8(11):1081–1083. doi: 10.1016/0019-2791(71)90496-4. [DOI] [PubMed] [Google Scholar]
- Frank E. D., Warren L. Aortic smooth muscle cells contain vimentin instead of desmin. Proc Natl Acad Sci U S A. 1981 May;78(5):3020–3024. doi: 10.1073/pnas.78.5.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Hanukoglu I. Unraveling the structure of the intermediate filaments. Cell. 1983 Sep;34(2):332–334. doi: 10.1016/0092-8674(83)90367-7. [DOI] [PubMed] [Google Scholar]
- Horisberger M., Rosset J., Bauer H. Colloidal gold granules as markers for cell surface receptors in the scanning electron microscope. Experientia. 1975 Oct 15;31(10):1147–1149. doi: 10.1007/BF02326761. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Lazarides E. Invariance and heterogeneity in the major structural and regulatory proteins of chick muscle cells revealed by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1450–1454. doi: 10.1073/pnas.74.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazarides E., Balzer D. R., Jr Specificity of desmin to avian and mammalian muscle cells. Cell. 1978 Jun;14(2):429–438. doi: 10.1016/0092-8674(78)90128-9. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Liem R. Simultaneous separation and purification of neurofilament and glial filament proteins from brain. J Neurochem. 1982 Jan;38(1):142–150. doi: 10.1111/j.1471-4159.1982.tb10865.x. [DOI] [PubMed] [Google Scholar]
- Moon R. T., Lazarides E. Canavanine inhibits vimentin assembly but not its synthesis in chicken embryo erythroid cells. J Cell Biol. 1983 Oct;97(4):1309–1314. doi: 10.1083/jcb.97.4.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Osborn M., Franke W., Weber K. Direct demonstration of the presence of two immunologically distinct intermediate-sized filament systems in the same cell by double immunofluorescence microscopy. Vimentin and cytokeratin fibers in cultured epithelial cells. Exp Cell Res. 1980 Jan;125(1):37–46. doi: 10.1016/0014-4827(80)90186-x. [DOI] [PubMed] [Google Scholar]
- Quinlan R. A., Franke W. W. Molecular interactions in intermediate-sized filaments revealed by chemical cross-linking. Heteropolymers of vimentin and glial filament protein in cultured human glioma cells. Eur J Biochem. 1983 May 16;132(3):477–484. doi: 10.1111/j.1432-1033.1983.tb07386.x. [DOI] [PubMed] [Google Scholar]
- Raju T., Bignami A., Dahl D. In vivo and in vitro differentiation of neurons and astrocytes in the rat embryo. Immunofluorescence study with neurofilament and glial filament antisera. Dev Biol. 1981 Jul 30;85(2):344–357. doi: 10.1016/0012-1606(81)90266-9. [DOI] [PubMed] [Google Scholar]
- Schmid E., Osborn M., Rungger-Brändle E., Gabbiani G., Weber K., Franke W. W. Distribution of vimentin and desmin filaments in smooth muscle tissue of mammalian and avian aorta. Exp Cell Res. 1982 Feb;137(2):329–340. doi: 10.1016/0014-4827(82)90034-9. [DOI] [PubMed] [Google Scholar]
- Sharp G., Osborn M., Weber K. Occurrence of two different intermediate filament proteins in the same filament in situ within a human glioma cell line. An immunoelectron microscopical study. Exp Cell Res. 1982 Oct;141(2):385–395. doi: 10.1016/0014-4827(82)90227-0. [DOI] [PubMed] [Google Scholar]
- Starger J. M., Goldman R. D. Isolation and preliminary characterization of 10-nm filaments from baby hamster kidney (BHK-21) cells. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2422–2426. doi: 10.1073/pnas.74.6.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Cabral F., Gottesman M. M., Goldman R. D. In vitro assembly of homopolymer and copolymer filaments from intermediate filament subunits of muscle and fibroblastic cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3692–3696. doi: 10.1073/pnas.78.6.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P., Idler W., Aynardi-Whitman M., Zackroff R., Goldman R. D. Heterogeneity of intermediate filaments assembled in vitro. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):465–474. doi: 10.1101/sqb.1982.046.01.043. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Hainfeld J. F., Trus B. L., Wall J. S., Steinert P. M. Epidermal keratin filaments assembled in vitro have masses-per-unit-length that scale according to average subunit mass: structural basis for homologous packing of subunits in intermediate filaments. J Cell Biol. 1983 Dec;97(6):1939–1944. doi: 10.1083/jcb.97.6.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travo P., Weber K., Osborn M. Co-existence of vimentin and desmin type intermediate filaments in a subpopulation of adult rat vascular smooth muscle cells growing in primary culture. Exp Cell Res. 1982 May;139(1):87–94. doi: 10.1016/0014-4827(82)90321-4. [DOI] [PubMed] [Google Scholar]
- Wang E., Cairncross J. G., Yung W. K., Garber E. A., Liem R. K. An intermediate filament-associated protein, p50, recognized by monoclonal antibodies. J Cell Biol. 1983 Nov;97(5 Pt 1):1507–1514. doi: 10.1083/jcb.97.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Cross R. K., Choppin P. W. Involvement of microtubules and 10-nm filaments in the movement and positioning of nuclei in syncytia. J Cell Biol. 1979 Nov;83(2 Pt 1):320–337. doi: 10.1083/jcb.83.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. H., Fields K. L. Antibodies to neurofilament, glial filament, and fibroblast intermediate filament proteins bind to different cell types of the nervous system. J Cell Biol. 1981 Jan;88(1):115–126. doi: 10.1083/jcb.88.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]