Abstract
Estrogen receptor (ER) antagonists have been widely used for breast cancer treatment, but the efficacy and drug resistance remain to be clinical concerns. The purpose of this study was to determine whether the extracts of coptis, an anti-inflammatory herb, improve the anticancer efficacy of ER antagonists. The results showed that the combined treatment of ER antagonists and the crude extract of coptis or its purified compound berberine conferred synergistic growth inhibitory effect on MCF-7 cells (ER+), but not on MDA-MB-231 cells (ER-). The similar results were observed in the combined treatment of fulvestrant, a specific aromatase antagonist. Analysis of the expression of breast cancer related genes indicated that EGFR, HER2, bcl-2 and COX-2 were significantly downregulated, while IFN-β and p21 were remarkably upregulated by berberine. Our results suggest that coptis extracts could be promising adjuvant to ER antagonists in ER positive breast cancer treatment through regulating expression of multiple genes.
Keywords: breast cancer, estrogen receptor antagonist, coptis, gene expression, cell growth
Introduction
Over 60% of breast cancers are estrogen receptor (ER) positive and depend on ER for growth. The ER antagonists, tamoxifen and fulvestrant, are therefore widely used for the treatment of ER positive breast cancer. Tamoxifen has been approved to be the first line anti-estrogen therapy since the early 1970s[1]. However, Most tamoxifen responsive breast cancer patients succumb to tamoxifen resistance[2]. Compelling evidences suggest that the resistance is mainly from the existence of the crosstalk between ER and type I tyrosine kinase receptors, including epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2) which may become upregulated during tamoxifen treatment and result in breast cancer growth[3; 4; 5]. In addition, upregulation of bcl-2 and cyclooxygenase-2 (COX-2) also contribute to tamoxifen resistance[6; 7]. To cope with the resistance, fulvestrant was developed as the second line anti-estrogen therapy[8]. Fulvestrant is an approved effective aromatase antagonist for tamoxifen resistance breast cancer by degrading ER and complete abrogating estrogen sensitive gene transcription[9]. However, the drug resistance of fulvestrant becomes to be a concern too. The loss of ER and ER regulated genes leads to upregulation of growth factor signalings and results in drug resistance and tumor growth promotion[10]. For this reason, several adjuvant agents, including the antagonists of tyrosine kinases, EGFR and COX-2, have been under intensive investigation in order to enhance the efficacy and reduce the resistance of the ER antagonists. The combined treatment begins to show potentials in improving breast cancer treatment[10].
Coptis (known as Gold Thread), a widely used herb in traditional Chinese medicine, attracted much attention because of its multiple pharmacological effects, including anti-infection, anti-inflammation and anticancer effects[11]. Beberine is the major alkaloid extracted from coptis[12]. The results from DNA microarray demonstrated that Bererine regulated almost the same profile of cancer related gene as the crude extract of coptis[13]. It was shown that the anti-inflammation and anticancer effects may be related with the downregulation of COX-2 by berberine[14]. In addition, berberine inhibited EGFR and bcl-2 expression in smooth muscle cells and hepatoma cells respectively[15]. Our previous studies demonstrated that the ethanol extract of coptis, which include berberine and other components of coptis, and purified berberine inhibited proliferation and induced apoptosis of MCF-7 breast cancer cells[16]. Therefore, it is feasible to speculate that coptis may have potentials in improving efficacy of the ER antagonists in breast cancer treatment.
In this study, we investigated whether the anticancer effects of tamoxifen and fulvestrant on breast cancer cells are enhanced by coptis extracts and its possible molecular mechanisms.
Materials and Methods
Materials
Coptis rhizoma powder (Mayway Inc., CA) was made from coptidis japonica and extracted as described previously[16]. Briefly, the powder was first dissolved in 70% ethanol and subsequently diluted in 35% ethanol at a stock concentration of 10 mg/ml. The mixture was vortexed rigorously for 2 min followed by 5 min ultrasonication. After centrifugation (2,000g, 10 min), the supernatant was collected and stored at -20°C until use. Pure berberine (>97%), the main active constituent in coptis extract, fulvestrant, the selective estrogen receptor antagonist, were purchased from Sigma-Aldrich Co. (St. Louis, MO). Berberine and fulvestrant were dissolved in DMSO. All above reagents were diluted in the complete medium (as described below) before use. The final concentration of DMSO in the medium was less than 0.1 %.
Cell culture
MCF-7 and MDA-MB-231 human breast cancer cell lines were from ATCC (Manassas, VA) and grown in DMEM/F-12 medium (Invitrogen, Carlsbad, CA, USA). All mediums were supplemented with 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% inactivated fetal calf serum (FBS; HyClone, South Logan, UT). Cells were incubated at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were sub-cultured twice weekly.
Cell viability assay
The cell viability was evaluated by a colorimetric XTT assay[17]. Briefly, cells (3 × 103 per well) were seeded in 96-well plastic plates and incubated at 37°C for 16 h. Then the drugs were added and incubated with cells for 72 h. The medium was replaced with 100 μl of phenol red-free MEM (Invitrogen) containing 0.45 mM XTT (Invitrogen) and 8 μM electron-coupling reagent 5-Methylphenazinium methyl sulfate (PMS; Sigma-Aldrich). After 4 h incubation at 37 °C, 5 % CO2, the spectrophotometrical absorbance (A490) of samples was measured using a microplate reader (PerkinElmer, Waltham, MA, USA) with measurement wavelength of 490nm and reference wave length of 690nm. Each measurement was performed in triplicate or quadruplicate. The inhibitory effect was calculated according to the equation: Inhibition (%) = [1-(A490 of treated wells/A490 of control wells)] × 100. The calculated theoretical additive inhibitory effect of agents a and b was calculated as following[18]: cIab = 100 × [1 − (1 − Ia/100) × (1 − Ib/100)]. cIab is the calculated additive inhibitory effect of combinations. Ia, Ib and mIab are measured inhibitory effect of each agent used alone and combination. The growth inhibitory effect of combination treatment was determined as following:
Synergistic effect: mIab > cIab
Additive effect: mIab = cIab
Antagonistic effect: mIab < cIab
Quantitative real-time PCR
Total RNA was extracted using an RNeasy Mini kit (Qiagen, Valencia, CA) from MCF-7 cells treated with or without berberine. Concentration and purity of extracted RNA were determined using a Shimadzu UV1600 spectrophotometer (Shimadzu, Kyoto, Japan). The quality of RNA was checked by the 1% agarose gel electrophoresis. Total RNA (2 μg) was used for synthesis of cDNA using reverse transcription kit (Promega, Madison, WI) following the manufacturer's protocol. Oligonucleotide primers (Table 1) were designed using the Primer Express software (Applied Biosystems, Foster City, CA) and synthesized by Invitrogen. GAPDH gene was used as an endogenous control to normalize the expression of the target genes. SYBR Green with low ROX qPCR mix was purchased from Thermo Scientific (Epsom, Surrey, UK). Quantitative real-time RT-PCR was performed in triplicate using a 96-well optic tray on an ABI Prism 7000 sequence detection system (Applied Biosystems). Data collection and analysis was performed with SDS v1.2x System Software (Applied Biosystems). Data were then exported and further analyzed in Microsoft Excel. Normalized cycle threshold (ΔCt) was calculated by subtracting Ct value of GAPDH from Ct value of target genes. Relative gene expression level of berberine treated cells to untreated cells (control) was determined by the formula: 2|ΔCt(control)- ΔCt(treatment)|.
Table 1.
Genes and oligonucleotide primers for quantitative real-time RT-PCR
| Genes | Forward primer | Reverse primer |
|---|---|---|
| bcl-2 | GGGGAGGATTGTGGCCTTC | CAGGGCGATGTTGTCCACC |
| COX-2 | GTGCAACACTTGAGTGGCTAT | AGCAATTTGCCTGGTGAATGAT |
| cyclin-D1 | GAACAAACAGATCATCCGCAAAC | GCGGTAGTAGGACAGGAAGTTG |
| EGFR | AAGGAAATCCTCGATGAAGCCT | TGTCTTTGTGTTCCCGGACATA |
| FLIP | AATTCAAGGCTCAGAAGCGA | GGCAGAAACTCTGCTGTTCC |
| GAPDH | TCCTGCACCACCAACTGCTTAG | GGCATGGACTGTGG TCATGAGT |
| HER2 | TCCAACTGGACAACCTCTCTC | TGAAGTACCGGGGTTTCCATT |
| IFN-β | AAGCAGCAATTTTCAGTGTCA | CCTCAGGGATGTCAAAGTTCA |
| p21 | CCTGTCACTGTCTTGTACCCT | GCGTTTGGAGTGGTAGAAATCT |
| Survivin | ATTTGAATCGCGGGACCC | GAGAAAGGGCTGCCAGGC |
| Tollip | GCCAAGAATTACGGCATGACC | GTGGATGACCTTATTCCAGCG |
| ZO-1 | ATTCACGCAGTTACGAGCAAG | AGATGAAGGTATCAGCGGAGG |
Statistic analysis
The data were expressed as means ± SD. One-way analysis of variance (ANOVA) was performed to determine the difference between control, agents alone and combinations using GraphPad Prism 4 software package (San Diego, CA, USA). Newman-Keuls test was used for multiple comparisons of variance. Statistical significance was accepted at the level of P < 0.05. Each experiment was repeated at least three times.
Results
1. Effect of combined treatment of ER antagonists plus coptis extracts on the growth of breast cancer cells
To evaluate whether combined treatment of ER antagonists and coptis extracts exerts an enhanced anticancer effect on breast cancer cells, cell viability was examined by XTT assay on human ER+ MCF-7 and ER- MDA-MB-231 cells treated with tamoxifen (TAM) alone or combined with crude extract of coptis (COP) or its pure major constituent berberine (BER). As shown in Figure 1A and 1B, the combined treatment of TAM and COP resulted in synergistic inhibitory effect on MCF-7 cells, e.g. TAM (1.5 μM) and COP (20 μg/ml), when used alone, induced only 10% and 30% of cell growth inhibitory effect respectively, while combined use of the same dose of TAM and COP resulted in an inhibitory effect of 85%, P = 0.0005 compared to the calculated additive inhibitory effect of 39%. Similarly, the combined use of TAM (1.5 μM) and BER (16 μg/ml) led to a synergistic growth inhibitory effect of 86%, P = 0.002 compared to the calculated additive inhibitory effect of 54%. However, combination treatment of TAM and COP did not show synergistic effect on ER negative MDA-MB-231 cells (Fig 2A and 2B).
Fig 1.
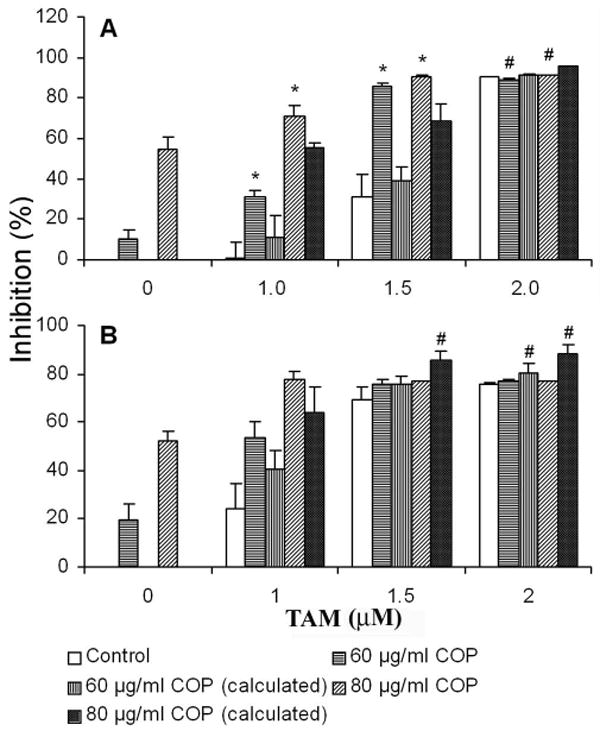
Effects of combined treatment of COP with TAM on the growth of MCF-7 cells (A) and MDA-MB-231 cells (B). The drugs were added into cell culture after the cells were inoculated in 96-well plate for 16 h. Cell growth was examined using XTT colorimetric assay as described in Materials and Methods after 72 h exposure to reagents. * represents the synergistic effects while # indicates antagonistic effects, P < 0.05 compared to calculated theoretical additive inhibitory effect of each combination. Data are represented as means ± SD of 3 to 5 independent experiments.
Fig 2.
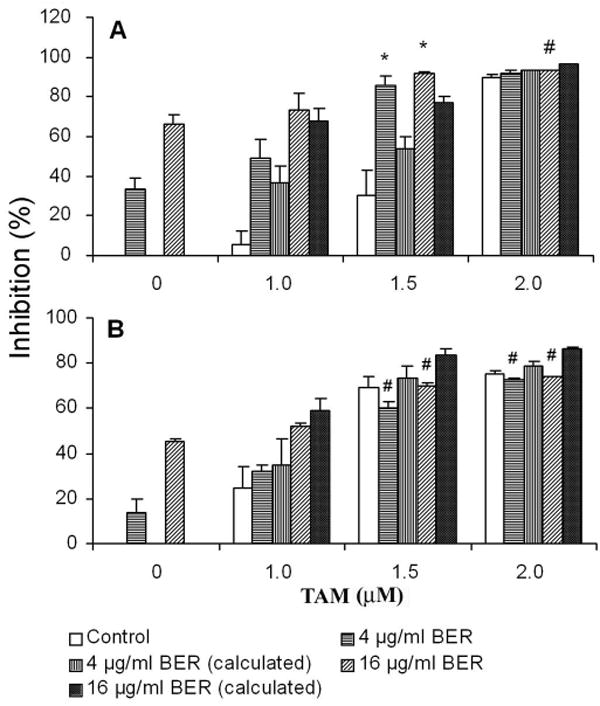
Effects of combined treatment of BER with TAM on the growth of MCF-7 cells (A) and MDA-MB-231 cells (B). The drugs were added into cell culture after the cells were inoculated in 96-well plate for 16 h. Cell growth was examined using XTT colorimetric assay as described in Materials and Methods after 72 h exposure to reagents. * represents the synergistic effects while # indicates antagonistic effects, P < 0.05 compared to calculated theoretical additive inhibitory effect of each combination. Data are represented as means ± SD of 3 to 5 independent experiments.
To further investigate whether there is synergistic inhibitory effect in combined treatment of other ER antagonist plus COP or BER, we next examined the effect of combined treatment of COP or BER with fulvestrant (FUL), a specific ER antagonist, on MCF-7 cell growth. The results showed that the combined use of COP or BER with FUL at 10 nM, which had no detectable inhibitory effect when used alone, resulted in significantly synergistic inhibitory effects on MCF-7 cell growth, P < 0.01 compared to COP or BER used alone (Fig 3A, 3B).
Fig 3.

Effects of combined treatment of COP or BER and FUL on the growth of MCF-7 cells. Cell growth was examined by XTT assay. Cells were treated with COP (A) or BER (B) at the indicated concentrations and FUL at a sub-inhibitory dose of 10 nM for 72 h before XTT assay. Data are represented as means ± SD of 3 to 5 independent experiments.
2. Effect of BER on the gene expression in MCF-7 cells
The possible mechanism for the synergistic inhibitory effects of combined treatment of coptis extracts and ER antagonists was primary investigated through analysis of gene expression by quantitative real time RT-PCR. Instead of using crude extract of coptis, we used the pure compound BER which is the major active compound in the anticancer effect of coptis in this experiment to avoid confounding factors created by unknown compounds in coptis. The regulation of gene expression by BER was expressed as fold differences between treatment and control groups as shown in Table 2. The results demonstrated that BER significantly downregulated the expression of EGFR, HER2, bcl-2, COX-2, FLIP, Surviving, cyclin-D1 and Tollip, while upregulated the expression of IFN-β, p21 and ZO-1 in MCF-7 cells. Notably, expression of EGFR remarkably decreased 16-fold, and IFN-β and p21 increased 35- and 21-fold respectively in MCF-7 cells treated with BER (16 μg/ml) for 48h, suggesting their important roles in the synergistic effects of combined treatment of coptis extracts and ER antagonists.
Table 2.
Effect of berberine (BER) on the expression of breast cancer related genes in MCF-7 cells
| Genes | Ct | ΔCt | Fold differences | ||
|---|---|---|---|---|---|
| Control | BER | Control | BER | ||
| GAPDH | 15.08 ± 0.13 | 15.70 ± 0.29 | |||
| bcl-2 | 22.24 ± 0.03 | 24.89 ± 0.31 | 7.16 ± 0.11 | 9.19 ± 0.15 | -4.08 ± 0.43 |
| COX-2 | 28.41 ± 0.14 | 31.27 ± 0.84 | 13.28 ± 0.23 | 15.41 ± 0.71 | -4.66 ± 2.20 |
| cyclin-D1 | 20.54 ± 0.24 | 21.67 ± 0.63 | 5.46 ± 0.11 | 5.97 ± 0.34 | -1.44 ± 0.32 |
| EGFR | 30.18 ± 0.29 | 34.59 ± 0.24 | 15.10 ± 0.26 | 19.02 ± 0.68 | -15.93 ± 7.22 |
| FLIP | 25.28 ± 0.07 | 26.67 ± 0.48 | 10.21 ± 0.10 | 10.97 ± 0.19 | -1.71 ± 0.22 |
| HER2 | 20.95 ± 0.40 | 23.59 ± 0.24 | 5.87 ± 0.28 | 9.19 ± 0.15 | -4.18 ± 1.31 |
| IFN-β | 37.55 ± 0.16 | 33.06 ± 0.79 | 22.47 ± 0.28 | 17.36 ± 0.79 | 34.54 ± 8.14 |
| p21 | 21.76 ± 0.43 | 17.96 ± 0.28 | 6.68 ± 0.33 | 2.26 ± 0.01 | 21.49 ± 0.16 |
| Survivin | 23.52 ± 0.44 | 25.39 ± 0.08 | 8.44 ± 0.32 | 9.70 ± 0.28 | -2.24 ± 0.48 |
| Tollip | 25.20 ± 0.32 | 26.78 ± 0.51 | 10.12 ± 0.23 | 11.08 ± 0.22 | -1.96 ± 0.30 |
| ZO-1 | 24.16 ± 0.65 | 23.70 ± 0.32 | 9.09 ± 0.78 | 8.01 ± 0.49 | 2.19 ± 0.69 |
Note: Regulation of gene by BER are expressed as fold differences of gene expression between BER treated cells and untreated cells. ΔCt was calculated by subtracting Ct value of GAPDH from Ct value of target genes, and fold differences (N) were calculated by the formula: N = 2|ΔCt(control)- ΔCt(treatment)|. If ΔCt(control) > ΔCt(treatment), then N is positive which means the gene is upregulated, otherwise, N is negative which means the gene is downregulated by the treatment of BER. The changes of all target genes are statistically significant at the level of P < 0.05 compared BER to control (One-way ANOVA). Values are means ± SD of three to five independent assays.
Discussion
Clinically, most tamoxifen responsive breast cancer patients succumb to drug resistance[2]. Similarly, fulvestrant resistance start to be concerned, although fulvestrant is approved an effective ER antagonist for tamoxifen resistance breast cancer[9]. It is necessary to develop combined treatment regimes to enhance the efficacy of ER antagonists. By recognizing the multiple targeting manner of coptis, particularly inhibiting inflammation and expression of cancer related genes (e.g. COX-2, bcl-2, EGFR, etc.), and our previous report that coptis extracts markedly inhibited cell proliferation and induced apoptotic cell death of MCF-7 cells through upregulating the expression of IFN-β and TNF-α[16], we speculate the coptis extracts may be potential in enhancing the efficacy of ER antagonists. The results of the present study demonstrated that combined treatment of tamoxifen and coptis extracts (i.e. the crude extract of coptis and the pure compound berberine) elicited enhanced growth inhibitory effects on ER positive MCF-7 cells, but not on ER negative MDA-MB-231 cells. Similar results were observed in the combination treatment of fulvestrant and coptis extracts on MCF-7 cells.
In order to determine the possible molecular mechanisms underlying the synergistic anticancer effects of coptis extracts on ER antagonists, we analyzed the expression level of several breast cancer related genes in MCF-7 cells treated with berberine. The results indicated that berberine significantly downregulated the expression of EGFR, HER2, bcl-2 and COX-2, while upregulated IFN-β and p21. Notably, expression of EGFR, IFN-β and p21 were changed by more than 15 times (Table 2). Compelling evidences suggested that multiple genes, particularly EGFR, HER2, COX-2, bcl-2 and IFN-β may play important roles in modulating the efficacy and resistance of ER-antagonists. Studies indicated that EGFR and HER2 antagonize the anticancer effect of tamoxifen and induce drug resistance through activating ER and co-regulatory proteins, inducing a cross talk that leads to enhanced ER positive breast cancer survival and proliferation[19; 20]. In addition, tamoxifen behaves as an estrogen agonist in the context of overexpression of HER2 in breast cancer cells, resulting in de novo resistance, and Gefitinib (an EGFR tyrosine kinase inhibitor) pretreatment blocked receptor cross-talk, eliminated the agonist effects of tamoxifen, and restored its antitumor activity[4]. Moreover, EGFR and HER2 inhibitors can restore tamoxifen sensitivity and delay resistance to tamoxifen and fulvestrant[10]. Bcl-2 protein exerts strong anti-apoptosis activity, and high expression of bcl-2 is also responsible for the tamoxifen resistance[21]. Our previous study indicated that upregulation of IFN-β was responsible for the antiproliferative effect of crude extract of coptis[16], and combination use of IFN-β and tamoxifen was suggested to overcome clinical resistance to tamoxifen in advanced breast cancer[22]. P21 is a potent cyclin-dependent kinase inhibitor mediating the p53-dependent G1 and S phase arrest. A very recent study revealed that loss of p21 expression was associated with resistant breast cancers which proliferatively respond to tamoxifen[3]. It has been suggested that anti-inflammation may be an important strategy in cancer prevention and treatment[23]. Coptis has been traditionally used as an anti-inflammatory agent for generations. Our results indicated that berberine inhibited COX-2 gene expression, further explained the mechanism of anti-inflammation of coptis. It was shown that COX-2 reduced inhibitory effects of tamoxifen on breast cancer cell growth[7].
Taken together, the modulation of berberine on multiple genes suggests its unique property and may explain, at least partially, the synergistic anticancer effects of combined use of coptis extracts and ER antagonists, and suggests the promising potential use in the adjuvant treatment of breast cancer. Targeting one or two pathways may not be efficient to inhibit the growth of cancer since cancer cells are heterogeneous and endowed with complex, redundant, converging and diverging pathways spanning both the genetic and metabolic networks to gain growth advantages, as evidenced that cyclin E kinase complexes can function redundantly and replace the loss of cyclin D-dependent kinase complexes that functionally inactivate pRb[24]. Studies indicated multiple targeted anticancer agents may have better potential in improving the efficacy of ER antagonists[19; 25].
Acknowledgments
We thank J. Wan for assistance in cell viability assay and data analysis. This work was partially supported by the Starr Foundation and the American Cancer Society (RSG-03-140-01-CNE to J.X.K.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jordan VC. Tamoxifen: catalyst for the change to targeted therapy. Eur J Cancer. 2008;44:30–8. doi: 10.1016/j.ejca.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee WL, Cheng MH, Chao HT, Wang PH. The role of selective estrogen receptor modulators on breast cancer: from tamoxifen to raloxifene. Taiwan J Obstet Gynecol. 2008;47:24–31. doi: 10.1016/S1028-4559(08)60051-0. [DOI] [PubMed] [Google Scholar]
- 3.Abukhdeir AM, Vitolo MI, Argani P, De Marzo AM, Karakas B, Konishi H, Gustin JP, Lauring J, Garay JP, Pendleton C, Konishi Y, Blair BG, Brenner K, Garrett-Mayer E, Carraway H, Bachman KE, Park BH. Tamoxifen-stimulated growth of breast cancer due to p21 loss. Proc Natl Acad Sci U S A. 2008;105:288–93. doi: 10.1073/pnas.0710887105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96:926–35. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez MC, Detre S, Johnston S, Mohsin SK, Shou J, Allred DC, Schiff R, Osborne CK, Dowsett M. Molecular changes in tamoxifen-resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinase. J Clin Oncol. 2005;23:2469–76. doi: 10.1200/JCO.2005.01.172. [DOI] [PubMed] [Google Scholar]
- 6.Planas-Silva MD, Bruggeman RD, Grenko RT, Smith JS. Overexpression of c-Myc and Bcl-2 during progression and distant metastasis of hormone-treated breast cancer. Exp Mol Pathol. 2007;82:85–90. doi: 10.1016/j.yexmp.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Tari AM, Simeone AM, Li YJ, Gutierrez-Puente Y, Lai S, Symmans WF. Cyclooxygenase-2 protein reduces tamoxifen and N-(4-hydroxyphenyl)retinamide inhibitory effects in breast cancer cells. Lab Invest. 2005;85:1357–67. doi: 10.1038/labinvest.3700339. [DOI] [PubMed] [Google Scholar]
- 8.Perey L, Paridaens R, Hawle H, Zaman K, Nole F, Wildiers H, Fiche M, Dietrich D, Clement P, Koberle D, Goldhirsch A, Thurlimann B. Clinical benefit of fulvestrant in postmenopausal women with advanced breast cancer and primary or acquired resistance to aromatase inhibitors: final results of phase II Swiss Group for Clinical Cancer Research Trial (SAKK 21/00) Ann Oncol. 2007;18:64–9. doi: 10.1093/annonc/mdl341. [DOI] [PubMed] [Google Scholar]
- 9.Dowsett M, Nicholson RI, Pietras RJ. Biological characteristics of the pure antiestrogen fulvestrant: overcoming endocrine resistance. Breast Cancer Res Treat. 2005;93 1:S11–8. doi: 10.1007/s10549-005-9037-3. [DOI] [PubMed] [Google Scholar]
- 10.Massarweh S, Osborne CK, Jiang S, Wakeling AE, Rimawi M, Mohsin SK, Hilsenbeck S, Schiff R. Mechanisms of tumor regression and resistance to estrogen deprivation and fulvestrant in a model of estrogen receptor-positive, HER-2/neu-positive breast cancer. Cancer Res. 2006;66:8266–73. doi: 10.1158/0008-5472.CAN-05-4045. [DOI] [PubMed] [Google Scholar]
- 11.Hsieh YS, Kuo WH, Lin TW, Chang HR, Lin TH, Chen PN, Chu SC. Protective effects of berberine against low-density lipoprotein (LDL) oxidation and oxidized LDL-induced cytotoxicity on endothelial cells. J Agric Food Chem. 2007;55:10437–45. doi: 10.1021/jf071868c. [DOI] [PubMed] [Google Scholar]
- 12.Iizuka N, Miyamoto K, Okita K, Tangoku A, Hayashi H, Yosino S, Abe T, Morioka T, Hazama S, Oka M. Inhibitory effect of Coptidis Rhizoma and berberine on the proliferation of human esophageal cancer cell lines. Cancer Lett. 2000;148:19–25. doi: 10.1016/s0304-3835(99)00264-5. [DOI] [PubMed] [Google Scholar]
- 13.I N, Hara A, Hamamoto Y, Uchimura S, Miyamoto T, Tsunedomi R, Miyamoto K, Hazama S, Okita K, Oka M. Molecular dissection of a medicinal herb with anti-tumor activity by oligonucleotide microarray. Life Sci. 2005;77:991–1002. doi: 10.1016/j.lfs.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Kuo CL, Chi CW, Liu TY. Modulation of apoptosis by berberine through inhibition of cyclooxygenase-2 and Mcl-1 expression in oral cancer cells. In Vivo. 2005;19:247–52. [PubMed] [Google Scholar]
- 15.Hwang JM, Kuo HC, Tseng TH, Liu JY, Chu CY. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch Toxicol. 2006;80:62–73. doi: 10.1007/s00204-005-0014-8. [DOI] [PubMed] [Google Scholar]
- 16.Kang JX, Liu J, Wang J, He C, Li FP. The extract of huanglian, a medicinal herb, induces cell growth arrest and apoptosis by upregulation of interferon-beta and TNF-alpha in human breast cancer cells. Carcinogenesis. 2005;26:1934–9. doi: 10.1093/carcin/bgi154. [DOI] [PubMed] [Google Scholar]
- 17.S R, Scudiero DA, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988;48:7. [PubMed] [Google Scholar]
- 18.Koren R, Rocker D, Kotestiano O, Liberman UA, Ravid A. Synergistic anticancer activity of 1,25-dihydroxyvitamin D(3) and immune cytokines: the involvement of reactive oxygen species. J Steroid Biochem Mol Biol. 2000;73:105–12. doi: 10.1016/s0960-0760(00)00068-6. [DOI] [PubMed] [Google Scholar]
- 19.Witters LM, Witkoski A, Planas-Silva MD, Berger M, Viallet J, Lipton A. Synergistic inhibition of breast cancer cell lines with a dual inhibitor of EGFR-HER-2/neu and a Bcl-2 inhibitor. Oncol Rep. 2007;17:465–9. [PubMed] [Google Scholar]
- 20.Fox EM, Bernaciak TM, Wen J, Weaver AM, Shupnik MA, Silva CM. Signal Transducer and Activator of Transcription 5b, c-Src, and Epidermal Growth Factor Receptor Signaling Play Integral Roles in Estrogen-Stimulated Proliferation of Estrogen Receptor-Positive Breast Cancer Cells. Mol Endocrinol. 2008;22:1781–96. doi: 10.1210/me.2007-0419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim R, Tanabe K, Emi M, Uchida Y, Toge T. Modulation of tamoxifen sensitivity by antisense Bcl-2 and trastuzumab in breast carcinoma cells. Cancer. 2005;103:2199–207. doi: 10.1002/cncr.21029. [DOI] [PubMed] [Google Scholar]
- 22.Buzzi F, Brugia M, Rossi G, Giustini L, Scoponi C, Sica G. Combination of beta-interferon and tamoxifen as a new way to overcome clinical resistance to tamoxifen in advanced breast cancer. Anticancer Res. 1992;12:869–71. [PubMed] [Google Scholar]
- 23.Puntoni M, Marra D, Zanardi S, Decensi A. Inflammation and cancer prevention. Ann Oncol. 2008;19 7:vii225–9. doi: 10.1093/annonc/mdn442. [DOI] [PubMed] [Google Scholar]
- 24.Gray-Bablin J, Zalvide J, Fox MP, Knickerbocker CJ, DeCaprio JA, Keyomarsi K. Cyclin E, a redundant cyclin in breast cancer. Proc Natl Acad Sci U S A. 1996;93:15215–20. doi: 10.1073/pnas.93.26.15215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tortora G, Bianco R, Daniele G, Ciardiello F, McCubrey JA, Ricciardi MR, Ciuffreda L, Cognetti F, Tafuri A, Milella M. Overcoming resistance to molecularly targeted anticancer therapies: Rational drug combinations based on EGFR and MAPK inhibition for solid tumours and haematologic malignancies. Drug Resist Updat. 2007;10:81–100. doi: 10.1016/j.drup.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]


