Abstract
Objective
Little is known about neural mechanisms of postpartum depression(PPD). Previous research notes ventral striatal activity and dopamine release increases with maternal attachment but decreases in major depressive disorder. This study tests the hypothesis that striatal response to reward is altered in PPD.
Method
Subjects underwent fMRI BOLD acquisition during a fast event-related card guessing, monetary reward task. Time series data from an independent sample of 10 healthy mothers were used to establish the ventral striatal region of interest (ROI). Repeated-measures ANOVA of time series data in the established ROI was then conducted for a discrete group of healthy (n=12) and depressed, unmedicated mothers(n=12).
Results
Data from the independent sample of 10 healthy mothers established an ROI in the left ventral striatum [−13, 12, −4, 477 mm3], with cluster significance p<0.01, corrected. There was a significant quadratic interaction of time*group (F[1,22]=5.22, p=0.032) in this ROI in the healthy(n=12) and depressed mothers(n=12). This effect represents a nonlinear attenuation of ventral striatal response with time that was greater in depressed than healthy mothers.
Conclusions
Rapid attenuation of ventral striatal response to reward receipt in postpartum depression may represent an important neural mechanism of postpartum depression. Additional study with infant stimuli and in relationship to mother-infant behavior is needed.
Keywords: postpartum depression, monetary reward, fMRI, ventral striatum
Introduction
Postpartum depression (PPD) is a common illness of reproductive-aged women(1) that poses risk for maternal health and infant development(2). While mechanistic conceptualization of PPD and PPD treatment approaches are largely based upon those for non-postpartum major depressive disorder (MDD)(3), it remains unknown whether PPD is a unique neurobiological entity compared to non-postpartum MDD(4). Given shortcomings of extant treatments for PPD(3), mechanistic study of PPD has the potential to not only improve nosological classification, but also guide development of more effective treatments.
Candidate mechanisms of great interest in PPD include impaired positive emotion and approach behaviors. Because maternal positive emotions increase mother-infant sensitivity(5) and because maternal behavior relies upon intact infant-approach/motivation functions, greater understanding of positive emotion processing and reward in PPD is highly relevant for the health of the mother-infant dyad, and ultimately infant development. Reward-related appetitive functions in maternal rodents(which encompass approach, grooming, feeding, and retrieving behaviors) rely upon intact medial preoptic area efferent connections to the mesostriatal dopamine system(6) and are proportional to striatal dopaminergic activity(7). fMRI studies in non-depressed mothers confirm that human ventral striatum is activated to the pleasant stimulus of one’s own infant smiling(8), which is modulated by the quality of maternal attachment security(9). In contrast, women with PPD had reduced ventral striatal activity to positive words(10) and impaired memory for positive events(11), suggesting impaired positive emotion processing in PPD, similar to what has been shown in MDD.
Mechanistic study PPD is also informed by non-postpartum MDD given similarity in phenomenology(12), treatment response(3), and the history of non-postpartum MDD in many women with PPD. An important characteristic of non-postpartum MDD is reduced positive affect(13), which itself is comprised of several key neural components: initial striatal activation, sustainment of activation, and striatal input to prefrontal cortical regions for the purpose of reward-based motivation and learning. Neural biomarkers of MDD during striatal engagement include ventral striatum hypoactivity during viewing of pleasant stimuli(14,15) and during anticipatory and consummatory phases of reward processing(16,17). There has been little study of the early temporal dynamics of striatal responses to pleasant stimuli in MDD; however, psychophysiological and behavioral studies suggest that healthy individuals have positive emotional bias and more sustained responses to positive stimuli, while depressed individuals have less sustained responses to positive stimuli due to interference from negative affective processes(18, 19). Indeed, in an emotion regulation task during viewing of affective pictures, individuals with MDD had reduced ventral striatal activity to positive pictures when asked to sustain the emotion, relative to controls(20). Furthermore, the relationship between reduced ventral striatal activity and reduced self-reported positive affect in the MDD group(20) suggests an important link between early temporal dynamics of striatal response with longer-term depressive behaviors, likely mediated by impaired prefrontal cortical processes that support reward-based motivation and learning(21).
As an initial approach to examine reward-related deficits in PPD, a previously unexplored area, we examined the consummatory phase of reward using a novel, fast-event-related version of a well-established monetary reward paradigm(22). We tested the hypothesis that depressed relative to healthy mothers would have less total and less sustained(19) striatal activity during reward processing.
Methods
Subjects provided written informed consent as approved by the University of Pittsburgh Biomedical Institutional Review Board. The first 10 healthy mothers enrolled comprised an independent sample from which the ROI was established (23). Group comparison was conducted on the next 12 healthy mothers enrolled versus all 12 depressed mothers enrolled, without prospective subject matching. The structured clinical interview for DSM-IV(24) was used to assess psychiatric status. Healthy subjects had no present or past history of an Axis I disorder, no family history of a mood or psychotic disorder, and a 25-item Hamilton Rating Scale for Depression score (HAM25) ≤ 7. Depressed subjects had no psychotic or bipolar illness, met DSM IV criteria for major MDD, and had a HAM25 ≥ 15 in the past month. Both prevalent(beginning antenatally) and incident(new onset postpartum) cases of postpartum depression were included to maximize generalizability, since the disorder commonly begins antenatally(25). Subjects were excluded if they had medical or neurological illnesses likely to affect cerebral physiology or anatomy, gross abnormalities of brain structure evident by magnetic resonance images, suicidal intent, substance abuse within one year, lifetime history of substance dependence(other than nicotine), eating disorders, use of hormonal contraception, or exposure to medications likely to alter cerebral physiology within 3 weeks. Subjects, of whom 50% were primiparous, delivered a healthy, term infant in the preceding 10 weeks, were medication-free, and breastfeeding or bottlefeeding.
On the scan day, clinical severity was established with the Hamilton depression scale, the Fawcett-Clark Pleasure Scale, the Edinburgh Postnatal Depression Scale (a well-validated, 10-item self-report measure of perinatal depression, anxiety, and function(26)), and the parent-to-infant attachment questionnaire(a reliable and valid self-report of attachment quality, hostility, and pleasure in interaction during the first postpartum year(27)). Statistical tests on group differences in demographic, reproductive, psychiatric, and behavioral data were performed with Pearson chi-square for categorical and Mann-Whitney U exact tests for continuous variables (Table).
Table.
|
|
|
|||||
|---|---|---|---|---|---|---|
| Sample characteristics (mean ± SD) | Healthy Mothers | Depressed Mothers | ||||
|
|
|
|
||||
| n | 12 | 12 | ||||
| Mean or Median | SD | % or IQR | Mean or Median | SD | % or IQR | |
| Demographic characteristics | ||||||
|
| ||||||
| Age | 28.6 | 6.4 | -- | 27.5 | 4.7 | -- |
| Right handed | -- | -- | 100 | -- | -- | 91.7 |
| Body mass index | 25.8 | 3.3 | -- | 29.6 | 5.7 | -- |
| Smoker | -- | -- | 25 | -- | -- | 25 |
| Primiparous | -- | -- | 50 | -- | -- | 50 |
| Breastfeeding | -- | -- | 66.7 | -- | -- | 83.3 |
| Time since childbirth (weeks)* | 10.3 | 2.3 | -- | 8.4 | 2.1 | -- |
| Total annual household income < $50,000 | 50 | 50 | ||||
| Mood/Pleasure/Attachment scores | ||||||
|
| ||||||
| Hamilton depression rating scale score (25-item)** | 3.0 | 1.9 | -- | 21.3 | 7.2 | -- |
| Edinburgh postnatal scale for depression score** | 1.3 | 1.6 | -- | 14.9 | 4.5 | -- |
| Fawcett-Clark Pleasure Scale Total† | 139.9 | 18.7 | -- | 126.3 | 17.0 | -- |
| Quality of mother-infant attachment**a | 43.3 | 2.0 | -- | 35.1 | 7.3 | -- |
| Absence of maternal-infant hostility**a | 22.1 | 1.8 | -- | 15.8 | 4.6 | -- |
| Pleasure in maternal-infant interaction†a | 23.0 | 1.6 | -- | 18.2 | 6.2 | -- |
| fMRI task behavioral performance | ||||||
|
| ||||||
| Number missed trials b | 3.5 | -- | 1–8.5 | 7.5 | -- | 3.5–17 |
| reaction time (msec) | 699.36 | 66.87 | -- | 747.12 | 148.29 | -- |
0.05 < p < 0.10
p < 0.05
p < 0.001
Condon scale for parent-infant attachment (27)
Due to skewed distribution, median and interquartile range (IQR)are provided in lieu of mean and SD. For sensitivity analysis Median (IQR) for Depressed mother subsample (n=10) was 5.5 (5.5 – 9).
We used a fast event-related version of a well known monetary reward number guessing task that activates ventral striatum (22, 28) and that was previously used in a block design in MDD(29). Subjects guess whether the value of a hidden card is less than or greater than 5(range 1–9). Subjects receive monetary gains(rewards) for correct guesses and incur monetary losses(punishment) for incorrect ones. Gains and losses may be high (+$0.80, −$0.50) or low magnitude(+$0.30, −$0.20). Unbeknownst to the subjects trial outcomes are predetermined and there is no way to optimize winning. Feedback is given as upward point green (reward) or downward pointing red (punishment) arrows that are presented for 750 ms. The size of the arrow indicates magnitude(high or low). The task consists of 160 trials with 40 trials per outcome.
Scanning was performed on a Siemens 3 Tesla Trio (Erlangen, Germany). High-resolution, T1-weighted anatomical images were acquired using an MPRAGE sequence (TR=1630ms; TE=2.48 ms; FOV=20.4 cm; α=8°; image matrix=2562; voxel size = 0.8×0.8×0.8 mm; 224 slices). High resolution functional scans(blood oxygenation level-dependent: BOLD) were taken in the same plane as the anatomical images(28, 3 mm slices, TR 1.5s, 3.5 mm in plane resolution). In each run, 160 successive brain volumes were acquired (5 runs, total=800).
The NeuroImage Software package(NIS) and AFNI were used to preprocess and analyze the data. Data was transformed using standard anatomical landmarks(anterior and posterior commisures) to conform to the atlas of Talairach and Tournoux. Functional data were concatenated across runs and analyzed using a general linear model (3dDeconvolve). Covariates for the model included time onsets for each trial type(positive and negative feedback, high and low) with separate parameters estimated for each time point from 0 to 16.5s after trial onset(TRs 1–11), motion estimates, a model of linear drift and baseline activity. Beta values for each of the time point covariates were calculated by least squares regression to the BOLD signal. These values constitute a time series from time 0–16.5s for each voxel in the brain and were used for all subsequent within and between subject statistical analyses.
The analysis of the functional data from the independent sample of ten healthy mothers was used to localize a functional region of interest(ROI) in the striatum. For each subject, we generated a voxel-wise contrast between the estimated BOLD response to reward trials(high and low magnitude) and the estimated BOLD response to punishment trials(high and low magnitude). A voxelwise t-test versus the null hypothesis then determined clusters of voxels where this difference was significant. The AFNI AlphaSim program(Montecarlo method) was used on the contrast T-maps to set the contiguity thresholds such that the map wise probability of a false detection remained lower than 0.01(30). Repeated-measures ANOVA of time series data extracted from this ROI was next used to compare depressed(n=12) with healthy mothers(n=12), separately for each trial type(low reward, high reward, low punishment, or high punishment). A sensitivity analysis was conducted in which subjects were matched on behavioral performance(depressed n=10, healthy n=12).
Results (Table)
Demographic characteristics were similar among the groups; however, healthy mothers were 2 weeks further postpartum (10.3 vs. 8.4 wks) compared to depressed mothers (p=0.04). Symptoms were higher and pleasure and mother-infant attachment scores were lower in depressed mothers. Healthy subject characteristics did not differ on the basis of assignment to the independent sample ROI group versus the comparison group(data not shown). Comorbid disorders in the depressed group included ADHD (n=1), panic disorder(n= 3), social phobia(n=3), generalized anxiety(n=4), anxiety disorder NOS (n=1), and eating disorder NOS (n=2). There was no group difference in reaction times or number of missed trials during task performance (Table). Mean reaction time was less than 725 ms within the full cohort. The number of missed trials ranged from 0 to 37 with a median of 4 and 2–10.5 interquartile range within the full cohort.
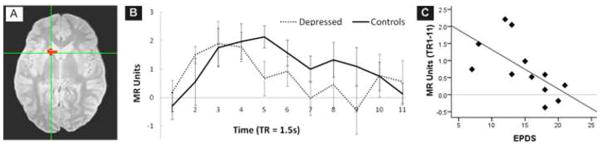
The Reward minus Punishment contrast in the independent sample of 10 healthy mothers revealed a region of significant difference in the left ventral striatum [−13, 12, −4, 477 mm3], cluster significance of p<0.01, corrected. The subsequent between group ANOVA for the high reward trial type revealed a significant quadratic main effect of time (F[1,22]=7.26, p=0.013), as expected, and a significant quadratic interaction of time*group(F[1,22]=5.22, p=0.032) that was confirmed in the sensitivity analysis(F[1,20]=5.01, p=0.037). This interaction effect represents a nonlinear attenuation of the BOLD response in the left ventral striatum response over time that is greater in depressed than healthy mothers (Figure). There were no significant time*group interactions in separate ANOVAs completed for low reward, low punishment, or high punishment trial types. In an exploratory correlation between time series and clinical data, the average MR unit for TR 1 through 11 was inversely correlated with depression severity, measured with the self-report Edinburgh postnatal scale for depression(Spearman rho = −0.80; p=0.002). There were no other significant correlations.
Figure 1.
A. Left ventral striatal region of interest [Talairach coordiantes for center voxel −13, 12, −4, volume 477 mm3] established through the Reward minus Punishment contrast in the independent sample of 10 healthy mothers.
B. Time course data for healthy (n=12) and depressed (n=12) mothers during high reward trials, extracted from the left ventral striatum regions of interest (figure A). Left ventral striatal BOLD activity associated with rewarding feedback increased in both depressed and healthy mothers from TR 1 to TR 4–5; however, the depressed mothers showed a more rapid attenuation of left ventral striatal response. Errors bars show standard error of the mean.
C. Time course data for depressed mothers averaged from TR 1 through 11 is shown relative to depressive severity, measured with the self-report Edinburgh postnatal scale for depression (Spearman rho = −0.80; p=0.002).
Discussion
We examined striatal response to monetary reward receipt following a number guessing task. In this task, striatal responses to positive feedback (reward) typically show an initial positive BOLD peak followed by a slow return to baseline(“sustain” component)(22, 31). In our sample of depressed mothers, there was a normal initial positive activity peak in the left ventral striatum in response to monetary reward; however, this group showed a rapid attenuation back to baseline, unlike the healthy mothers who revealed the expected sustain component. Although not well studied, it is likely that unsustained consummatory reward-related striatal activity in MDD contributes to longer term difficulties in motivation and goal-directed behavior, as mediated by deficient activation of prefrontal cortex systems for reward-based motivation and learning(18, 21). This concept is illustrated by the findings of Heller and colleagues(20) in non-postpartum MDD, in which lower self-reported levels of positive affect in MDD(over hours) was associated with reduced ability to sustain striatal activation(over 20 seconds) when viewing positive IAPS pictures.
Our finding of an inverse correlation between left striatal activity and depressive severity measured by the Edinburgh Postnatal Depression Scale(EPDS) increases biological plausibility for a specific role of altered striatal activity to reward in PPD. The lack of correlation between neural activity and other clinical measures suggests that the affective component of PPD(and not purely hedonic function, somatic symptoms, or mother-infant attachment) is strongly associated with striatal activity.
This is among the first studies of neural activity during positive emotion, and specifically reward processing, in PPD. The specific task version we used has not been applied to non-postpartum MDD, and therefore our results cannot be directly compared to prior reward studies in MDD. It is noteworthy, however, that reductions of time-based striatal activity to reward in PDD reported here bears similarity to reductions in striatal activity to reward in non-postpartum MDD(15–17, 32). Although our sample is small, it comprised unmedicated and largely antidepressant naïve women. We measured ventral striatal activity to reward receipt; there remain additional domains of reward to study in PPD, including motivational, “wanting,”(16) aspects of reward, as well as the cognitive processing components that drive motivation and relate to longer-term behavior.
The study design employed a monetary reward task well-known for activating striatum as a first step toward understanding reward circuitry in PPD. It remains unknown whether the reported neural responses in PPD would generalize to other positive stimuli, such as infant stimuli, which is an important area for further investigation. Adaptation of the paradigm to employ infant stimuli will be important for understanding how reward processing affects the mother-infant dyad. Our study design cannot determine whether unsustained striatal activity in PPD is a cause or effect of illness; nevertheless, if these findings are replicated, reward-enhancing behavioral therapies may have a role in PPD, as they do in MDD(33).
As we gain additional understanding of neural circuitry of PPD, we are acquiring evidence, that similar to other mood disorders, dysregulation in circuits that regulate both positive and negative emotion(34) is present in PPD. Additional research is warranted to investigate to the role of regulatory prefrontal cortical regions for sustaining ventral striatal activity to reward in PPD. This study was not designed to identify unique biomarkers of PPD and therefore does not answer the remaining important question of whether PPD and MDD are nosologically distinct. Additional research is needed to evaluate whether the postpartum timing of MDD, replete with social attachment hormones and behaviors, might confer a distinct neural signature for positive emotion processing relative to MDD that occurs at other times of the lifespan.
Acknowledgments
We thank all of the research participants and their families for their time dedicated to this study. We thank the University of Pittsburgh Research Center staff for performing the MR acquisition. We thank Medela, Inc for donation of a multi-use breast pump. We thank the CTSI of the University of Pittsburgh School of Medicine for assistance with recruitment.
This research was supported by National Institute of Mental Health R01 MH079164 and NARSAD Young Investigator Award to Dr. Moses-Kolko and NSF Supplemental Funding Grant SBE-0354420 and NSF Grant DRL-0815945 to Dr. Fiez. Dr. Moses-Kolko’s, Dr. Wisner’s, Mr James’, and Ms. Saul’s contribution to this work was supported by R01 MH079164. Drs Fraser’s and Fiez’s contribution to the work was supported by NSF Supplemental Funding Grant SBE-0354420 and NSF Grant DRL-0815945. Dr. Phillips’ contribution was supported by MH076971.
Footnotes
Financial Disclosures
Dr. Wisner has also received grant support from: Nova-Gyne (donation of transdermal placebo patches for an NIMH funded study of estradiol patch for postpartum depression treatment), and served on an advisory board for Eli Lilly Pharmaceutical Co. Dr. Wisner receives additional grant support from the National Institute of Mental Health. Dr. Phillips receives grant support from the National Institute of Mental Health, The United States Army Research Office, and The Pennsylvania Department of Health. Dr. Fiez receives grant support from the National Science Foundation and the NIH. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes. Evid Rep Technol Assess. 2005;119:1–8. doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beck CT. The effects of postpartum depression on child development: a meta-analysis. Arch Psychiatr Nurs. 1998;12:12–20. doi: 10.1016/s0883-9417(98)80004-6. [DOI] [PubMed] [Google Scholar]
- 3.Moses-Kolko EL, Berga SL, Kalro B, Sit DK, Wisner KL. Transdermal estradiol for postpartum depression: a promising treatment option. Clin Obstet Gynecol. 2009;52:516–529. doi: 10.1097/GRF.0b013e3181b5a395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne JL, Palmer JT, Joffe H. A reproductive subtype of depression: conceptualizing models and moving toward etiology. Harvard Review of Psychiatry. 2009;17:72–86. doi: 10.1080/10673220902899706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleming AS, Gonzalez A, Afonso VM, Lovic V. Plasticity in the maternal neural circuit: Experience, Dopamine, and Mothering. In: Bridges RS, editor. Neurobiology of the Parental Brain. Oxford: Elsevier; 2008. pp. 519–535. [Google Scholar]
- 6.Numan M, Stolzenberg DS. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Frontiers in Neuroendocrinology. 2009;30:46–64. doi: 10.1016/j.yfrne.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24:4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strathearn L, Li J, Fonagy P, Montague PR. What’s in a Smile? Maternal Brain Responses to Infant Facial Cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–2666. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, Goldstein M. Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectrums. 2007;12:853–862. doi: 10.1017/s1092852900015595. [DOI] [PubMed] [Google Scholar]
- 11.Hipwell A, Reynolds S, Crick E. Cognitive vulnerability to postnatal depressive symptomatology. Journal of Reproductive and Infant Psychology. 2004;22:211–227. [Google Scholar]
- 12.Wisner K, Gracious B, Piontek C, Peindl K, Perel J. Postpartum disorders: Phenomenology, treatment approaches, and relationship to infanticide. In: Spinelli M, editor. Psychosocial and Legal Perspectives on Mothers Who Kill. Elsevier, London: American Psychiatric Publishing, Inc; 2003. [Google Scholar]
- 13.Clark LA, Watson D. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- 14.Lawrence NS, Williams AM, Surguladze S, Giampietro V, Brammer MJ, Andrew C, Frangou S, Ecker C, Phillips ML. Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry. 2004;55:578–587. doi: 10.1016/j.biopsych.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 15.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry. 2006;163:1784–1790. doi: 10.1176/ajp.2006.163.10.1784. [DOI] [PubMed] [Google Scholar]
- 16.Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM, Brown SM, Ryan ND, Birmaher B, Axelson DA, Dahl RE. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. Am J Psychiatry. 2009;166:64–73. doi: 10.1176/appi.ajp.2008.07081336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomarken AJ, Keener AD. Frontal Brain Asymmetry and Depression: A Self-regulatory Perspective. Cognition & Emotion. 1998;12:387–420. [Google Scholar]
- 19.Davidson RJ. Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion. 1998;12:307–330. [Google Scholar]
- 20.Heller AS, Johnstone T, Shackman AJ, Light SN, Peterson MJ, Kolden GG, Kalin NH, Davidson RJ. Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:22445–22450. doi: 10.1073/pnas.0910651106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berridge KC, Robinson TE. Parsing reward. Trends in Neurosciences. 2003;26:507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- 22.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 23.Jollant F, Lawrence NS, Olie E, O’Daly O, Malafosse A, Courtet P, Phillips ML. Decreased activation of lateral orbitofrontal cortex during risky choices under uncertainty is associated with disadvantageous decision-making and suicidal behavior. NeuroImage. 2010;51:1275–1281. doi: 10.1016/j.neuroimage.2010.03.027. [DOI] [PubMed] [Google Scholar]
- 24.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1998. [Google Scholar]
- 25.Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. Americal Journal of Obstetrics and Gynecology. 2005;192:522–526. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 26.Cox J, Holden J, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 27.Condon JT, Corkindale CJ. The assessment of parent-to-infant attachment: development of a self-report questionnaire instrument. Journal of Reproductive and Infant Psychology. 1998;16:57–76. [Google Scholar]
- 28.Tricomi E, Delgado MR, McCandliss BD, McClelland JL, Fiez JA. Performance feedback drives caudate activation in a phonological learning task. Journal of Cognitive Neuroscience. 2006;18:1029–1043. doi: 10.1162/jocn.2006.18.6.1029. [DOI] [PubMed] [Google Scholar]
- 29.Forbes EE, Christopher May J, Siegle GJ, Ladouceur CD, Ryan ND, Carter CS, Birmaher B, Axelson DA, Dahl RE. Reward-related decision-making in pediatric major depressive disorder: an fMRI study. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2006;47:1031–1040. doi: 10.1111/j.1469-7610.2006.01673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cox RW. AFNI: Software for Analysis and Visualization of Functional Magnetic Resonance Neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 31.Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cogn Affect Behav Neurosci. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- 32.Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 33.Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ. The effects of psychotherapy on neural responses to rewards in major depression. Biological Psychiatry. 2009;66:886–897. doi: 10.1016/j.biopsych.2009.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala to negative emotional faces in postpartum depression. American Journal of Psychiatry. 2010 doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]