Abstract
Preclinical evidence implicates several neurotransmitter systems in the extinction of conditioned fear. These results are of great interest, because the reduction of acquired fear associations is critical in therapies for anxiety disorders. We tested whether findings with respect to the N-methyl-D-aspartate (NMDA) and cannabinoid receptor (CB) systems in animals carry over to healthy human subjects. To that end, we administered selected doses of D-cycloserine (partial NMDA receptor agonist, 250 mg), delta-9-tetrahydrocannabinol (THC, CB1 receptor agonist, 10 mg), or placebo prior to the extinction session of a 3-day conditioning protocol. D-cycloserine did not affect within-session extinction, or the retention of extinction in healthy human participants, in contrast with patient data but in line with previous reports in healthy volunteers. During extinction training, Δ9-THC reduced conditioned skin conductance responses, but not fear-potentiated startle. This effect was not retained at the retention test 2 days later, suggesting it was dependent on acute effects of the drug. Our findings implicate that facilitation of the CB1 or NMDA system with the substances used in this study does not affect conditioned fear extinction lastingly in healthy humans. The apparent discrepancy between these findings and the results from (pre-) clinical trials is discussed in terms of room for improvement in these systems in healthy volunteers, and the lack of specificity of THC as a CB1 agonist.
Keywords: Δ9-THC, d-cycloserine, extinction, fear conditioning, fear-potentiated startle, human
Introduction
Fear conditioning is considered central to the aetiology and maintenance of anxiety disorders (Mineka and Oehlberg, 2008). Accordingly, an initially neutral conditional stimulus (CS) comes to elicit fear after being associated with an unconditioned aversive stimulus (US). Exposure therapy, one of the most effective treatments for anxiety disorders, attempts to counteract the CS–US association through repeated exposure to fear-provoking stimuli in absence of negative consequences. Repeated exposure to the CS in the absence of the US (extinction training) leads to extinction, a reduction in conditioned fear responses. Because suppressing the fear reaction provoked by emotional and traumatic memories appears crucial to successful treatment of anxiety disorders, there is growing interest in pharmacological treatments that may facilitate extinction. Recent findings have identified receptor systems that may constitute pharmacological targets for facilitation of the extinction process (reviewed by Myers and Davis, 2007). While the ultimate test for treatment aimed at modulating extinction lies in clinical trials, screening substances in healthy human subjects can bridge the current gap between preclinical findings and application of these substances in humans. Tools with great potential for this cross-species translational research are the startle reflex and fear conditioning (Davis et al., 1993; Grillon and Baas, 2003).
Animal work has indicated that the N-methyl-D-aspartate (NMDA) receptor is a crucial link in fear extinction (Davis, 2002; Falls et al., 1992; see Myers et al., 2011 for review). D-cycloserine (DCS), a partial agonist at the glycine modulatory site of the NMDA receptor, enhances learning and memory in several animal models without producing obvious toxicity (Flood et al., 1992; Monahan et al., 1989; Steele et al., 1996; Thompson and Disterhoft, 1997). Moreover, DCS enhances extinction retention in rodents, both in fear-potentiated startle (FPS) (Walker et al., 2002), and in conditioned freezing. In addition, clinical trials have demonstrated that DCS administered during exposure therapy improves long-term extinction of pathological fear responses in patients with fear of heights (Ressler et al., 2004), social anxiety (Guastella et al., 2008; Hofmann et al., 2006), and obsessive–compulsive disorder (Kushner et al., 2007; Storch et al., 2010; Wilhelm et al., 2008; but see also Storch et al., 2007). In contrast, DCS did not affect extinction of fear measured with autonomic measures (skin conductance and heart rate) in healthy volunteers and subjects selected for subclinical phobic fear of spiders (Guastella et al., 2007a, 2007b). This result calls for more experimental work to elucidate the working mechanism of DCS on extinction. We set out to further test effects of DCS in a human fear extinction paradigm, including FPS as a physiological measure for fear (Lang et al., 1990). FPS indexes fear at a basic physiological level that may reflect more implicit processing than skin conductance (Hamm and Vaitl, 1996) and may be more sensitive to pharmacological treatments (Graham et al., 2005). Moreover, FPS is also used in the preclinical work (Davis et al., 2006), making it a more direct translational tool.
Converging findings indicate that the CB1 receptor is also crucial in the extinction of conditioned fear. This was demonstrated by blocking CB1 transmission in mice either by gene knockout or administration of the CB1 antagonist SR141716A (Marsicano et al., 2002). These findings were replicated and extended using an FPS protocol (Chhatwal et al., 2005) and contextual fear induced freezing in rats (Pamplona et al., 2006). In addition, increasing availability of endogenous cannabinoids (eCB) by blocking eCB reuptake and breakdown (Chhatwal et al., 2005) or activating this receptor with an agonist (Pamplona et al., 2006) both facilitate fear extinction retention in rodents. These findings suggest that enhancing CB1 transmission may also facilitate fear extinction in humans. Nevertheless, up to now there has been no study published on the effect of cannabinoid agents on conditioned fear in humans. The non-selective CB1/CB2 ligand delta-9-tetrahydrocannabinol (THC) exerts its central effects through agonism of the CB1 receptor. THC, the primary constituent of cannabis, produces cannabis-like subjective effects (Wachtel et al., 2002) and is readily available for administration in humans; it is prescribed as an anti-emetic and appetite stimulant.
In the current study, three groups of healthy volunteers participated in a 3-day conditioning protocol, in which on the second day, 2 h before extinction training, a single dose of either 250 mg DCS, 10 mg of the CB1 receptor agonist Δ9-THC or a placebo was administered. Two days later, extinction retention was tested without drug administration. We hypothesized that both DCS and Δ9-THC would increase retention of fear extinction as indexed by FPS.
Materials and methods
Subjects
Healthy volunteers age 18–30 years were recruited with flyers on a university campus. Exclusion criteria were: never having been exposed to marijuana, current drug use, low startle reactivity, pregnancy, and first or second-degree family members with a history of psychosis. In total 61 subjects were included in the trial. A complete data set was acquired of 18 subjects per group (total N = 54, 31 female, 23 male). Two subjects dropped out because of side effects of THC, one was removed after a positive drug test for cannabis. Electromyography (EMG) data of four subjects were not usable due to equipment failure. See Table 1 for an overview of the gender distribution, mean age and mean Spielberger trait anxiety scores (Spielberger, 1972) in each group.
Table 1.
Sample overview. Sample size (N), male/female distribution and mean (SD) age and state–trait anxiety inventory (STAI) trait score for each of the drug groups.
| DCS | Placebo | THC | |
|---|---|---|---|
| N | 18 | 18 | 18 |
| Gender (m/f) | 8/10 | 7/11 | 8/10 |
| Age | 21.9 (1.9) | 21.7 (1.8) | 21.0 (1.2) |
| STAI score | 29.4 (5.9) | 29.5 (5.6) | 30.8 (5.3) |
Treatments
Treatments were administered in a double-blind parallel placebo-controlled design. Capsules containing either 10 mg of delta-9- tetrahydrocannabinol (synthetic Δ9-THC, Marinol), 250 mg of DCS or placebo were administered orally, 2 h before the extinction training session. Previous work showed efficacy of 50–500 mg of DCS (Norberg et al., 2008; Ressler et al., 2004), which led us to select an intermediate dose. For THC, evidence from preclinical studies shows that relatively low doses of CB1 agonists are most effective in facilitating extinction (Chhatwal et al., 2005; Pamplona et al., 2006), and that high doses are anxiogenic (Moreira and Lutz, 2008; Viveros et al., 2005). Therefore we selected a relatively low dose of 10 mg which typically does not produce anxiety (Zuurman et al., 2009) or strong negative effects on cognition (Curran et al., 2002; McDonald et al., 2003).
Procedure
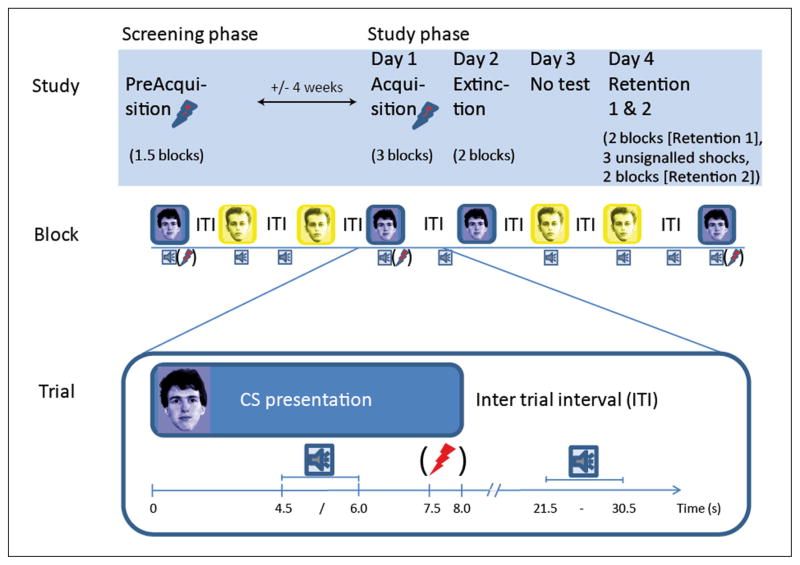
This study was conducted in accordance with the Declaration of Helsinki and approved by the medical ethical committee of the University Medical Centre Utrecht. Following telephone screening, eligible subjects were invited to the institute for informed consent, a thorough medical and psychiatric screening including the Dutch version of the Mini-International Neuropsychiatric Interview (Van Vliet et al., 2007), a startle test and an initial acquisition session (details of conditioning sessions described below). Approximately 4 weeks later, day 1 of the protocol consisted of a final acquisition phase. On day 2 extinction training took place, and the retention test was conducted 2 days later (day 4), separated into two parts (Retention 1 and Retention 2; see Figure 1 for overview). Retention sessions 1 and 2 were separated only by a 35 s interval during which three shocks were administered (details below).
Figure 1.
Illustration of the study set up. The top row illustrates the different study sessions, the middle row gives an example of one experimental block, and the bottom row exemplifies how a trial was construed. The flash of lightning represents administration of a shock (between brackets as shocks were only delivered during trials in the acquisition phase). The sound icon represents administration of a noise to elicit a startle reflex (startle probe).
Stimuli and apparatus
CS+ and CS− stimuli were two neutral faces taken from the Psychological Image Collection at Stirling (PICS) (http://pics.stir.ac.uk/), one coloured blue and one coloured yellow to increase distinctiveness. Each was presented against a black background. A white fixation cross replaced the CS during the inter-trial interval (ITI). A trial consisted of a CS presented for 8 s. In the reinforced CS+ trials, shocks were administered 7500 ms after CS onset on the right wrist by a constant current generator at 2.5 mA for 50 ms. Startle probes were presented at 4500 or 6000 ms after CS onset or at semi-random moments during the ITI. Inter-startle intervals were 17, 20 or 23 s with a mean of 20 s for each of the conditions (CS+, CS− and ITI). Also, the minimum interval between a shock presentation and the next startle probe was 17 s (see Figure 1 for illustration of the startle timing). ITIs between CSs were varied in duration between 4 and 22.5 s. The startle probes consisted of a 95 dB(A) burst of white noise with near instantaneous rise time and 50 ms duration presented binaurally through foam earplugs by Ear Link headphones (Aero Company).
Physiological recording and amplification was carried out using the Biosemi Active Two system (www.biosemi.nl). Startle was measured with EMG from the orbicularis oculi muscle under the right eye. The skin conductance response (SCR) was recorded by two electrodes on the palm of the left hand.
Conditioning procedure
To ensure acquisition of conditioned responses, an initial training session was included in the screening visit (approximately 4 weeks before the experimental phase) comprising six CS+ (all reinforced) and six CS− presentations. The sessions on experimental days 1, 2 and 4, started with a brief startle habituation phase consisting of nine startle probes followed by a single test shock to confirm proper electrode fixation and equipment function to the subject (incorporated after a pilot in which subjects alerted the experimenter during the extinction session because they supposed that the shock administration was not functioning properly). Directly afterwards, subjects completed subjective fearfulness and shock expectancy ratings for each cue as supplementary measures of conditioning (these data matched the physiological data and are therefore described in the supplemental material). After the ratings, the experimental phase commenced. Each experimental phase consisted of blocks of four trials of each CS (4 CS+ and 4 CS−) with three startles probe per condition in each block (CS+, CS−, ITI). Unless stated otherwise, blocks were not separated by delays. The details for each phase are given below, summarized in Figure 1.
Acquisition (day 1)
During day 1, subjects were presented with three blocks of four CS+ and four CS− presentations. Three out of the four CS+ trials were reinforced with a shock. Following the first block of acquisition, subjects received a written on-screen instruction that they could only receive shocks during the previously reinforced CS.
Extinction (day 2)
Day 2 started with drug ingestion, after which subjects filled out paper-and-pencil visual analogue scales on drug effects at time points 0, 1, 2, and 3 h post ingestion. The extinction phase commenced 2 h post ingestion, consisting of two blocks (no pause) of four CS+ and four CS− trials all without shock reinforcement.
Retention 1 and 2 (day 4)
The retention phases each comprised two blocks without shock reinforcement. After the first retention phase, a black screen with only a fixation cross was presented for 35 s during which subjects received three unsignalled shocks spaced 5 s apart. These isolated shocks were administered to assess differential impact on retention between substances.
Data processing and scoring
Startle and SCR data pre-processing is described in detail in the supplementary material. Startle amplitudes were quantified as the highest peak between 25 and 100 ms after probe onset and average amplitude was calculated for each block. Startle data were normalized with a within-subjects z-transformation (Grillon and Baas, 2002). First interval SCR responses (FIRs) were defined as the first peak with an onset 1–4 s after CS onset, and second interval responses (SIRs) as peaks between 4–8 s. SCR data were normalized with a square-root transformation.
Statistical analyses
All analyses were SPSS 18 repeated measures ANOVAs. Physiological data were analysed with Phase (Acquisition, Extinction, Retention 1, Retention 2), CS (CS+, CS−), and Block (1, 2) as within-subjects factors, and Drug as between-subjects factor. Conditioned responding was assessed by evaluating the difference in response to the CS+ and CS− for all measures, as indexed by the factor CS in our repeated measures model. To evaluate the development of the conditioned response across phases we compared the CS main effect in the extinction, retention 1 and retention 2 phases with the CS main effect during acquisition. Additional details are provided in the supplemental material. Finally, subjective effects of the drugs were assessed directly after drug administration and 1, 2 and 3 h after ingestion. These data were analysed with Time (0, 1, 2, 3 h after ingestion) as within-subjects factor, and Drug as between-subjects factor.
Results
Subjective drug effects
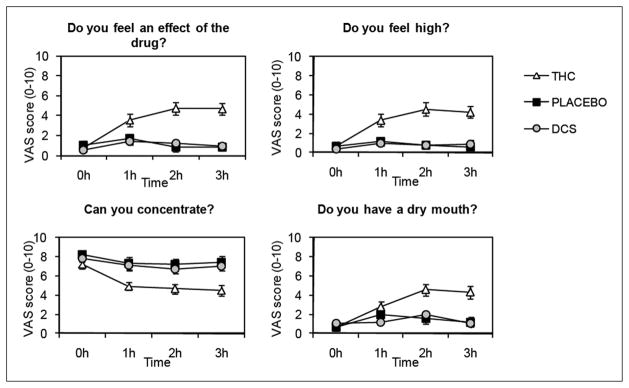
For all questions in Figure 2 there were significant Drug main effects (F(2,51)-values > 6.6, p-values < 0.005) and Time × Drug interactions (F(6,153)-values > 3.9, GG-ε corrected p-values < 0.005). THC, in comparison with placebo, resulted in significant increases in reportable effects of the drug (Time × Drug THC vs. placebo (F(3,102)-values > 4.8, all p-values < 0.01). Peak effects were reached at 2 h, immediately before the start of the extinction session (see Figure 2). DCS did not differ from placebo on any question (F-values all < 1.1, n.s.).
Figure 2.
Mean subjective ratings of drug effects scored with visual analogue scales (VAS). Anchors were ‘not at all’ (0) and ‘very much’ (10). Error bars are standard error of the mean (SEM).
Fear-potentiated startle
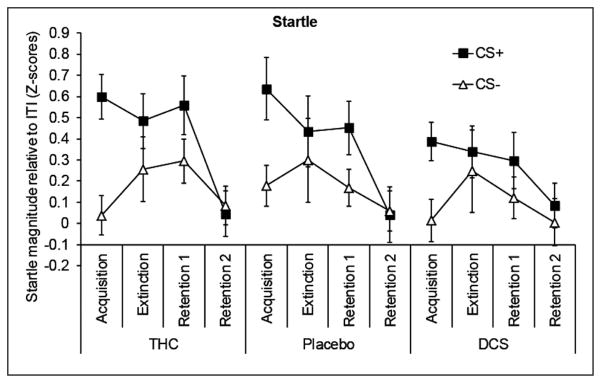
See Figure 3 for startle responses during CS+ and CS− relative to responses during the ITI for each of the conditioning phases. A plot containing ITI data is available in the supplemental material (Figure S2). The conditioning procedure produced the intended results as witnessed by the development of conditioned responding across phases (Phase × CS F(3,153; GGε = 0.75) = 4.4, p = 0.01). Compared with acquisition, the difference between CS+ and CS− (FPS) in the extinction phase was significantly reduced (F(1,51) = 4.8, p = 0.03). Compared with acquisition the first retention phase showed a marginally significant reduction in FPS (F(1,51) = 3.6, p = 0.06). In the second retention phase (Retention 2), no evidence of a reinstatement effect due to the uncoupled shocks was found. Instead, the conditioned FPS response was completely extinguished, reflected in a highly significant difference with acquisition (F(1,51) = 19.8, p < 0.001). See supplementary material (Table S1) for CS effects per phase.
Figure 3.
Mean startle magnitudes relative to inter-trial interval (ITI) in the three drug groups during the different phases of conditioning. The difference between CS+ and CS− constitutes fear-potentiated startle (FPS). Error bars are SEM.
Neither drug affected baseline startle as indexed by the mean raw startle amplitude (not T-transformed) during the habituation phase preceding the extinction session (THC M = 97.2 μV SD = 73.7, Placebo M = 100.5 μV SD = 35.8, DCS M = 100.3 μV SD = 55.1; ANOVA F-value < 1). Moreover, although conditioning was successful, our analyses suggested no effect of either drug treatment on the extinction of fear measured with startle. None of the interactions with Drug reached significance in the overall analysis (all Fs < 1.5, n.s.). More specific analyses also gave no significant results (for the specific contrasts including Drug and the different levels of the factors Phase and CS all F-values < 2.5, n.s.).
Skin conductance response
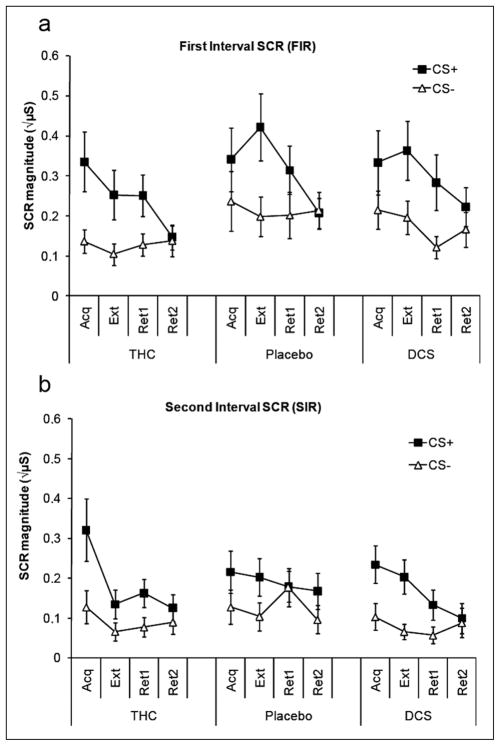
FIR (Figure 4a) showed sensitivity to the conditioning procedure as reflected in a Phase × CS interaction (F(3,153; GGε = 0.96) = 9.6, p < 0.001). Compared with acquisition, specific contrasts indicated no significant extinction of CS differentiation in the SCR data across extinction (F(1,51)= 1.7, n.s.), or early retention (F(1,51)= 0.5, n.s.). During late retention (retention 2), the conditioned response was significantly reduced with respect to acquisition (F(1,34)= 14.9, p < 0.001). See supplementary material (Table S1) for CS effects per phase.
Figure 4.
Mean skin conductance responses in the three drug groups during the different phases of conditioning. Skin conductance responses (SCRs) are divided into (a) early responses reflecting an orienting response to the CS (first interval responses) and (b) late responses reflecting anticipation of the US (second interval responses, panel B). Error bars are SEM. Acq, Acquisition; Ext, Extinction; Ret, Retention; CS, conditioned stimulus
Neither interactions in the overall analysis with Drug nor any of the specific contrasts between levels of the factors Phase and CS with Drug were significant (all Fs < 1.4, n.s.). One specific contrast, between CS+ and CS− in the difference between acquisition and extinction in interaction with drug, approached significance (CS × Drug on acquisition vs. extinction, F(1,34) = 2.7, p = 0.08). Testing per active drug condition against placebo revealed that this effect was caused by a CS (acquisition versus extinction) difference between THC and placebo (F(1,34) = 6.0, p = 0.02), while there was no difference in the comparison DCS versus placebo (F(1,34) = 0.7, n.s.). The THC group showed more evidence of extinction than the placebo group (see Figure 4). However, the effect did not carry over into the retention phase, as reflected in non-significant differences between drug groups in the comparisons of the CS effect between acquisition and both parts of the retention phase (Phase × CS × Drug interaction < 1.1, n.s.).
Similar conditioning effects were observed for the SIR (Figure 4b, omnibus statistics omitted for brevity). A comparable THC effect was observed in the comparison between acquisition and extinction, which again did not carry over to retention. Tested against placebo, the THC group differed from placebo in a marginally significant Phase (acquisition vs. extinction) × CS × Drug (F(1, 34) = 4.0, p = 0.05) interaction. There were again no effects in the corresponding specific comparisons between acquisition and the retention phases (all F-values < 2.7, p > 0.11).
Discussion
The current study sought to evaluate the effect of selected doses of THC and DCS on the extinction of fear conditioning in humans. Fear conditioning was successful in that there was consistent and significant cued conditioned responding. A significant reduction of conditioned responses was observed only at the last retention phase for several of the measures, leaving room for drug-induced extinction facilitation. However, neither DCS nor THC affected the retention of fear extinction.
This study may be seen as one of the initial steps in the development of paradigms that could be used for this purpose. Creating sufficient room for improvement in our dependent measures of fear extinction was crucial, as this could explain previous negative findings in healthy humans for DCS (Guastella et al., 2007b; Vervliet, 2008). In the current study we took several precautions to leave room for facilitation of extinction, as extinction in healthy participants can be fast (Vansteenwegen et al., 1998). The main measures that were taken to delay extinction were (i) acquisition training on two different occasions (screening and experimental day 1); (ii) partial reinforcement during the latter part of acquisition; and (iii) a relatively short extinction training phase compared with other human extinction protocols (e.g. Alvarez et al., 2007; Norrholm et al., 2006). Accordingly, the average conditioned response during the extinction session was only significantly reduced compared with acquisition in the startle data. Also, the presence of a significant conditioned response in all of the measures in the first retention phase indicates there was room for drug-induced improvement of extinction. Nevertheless, in contrast to our hypotheses, neither DCS nor THC convincingly affected any measure of fear extinction in this study. While reinstatement of conditioned responding has been demonstrated in humans after unsignalled US presentation following a single extinction session (Kindt et al., 2009; Norrholm et al., 2006), unsignalled shocks did not reinstate the conditioned response after two extinction training sessions, extinction and retention 1, that took place on separate days in our study. Therefore drug effects on reinstated fear could not be evaluated.
The absence of enhanced extinction following DCS administration could be explained in several ways. It has been suggested that the effects of DCS on extinction training may only be effective if extinction is well under way (Bouton et al., 2008; Weber et al., 2007). In line with this, there is evidence that DCS may strengthen the reconsolidation of fear memories rather than facilitate extinction when few extinction trials are presented (Lee et al., 2006). The relatively short extinction training phase and the limited reductions in conditioned responding that occurred as a result could thus be an explanation for the negative findings in this study. Nevertheless, alternative explanations should be considered, given the consistency of our finding with other negative results in healthy humans in paradigms that produced more robust reductions in fear during extinction training (Guastella et al., 2007b). While one explanation has been that DCS exerts its effects through lower-level mechanisms than assessed previously (Grillon, 2009), a lack of effects on our startle measure indicates that also when extinction is assessed using basic defensive measures that do not require explicit awareness (Weike et al., 2005) DCS treatment has no effect in healthy humans. A variety of conditioning studies in healthy participants to date thus do not confirm effects of DCS on fear extinction observed in animal and patient work.
Another explanation for the negative findings is that DCS produces facilitating effects only when there is resistance to extinction. In both patients and experimental animals anxiety is expected to reach higher levels. Moreover, because clinical anxiety typically does not extinguish naturally, patients may be characterized by a resistance to extinction. Rather than acting as cognitive enhancer, DCS may thus be better described as a substance that enables extinction resistance to be overcome. For example, DCS may only exert an augmenting effect on glutamatergic NMDA receptors when the receptors are not already saturated (Davis et al., 2006). In healthy individuals extinction, and thus the NMDA receptor activation necessary for this process, may already be optimal.
Following promising findings in the animal literature (Chhatwal et al., 2005; Marsicano et al., 2002) this was the first study to assess effects of a cannabinoid agonist on human fear extinction. Administration of THC resulted in less responsiveness to presentations of the CS+ in the skin conductance measure during the extinction phase. Although THC also has affinity for CB2 receptors, these are localized predominantly outside the central nervous system. The effect of THC was primarily observed in responding to the CS+, indicating it most plausibly emerged from stimulation of central CB1 receptor transmission. However, we could not confirm this pattern with our other measures of extinction, particularly startle, and therefore this result needs to be interpreted with caution. Moreover, no differences were observed compared with the placebo group during the retention phase, suggesting that THC only affected conditioned responding on the SCR measure acutely and not long term. Tentatively, this result seems consistent with the recently developed notion that CB1 transmission may primarily affect within-session extinction (Plendl and Wotjak, 2010), perhaps through habituation-like mechanisms (Kamprath et al., 2006). One potentially powerful option to scrutinize this further in humans would be to utilize a CB1 antagonist, as it may be easier to block rather than facilitate extinction in healthy humans already capable of fast fear extinction. Unfortunately, the CB1 antagonist rimonabant was recently taken off the market due to serious adverse events, making its use in a human trial problematic. Use of a drug aimed at prolonging availability of eCBs would arguably be a more elegant approach than use of THC. These drugs may render more confined effects (Moreira and Lutz, 2008), only acting when and where endocannabinoids are released. Moreover, depending on local endocannabinoid levels and CB1 receptor occupancy THC may exert antagonistic effects (Hoyer and Boddeke, 1993; Laaris et al., 2010). Unfortunately, substances that inhibit the reuptake or breakdown of endocannabinoids are currently not available for use in humans. An interesting alternative could be the use of cannabidiol (Bitencourt et al., 2008).
Importantly, the lack of drug effects on extinction performance in this study is not likely the result of insufficient drug availability at the time of testing. For DCS, plasma levels plateau for several hours after reaching a peak level approximately 1 h after ingestion (van Berckel et al., 1997,1998). Also, doses ranging from 50–500 mg have shown efficacy in human studies without clear evidence of dose dependency (Norberg et al., 2008), although one study did find stronger effects in 500 than 50 mg (Ressler et al., 2004). While the pharmacokinetics of orally administered THC such as peak concentration and time until peak concentration vary across individuals (Grotenhermen, 2003), subjective drug effects after THC administration arrived at peak levels at the time of the extinction test (2 h after ingestion) which is in line with previous work (Curran et al., 2002; McDonald et al., 2003; Phan et al., 2008). Nevertheless, our use of single, preselected doses for both drugs should be considered a limitation to the current study.
In conclusion, we implemented a human conditioning paradigm with FPS as primary outcome measure to test for substances facilitating extinction of conditioned fear. In line with previous work, DCS did not facilitate extinction of fear in healthy subjects. This suggests that DCS is not a cognitive enhancer per se, but may be most effective when there is resistance to extinction. Also, the cannabinoid agent THC did not facilitate extinction of fear lastingly. However, as this was only the first study exploring cannabinoid involvement in humans, more work is necessary to elucidate the role of the CB1 system in human fear extinction, preferably with more selective compounds.
Supplementary Material
Acknowledgments
Funding
This work was supported by a NWO Veni-grant awarded to JMPB, and by the NIMH Intramural Research Program (CG).
The authors would like to thank Linda van Ooijen for valuable assistance in subject screening.
Footnotes
Conflict of interest
None of the authors have potential conflict of interest in relation to the subject of this report.
References
- Alvarez RP, Johnson L, Grillon C. Contextual-specificity of short-delay extinction in humans: renewal of fear-potentiated startle in a virtual environment. Learn Mem. 2007;14:247–253. doi: 10.1101/lm.493707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol. 2008;18:849–859. doi: 10.1016/j.euroneuro.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Vurbic D, Woods AM. D-cycloserine facilitates context-specific fear extinction learning. Neurobiol Learn Mem. 2008;90:504–510. doi: 10.1016/j.nlm.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhatwal JP, Davis M, Maguschak KA, et al. Enhancing cannabinoid neurotransmission augments the extinction of conditioned fear. Neuropsychopharmacology. 2005;30:516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- Curran HV, Brignell C, Fletcher S, et al. Cognitive and subjective dose-response effects of acute oral Delta 9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology (Berl) 2002;164:61–70. doi: 10.1007/s00213-002-1169-0. [DOI] [PubMed] [Google Scholar]
- Davis M. Role of NMDA receptors and MAP kinase in the amygdala in extinction of fear: clinical implications for exposure therapy. Eur J Neurosci. 2002;16:395–398. doi: 10.1046/j.1460-9568.2002.02138.x. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, et al. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, et al. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Morley JE, Lanthorn TH. Effect on memory processing by D-cycloserine, an agonist of the NMDA/glycine receptor. Eur J Pharmacol. 1992;221:249–254. doi: 10.1016/0014-2999(92)90709-d. [DOI] [PubMed] [Google Scholar]
- Graham SJ, Scaife JC, Langley RW, et al. Effects of lorazepam on fear-potentiated startle responses in man. J Psychopharmacol. 2005;19:249–258. doi: 10.1177/0269881105051528. [DOI] [PubMed] [Google Scholar]
- Grillon C. D-cycloserine facilitation of fear extinction and exposure-based therapy might rely on lower-level, automatic mechanisms. Biol Psychiatry. 2009;66:636–641. doi: 10.1016/j.biopsych.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JMP. Comments on the use of the startle reflex in psychopharmacological challenges: impact of baseline startle on measurement of fear-potentiated startle. Psychopharmacol. 2002;164:236–238. doi: 10.1007/s00213-002-1164-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas JMP. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grotenhermen F. Pharmacokinetics and pharmacodynamics of cannabinoids. Clin Pharmacokinet. 2003;42:327–360. doi: 10.2165/00003088-200342040-00003. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Dadds MR, Lovibond PF, et al. A randomized controlled trial of the effect of D-cycloserine on exposure therapy for spider fear. J Psychiatr Res. 2007a;41:466–471. doi: 10.1016/j.jpsychires.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Lovibond PF, Dadds MR, et al. A randomized controlled trial of the effect of D-cycloserine on extinction and fear conditioning in humans. Behav Res Ther. 2007b;45:663–672. doi: 10.1016/j.brat.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Richardson R, Lovibond PF, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry. 2008;63:544–549. doi: 10.1016/j.biopsych.2007.11.011. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D. Affective learning: awareness and aversion. Psychophysiology. 1996;33:698–710. doi: 10.1111/j.1469-8986.1996.tb02366.x. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Meuret AE, Smits JA, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry. 2006;63:298–304. doi: 10.1001/archpsyc.63.3.298. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Boddeke HW. Partial agonists, full agonists, antagonists: dilemmas of definition. Trends Pharmacol Sci. 1993;14:270–275. doi: 10.1016/0165-6147(93)90129-8. [DOI] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, et al. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt M, Soeter M, Vervliet B. Beyond extinction: erasing human fear responses and preventing the return of fear. Nat Neurosci. 2009;12:256–258. doi: 10.1038/nn.2271. [DOI] [PubMed] [Google Scholar]
- Kushner MG, Kim SW, Donahue C, et al. D-cycloserine augmented exposure therapy for obsessive–compulsive disorder. Biol Psychiatry. 2007;62:835–838. doi: 10.1016/j.biopsych.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Laaris N, Good CH, Lupica CR. Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology. 2010;59:121–127. doi: 10.1016/j.neuropharm.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychol Rev. 1990;97:377–395. [PubMed] [Google Scholar]
- Lee JL, Milton AL, Everitt BJ. Reconsolidation and extinction of conditioned fear: inhibition and potentiation. J Neurosci. 2006;26:10051–10056. doi: 10.1523/JNEUROSCI.2466-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, et al. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- McDonald J, Schleifer L, Richards JB, et al. Effects of THC on behavioral measures of impulsivity in humans. Neuropsychopharmacology. 2003;28:1356–1365. doi: 10.1038/sj.npp.1300176. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychol (Amst) 2008;127:567–580. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Monahan JB, Handelmann GE, Hood WF, et al. D-cycloserine, a positive modulator of the N-methyl-D-aspartate receptor, enhances performance of learning tasks in rats. Pharmacol Biochem Behav. 1989;34:649–653. doi: 10.1016/0091-3057(89)90571-6. [DOI] [PubMed] [Google Scholar]
- Moreira FA, Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict Biol. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Jr, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–293. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norberg MM, Krystal JH, Tolin DF. A meta-analysis of D-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63:1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, et al. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn Mem. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pamplona FA, Prediger RD, Pandolfo P, et al. The cannabinoid receptor agonist WIN 55,212-2 facilitates the extinction of contextual fear memory and spatial memory in rats. Psychopharmacology (Berl) 2006;188:641–649. doi: 10.1007/s00213-006-0514-0. [DOI] [PubMed] [Google Scholar]
- Phan KL, Angstadt M, Golden J, et al. Cannabinoid modulation of amygdala reactivity to social signals of threat in humans. J Neurosci. 2008;28:2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plendl W, Wotjak CT. Dissociation of within- and between-session extinction of conditioned fear. J Neurosci. 2010;30:4990–4998. doi: 10.1523/JNEUROSCI.6038-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anxiety Inventory. Palo Alto: Consulting Psychologist Press; 1972. [Google Scholar]
- Steele RJ, Dermon CR, Stewart MG. D-cycloserine causes transient enhancement of memory for a weak aversive stimulus in day-old chicks (Gallus domesticus) Neurobiol Learn Mem. 1996;66:236–240. doi: 10.1006/nlme.1996.0064. [DOI] [PubMed] [Google Scholar]
- Storch EA, Merlo LJ, Bengtson M, et al. D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol. 2007;22:230–237. doi: 10.1097/YIC.0b013e32819f8480. [DOI] [PubMed] [Google Scholar]
- Storch EA, Murphy TK, Goodman WK, et al. A preliminary study of D-cycloserine augmentation of cognitive-behavioral therapy in pediatric obsessive-compulsive disorder. Biol Psychiatry. 2010;68:1073–1076. doi: 10.1016/j.biopsych.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LT, Disterhoft JF. Age- and dose-dependent facilitation of associative eyeblink conditioning by D-cycloserine in rabbits. Behav Neurosci. 1997;111:1303–1312. doi: 10.1037//0735-7044.111.6.1303. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Lipsch C, Gispende Wied C, et al. The partial NMDA agonist D-cycloserine stimulates LH secretion in healthy volunteers. Psychopharmacology (Berl) 1998;138:190–197. doi: 10.1007/s002130050662. [DOI] [PubMed] [Google Scholar]
- van Berckel BN, Lipsch C, Timp S, et al. Behavioral and neuroendocrine effects of the partial NMDA agonist D-cycloserine in healthy subjects. Neuropsychopharmacology. 1997;16:317–324. doi: 10.1016/S0893-133X(96)00196-0. [DOI] [PubMed] [Google Scholar]
- Van Vliet IM, De Beurs E. The MINI-International Neuropsychiatric Interview. A brief structured diagnostic psychiatric interview for DSM-IV en ICD-10 psychiatric disorders. Tijdschr Psychiatr. 2007;49:393–397. [PubMed] [Google Scholar]
- Vansteenwegen D, Crombez G, Baeyens F, et al. Extinction in fear conditioning: effects on startle modulation and evaluative self-reports. Psychophysiology. 1998;35:729–736. [PubMed] [Google Scholar]
- Vervliet B. Learning and memory in conditioned fear extinction: effects of D-cycloserine. Acta Psychol (Amst) 2008;127:601–613. doi: 10.1016/j.actpsy.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Viveros MP, Marco EM, File SE. Endocannabinoid system and stress and anxiety responses. Pharmacol Biochem Behav. 2005;81:331–342. doi: 10.1016/j.pbb.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Wachtel SR, ElSohly MA, Ross SA, et al. Comparison of the subjective effects of Delta(9)-tetrahydrocannabinol and marijuana in humans. Psychopharmacology (Berl) 2002;161:331–339. doi: 10.1007/s00213-002-1033-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, et al. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M, Hart J, Richardson R. Effects of D-cycloserine on extinction of learned fear to an olfactory cue. Neurobiol Learn Mem. 2007;87:476–482. doi: 10.1016/j.nlm.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Weike AI, Hamm AO, Schupp HT, et al. Fear conditioning following unilateral temporal lobectomy: dissociation of conditioned startle potentiation and autonomic learning. J Neurosci. 2005;25:11117–11124. doi: 10.1523/JNEUROSCI.2032-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm S, Buhlmann U, Tolin DF, et al. Augmentation of behavior therapy with D-cycloserine for obsessive–compulsive disorder. Am J Psychiatry. 2008;165:335–341. doi: 10.1176/appi.ajp.2007.07050776. quiz 409. [DOI] [PubMed] [Google Scholar]
- Zuurman L, Ippel AE, Moin E, et al. Biomarkers for the effects of cannabis and THC in healthy volunteers. Br J Clin Pharmacol. 2009;67:5–21. doi: 10.1111/j.1365-2125.2008.03329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.