Abstract
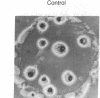
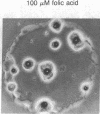
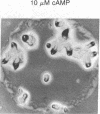
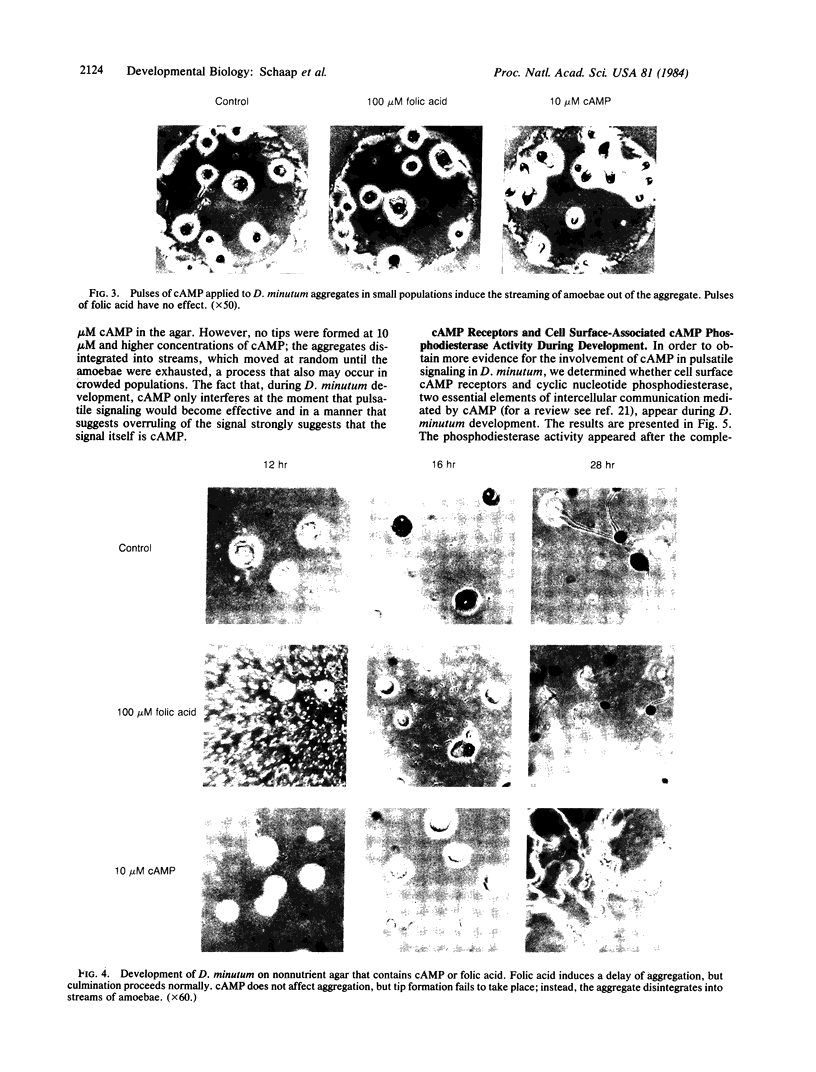
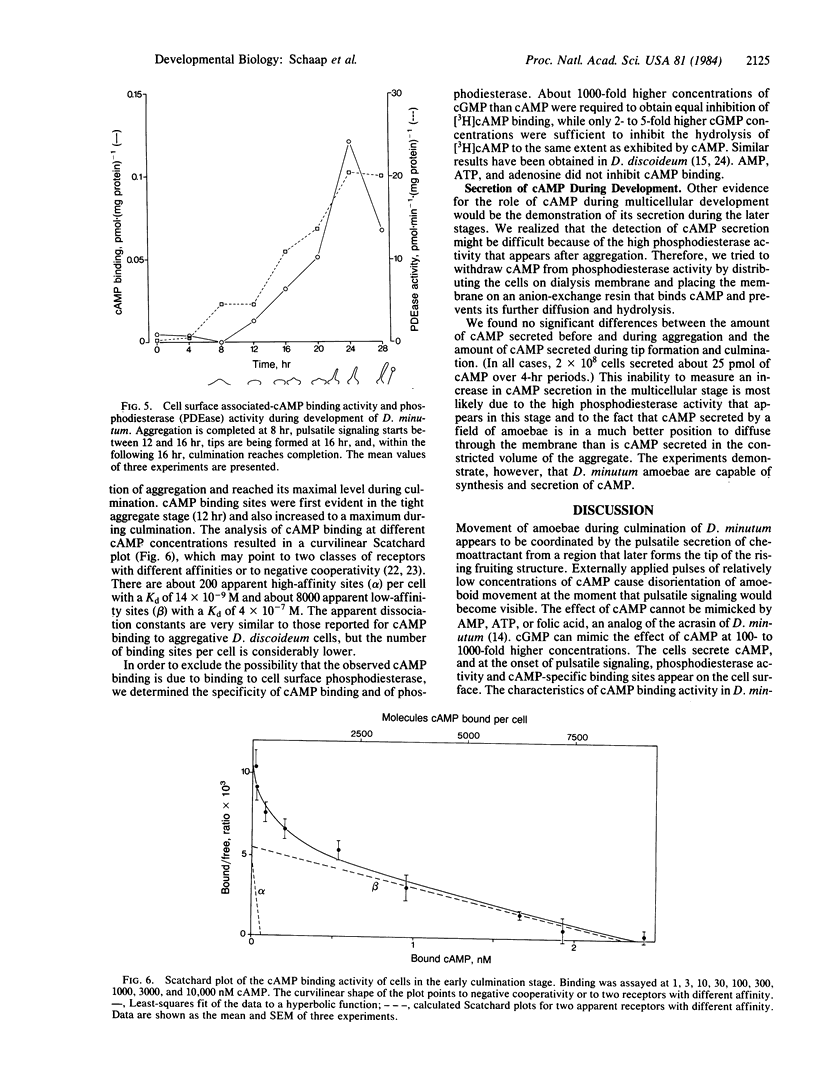
Aggregation in the primitive cellular slime mold Dictyostelium minutum proceeds by means of chemotaxis toward a continuously secreted folic acid analog [De Wit, R. J. W. & Konijn, T. M. (1983) Cell Differ. 12, 205-210]. The onset of culmination is marked by the appearance of concentric waves of cell movement on the aggregate surface. Culmination proceeds by the chemotactic attraction of amoebae to the center of wave propagation, which results in the accumulation of amoebae into a finger-like structure. Evidence is presented that the chemoattractant used during culmination is cAMP, which is secreted in pulses. The cells secrete cAMP themselves; cAMP receptors and phosphodiesterase activity appear on the cell surface just before the onset of culmination. Micromolar concentrations of externally applied cAMP induce disorientation of amoeboid movement at the onset of culmination. These observations are compatible with the hypothesis that the cAMP signaling system organizes multicellular development in both primitive and advanced cellular slime mold species. Advanced species such as Dictyostelium discoideum use this signaling system also in an earlier stage of development to organize the process of cell aggregation.
Keywords: cellular slime molds, multicellular stage, pulsatile signaling, chemotaxis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcantara F., Monk M. Signal propagation during aggregation in the slime mould Dictyostelium discoideum. J Gen Microbiol. 1974 Dec;85(2):321–334. doi: 10.1099/00221287-85-2-321. [DOI] [PubMed] [Google Scholar]
- Brenner M. Cyclic AMP levels and turnover during development of the cellular slime mold Dictyostelium discoideum. Dev Biol. 1978 Jun;64(2):210–223. doi: 10.1016/0012-1606(78)90073-8. [DOI] [PubMed] [Google Scholar]
- Durston A. J., Cohen M. H., Drage D. J., Potel M. J., Robertson A., Wonio D. Periodic movements of Dictyostelium discoideum sorocarps. Dev Biol. 1976 Sep;52(2):173–180. doi: 10.1016/0012-1606(76)90237-2. [DOI] [PubMed] [Google Scholar]
- Durston A. J. Pacemaker activity during aggregation in Dictyostelium discoideum. Dev Biol. 1974 Apr;37(2):225–235. doi: 10.1016/0012-1606(74)90144-4. [DOI] [PubMed] [Google Scholar]
- Gerisch G. Cell aggregation and differentiation in Dictyostelium. Curr Top Dev Biol. 1968;3:157–197. doi: 10.1016/s0070-2153(08)60354-3. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green A. A., Newell P. C. Evidence for the existence of two types of cAMP binding sites in aggregating cells of Dictyostelium discoideum. Cell. 1975 Oct;6(2):129–136. doi: 10.1016/0092-8674(75)90003-3. [DOI] [PubMed] [Google Scholar]
- Jones M. E., Robertson A. Cyclic adenosine monophosphate and the development of Polysphondylium. J Cell Sci. 1976 Oct;22(1):41–47. doi: 10.1242/jcs.22.1.41. [DOI] [PubMed] [Google Scholar]
- Konijn T. M. Microbiological assay of cyclic 3',5'-AMP. Experientia. 1970 Apr 15;26(4):367–369. doi: 10.1007/BF01896891. [DOI] [PubMed] [Google Scholar]
- Konijn T. M., Van De Meene J. G., Bonner J. T., Barkley D. S. The acrasin activity of adenosine-3',5'-cyclic phosphate. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1152–1154. doi: 10.1073/pnas.58.3.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Malkinson A. M., Ashworth J. M. Adenosine 3':5'-cyclic monophosphate concentrations and phosphodiesterase activities during axenic growth and differentiation of cells of the cellular slime mould Dictyostelium discoideum. Biochem J. 1973 May;134(1):311–319. doi: 10.1042/bj1340311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullens I. A., Newell P. C. cAMP binding to cell surface receptors of Dictyostelium. Differentiation. 1978 May 26;10(3):171–176. doi: 10.1111/j.1432-0436.1978.tb00960.x. [DOI] [PubMed] [Google Scholar]
- Nestle M., Sussman M. The effect of cyclic AMP on morphogernesis and enzyme accumulation in Dictyostelium discoideum. Dev Biol. 1972 Aug;28(4):545–554. doi: 10.1016/0012-1606(72)90002-4. [DOI] [PubMed] [Google Scholar]
- Robertson A., Cohen M. H. Control of developing fields. Annu Rev Biophys Bioeng. 1972;1:409–464. doi: 10.1146/annurev.bb.01.060172.002205. [DOI] [PubMed] [Google Scholar]
- Roos W., Nanjundiah V., Malchow D., Gerisch G. Amplification of cyclic-AMP signals in aggregating cells of Dictyostelium discoideum. FEBS Lett. 1975 May 1;53(2):139–142. doi: 10.1016/0014-5793(75)80005-6. [DOI] [PubMed] [Google Scholar]
- SHAFFER B. M. Aggregation in cellular slime moulds: in vitro isolation of acrasin. Nature. 1953 May 30;171(4361):975–975. doi: 10.1038/171975a0. [DOI] [PubMed] [Google Scholar]
- Schaap P., van der Molen L., Konijn T. M. The organisation of fruiting body formation in Dictyostelium minutum. Cell Differ. 1983 May;12(5):287–297. doi: 10.1016/0045-6039(83)90025-8. [DOI] [PubMed] [Google Scholar]
- Shaffer B. M. Secretion of cyclic AMP induced by cyclic AMP in the cellular slime mould Dictyostelium discoideum. Nature. 1975 Jun 12;255(5509):549–552. doi: 10.1038/255549a0. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Suthers H. L., Bonner J. T. Chemical identity of the acrasin of the cellular slime mold Polysphondylium violaceum. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7376–7379. doi: 10.1073/pnas.79.23.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson W. J., Brooker G., Appleman M. M. Assay of cyclic nucleotide phosphodiesterases with radioactive substrates. Methods Enzymol. 1974;38:205–212. doi: 10.1016/0076-6879(74)38033-0. [DOI] [PubMed] [Google Scholar]
- Tomchik K. J., Devreotes P. N. Adenosine 3',5'-monophosphate waves in Dictyostelium discoideum: a demonstration by isotope dilution--fluorography. Science. 1981 Apr 24;212(4493):443–446. doi: 10.1126/science.6259734. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J., Dijkgraaf P. A., Konijn T. M., Abbad E. G., Petridis G., Jastorff B. Substrate specificity of cyclic nucleotide phosphodiesterase from beef heart and from Dictyostelium discoideum. Eur J Biochem. 1983 Apr 5;131(3):659–666. doi: 10.1111/j.1432-1033.1983.tb07314.x. [DOI] [PubMed] [Google Scholar]
- Van Haastert P. J., Kien E. Binding of cAMP derivatives to Dictyostelium discoideum cells. Activation mechanism of the cell surface cAMP receptor. J Biol Chem. 1983 Aug 25;258(16):9636–9642. [PubMed] [Google Scholar]
- de Wit R. J., Konijn T. M. Identification of the acrasin of Dictyostelium minutum as a derivative of folic acid. Cell Differ. 1983 Apr;12(4):205–210. doi: 10.1016/0045-6039(83)90029-5. [DOI] [PubMed] [Google Scholar]
- van Haastert P. J., Konijn T. M. Signal transduction in the cellular slime molds. Mol Cell Endocrinol. 1982 Apr;26(1-2):1–17. doi: 10.1016/0303-7207(82)90002-8. [DOI] [PubMed] [Google Scholar]