Abstract
Binge alcohol exposure in adolescent rats potently inhibits adult hippocampal neurogenesis by altering neural progenitor cell (NPC) proliferation and survival; however, it is not clear whether alcohol results in an increase or decrease in net proliferation. Thus, the effects of alcohol on hippocampal NPC cell cycle phase distribution and kinetics were assessed in an adolescent rat model of an alcohol use disorder. Cell cycle distribution was measured using a combination of markers (Ki-67, bromo-deoxy-uridine incorporation, and phospho-histone H3) to determine the proportion of NPCs within G1, S, and G2/M phases of the cell cycle. Cell cycle kinetics were calculated using a cumulative bromo-deoxy-uridine injection protocol to determine the effect of alcohol on cell cycle length and S-phase duration. Binge alcohol exposure reduced the proportion of NPCs in S-phase, but had no effect on G1 or G2/M phases, indicating that alcohol specifically targets S-phase of the cell cycle. Cell cycle kinetics studies revealed that alcohol reduced NPC cell cycle duration by 36% and shortened S-phase by 62%, suggesting that binge alcohol exposure accelerates progression through the cell cycle. This effect would be expected to increase NPC proliferation, which was supported by a slight, but significant increase in the number of Sox-2+ NPCs residing in the hippocampal subgranular zone following binge alcohol exposure. These studies suggest the mechanism of alcohol inhibition of neurogenesis but also reveal the earliest evidence of the compensatory neurogenesis reaction that has been observed a week after binge alcohol exposure.
Keywords: adult neurogenesis, alcoholism, ethanol, neural stem cell, neurodegeneration
INTRODUCTION
Alcohol use disorders (AUDs) are a pervasive part of our society with over 17 million Americans meeting the diagnostic criteria for an AUD at any moment (Hasin et al., 2007). Initiation of alcohol consumption during adolescence greatly increases the probability that an individual will develop an AUD (Grant and Dawson, 1997). When this is coupled with the alarmingly high alcohol use rate among adolescents (Johnston et al., 2007), it becomes clear that alcohol’s interaction with the developing adolescent brain holds important clues as to how AUDs develop. More than half of 12–17 year olds who consume alcohol do so in a binge pattern (>5/4 drinks for males and females, respectively), which is associated with greater risk for alcohol-induced neurodegeneration and has been hypothesized to be a key step in the development of addiction (Koob and Moal, 1997; Crews, 1999; Johnston et al., 2007; Crews and Nixon, 2009).
The adolescent brain responds differently to alcohol than the adult brain likely due to its unique developmental state (Spear, 2000; Bava et al., 2010). Compared to adults, adolescents are resistant to the motor impairing and sedative effects of alcohol, but more sensitive to its rewarding and reinforcing properties (Little et al., 1996; Doremus-Fitzwater et al., 2010), a combination that may drive excessive alcohol consumption to neurotoxic levels. In addition, the adolescent limbic system is more susceptible to alcohol-induced damage and alcohol’s memory-impairing properties, which suggest a particular vulnerability of the adolescent hippocampus (Crews et al., 2000; White and Swartzwelder, 2004; 2005). Indeed, MRI studies have consistently reported reductions in hippocampal volume in binge drinking and alcohol dependent adolescents, while functional studies have suggested potential impairments in hippocampal-dependent learning and memory processes (De Bellis et al., 2000; Nagel et al., 2005; Medina et al. 2007; Schulteis et al., 2008; Schweinsburg et al., 2010). These findings have been extended to adolescent animal models which show alcohol-induced deficits in spatial memory acquisition, trace fear conditioning, and contextual fear conditioning, all of which depend on hippocampal function (Markwiese et al., 1998; Gould, 2003; Weitemier and Ryabinin, 2003; Sircar and Sircar, 2005; Hunt et al., 2009; Sircar et al., 2009).
The hippocampus is one of two brain regions in which neurogenesis persists throughout life. Here, neural progenitor cells (NPCs) located along the subgranular zone (SGZ) generate new granule neurons found in the hippocampal dentate gyrus (Kuhn et al., 1996; Zhao et al., 2008). The continuous generation of new neurons results in a steady increase in the size of the dentate gyrus granule layer, demonstrating the important contribution of adult neurogenesis in shaping hippocampal structure (Imayoshi et al., 2008). Although the function of adult hippocampal neurogenesis has not been firmly established, accumulating evidence indicates that these new neurons play a role in hippocampal-dependent learning and memory. This is supported by studies demonstrating learning-dependent regulation of adult neurogenesis and the development of specific learning deficits following ablation of neurogenesis (Deng et al., 2010). In addition, hippocampal neurogenesis may regulate drug reward and drug seeking behavior, potentially linking neurogenesis to the development of addiction (Eisch et al., 2008; Noonan et al., 2010). Given the structural and functional importance of adult neurogenesis, alcohol’s effects on this process could be an important factor contributing to alcohol-induced disruption of hippocampal structure and function in adolescents (Nixon, 2006; Nixon et al., 2010). In an adult rat binge model, alcohol intoxication inhibits neurogenesis by reducing the number of proliferating NPCs and survival of newly born granule neurons (Nixon and Crews, 2002). In adolescents, alcohol also potently attenuates new neuron survival, but its effects on NPC proliferation appear more complex (Morris et al., 2010a). For instance, binge alcohol intoxication decreased bromo-deoxy-uridine (BrdU) incorporation without affecting the overall number of proliferating NPCs, suggesting that alcohol may target cell cycle progression in adolescents.
Therefore, the current study examined the effects of alcohol on hippocampal NPC cell cycle progression in adolescent rats. This was accomplished with two stereology-based anatomical studies: the first sought to determine whether binge alcohol exposure altered the distribution of dividing NPCs within the different phases of the cell cycle; the second aimed to measure cell cycle and S-phase duration following binge alcohol intoxication.
MATERIALS AND METHODS
Subjects
Sixty adolescent (postnatal day 30) male Sprague-Dawley rats (Charles River Laboratories, Portage, MI) were used for these experiments. Rats were individually housed and maintained on a 12h light/dark cycle with food and water available ad libitum. Rats were acclimated to the vivarium for 5 days prior to the start of ethanol administration. All experiments were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (NRC, 1996) and were approved by the University of Kentucky’s Institutional Animal Care and Use Committee.
Adolescent Rat AUD Model
Binge ethanol administration followed a procedure modified from Majchrowicz (1975) and began at postnatal day 35 as described previously (Morris et al., 2010a; b). Food was removed immediately prior to the start of the experiment, while water remained available throughout. Nutritionally complete ethanol (25% ethanol w/v in Vanilla Ensure Plus) or isocaloric dextrose diet was delivered intragastrically using stainless steel gavage needles (Popper and Sons, New Hyde Park, NY) 3 times a day for 4 days with doses spaced out over 8h intervals. The initial ethanol dose was 5g/kg with subsequent doses titrated based on the following 6-point intoxication behavior scale: 0-normal rat (5g/kg), 1-hypoactive (4g/kg), 2-ataxic (3g/kg), 3-delayed righting reflex (2g/kg), 4-loss of righting reflex (1g/kg), and 5-loss of eye blink reflex (0g/kg). This paradigm results in rapid development of alcohol dependence as evidenced by increased ethanol tolerance and the appearance of symptoms consistent with severe withdrawal once ethanol is cleared.
Blood Ethanol Concentration
Tail blood samples were collected 90 min following the first ethanol dose on the third binge day. Samples were centrifuged for 5 min at 1800 × g and then were stored at −20°C. Blood ethanol concentrations were determined in serum and performed in triplicate using a GM7 Alcohol Analyzer calibrated with a 300mg/dl external standard (Analox, Lunenberg, MA).
Tissue Preparation
Rats were euthanized with a pentobarbital overdose (Nembutal®, MWI Veterinary Supply, Nampa, ID) followed by transcardial perfusion with 0.1M phosphate buffered saline (PBS; pH 7.4) and 4% paraformaldehyde (in 0.1M phosphate buffer, pH 7.4). Brains were extracted, post-fixed for 24h in 4% paraformaldehyde, and then stored in 0.1M PBS at 4°C. Free-floating, 40μm coronal sections were obtained from the entire length of the cerebral cortex in a 1:12 series with a vibrating microtome (Leica Microsystems, Wetzlar, Germany). This collection procedure results in tissue sections from the entire rostral to caudal extent of the hippocampus. Sections were stored in cryoprotectant at −20°C.
Antibody Characterization
Antibodies used for immunohistochemistry are listed in Table 1. Mouse monoclonal anti-Ki-67 clone MM1 (Vector Laboratories, Burlingame, CA) was generated against a prokaryotic recombinant fusion protein corresponding to amino acids 1159–1522 of the human Ki-67 sequence (Komitova et al., 2009). According to the manufacturer, the MM1 clone recognizes bands of 345 and 395 kDa on western blots of MCF-7 cell lysates, matching reports using other Ki-67 clones (Key et al., 1993). Within the rat SGZ, the Ki-67 antibody only labels dividing cells as was shown in a cumulative BrdU study in which Ki-67 expressing cells eventually all become co-labeled with BrdU (Olariu et al., 2007). Similarly, the Ki-67 antibody stained clusters of cells along the SGZ in the current study.
Table 1.
Primary Antibody Details
| Antibody | Host Species | Titer | Time in Primary | Company, Catalog No. | Lot No. | Immunogen |
|---|---|---|---|---|---|---|
| BrdU | Mouse | 1:5000 | 16h | Millipore, MAB3424 | NMM1645458 | Bromodeoxyuridine-bovine serum albumin |
| Ki-67 | Mouse | 1:200 | 41–43h | Vector, VP-K452 | L111855, L111560 | Recombinant protein corresponding to amino acids 1159–1522 of human Ki-67 |
| pHis-H3 | Rabbit | 1:1000 | 16h | Millipore, 06–570 | DAM1545035 | Peptide corresponding to amino acids 7–20 of human S10 phosphorylated histone H3 |
| Sox-2 | Rabbit | 1:1000 | 16h | Millipore, AB5603 | LV1622755 | Synthetic peptide corresponding to amino acids 249–265 of human Sox-2 |
Mouse monoclonal anti-BrdU clone AH4H7-1/131-14871 (Millipore, Bellerica, MA) labels only cells that have incorporated BrdU into their DNA and does not recognize any endogenous proteins or other cellular components. No labeling is observed with this BrdU antibody in brain sections obtained from rats that were not injected with BrdU, and we have used this antibody previously to assess NPC cell proliferation within the SGZ following binge ethanol intoxication in adolescent rats (Morris et al., 2010a).
Rabbit polyclonal anti-phospho-histone H3 (pHis-H3; Millipore, Bellerica, MA) was generated from a KLH-conjugated peptide (ARKpSTGGKAPRKQLC) corresponding to amino acids 7–20 of S10 phosphorylated human histone H3. According to the manufacturer, this antibody recognizes a single band of protein at 17kDa in colcemid treated HeLa cells. In determining mitotic indices, results obtained with this anti-pHis-H3 antibody strongly correlated with results obtained from H & E staining, showing that this antibody reliably labels proliferating cells (Brenner et al., 2003). Consistent with its specificity for labeling proliferating cells, this antibody detects clusters of cells within the neurogenic regions of the brain, similar to staining patterns for Ki-67 and BrdU (Ackman et al., 2007).
Affinity purified rabbit polyclonal anti-Sox-2 (Millipore, Bellerica, MA) was produced from a synthetic peptide with the sequence SSSPPVVTSSSHSRAPC, which corresponds to amino acids 249–265 of the C-terminal region of human Sox-2. In the NT2/D1 human embryonic stem cell line, this antibody recognizes a 34 kDa protein present only in nuclear extracts, matching the predicted location and size of Sox-2. This antibody labeled cells along the SGZ following lentiviral transduction with a GFP/cre fusion protein, whose expression was driven by the Sox-2 promoter, confirming that the antibody specifically labels Sox-2 expressing cells (Suh et al., 2007). Importantly, fate tracing studies showed that Sox-2 expressing cells in the SGZ could differentiate into neurons and astrocytes, indicating that these cells are multi-potent NPCs (Suh et al., 2007). In addition, a small proportion of Sox-2 positive cells along the SGZ co-localize with Ki-67 and BrdU (Mathews et al., 2010).
Biotinylated horse anti-mouse rat absorbed IgG and goat anti-rabbit IgG (Vector Laboratories, Burlingame, CA) were used as secondary antibodies. The manufacturer states that both secondary antibodies show less than 1% cross-reactivity with rat immunoglobulins. For all antibodies, optimal antibody concentrations were determined from dilution curves, while specificity of antibody immunoreactivity was confirmed with control tissue in which either the primary or secondary antibody was omitted.
Immunohistochemistry
For all antibodies, tissue sections were washed 2 × 5 min in 1x tris-buffered saline (TBS, pH 7.5) to remove traces of the cryoprotectant. In general, sections were incubated in 0.6-1.0% H2O2 for 30 min to quench endogenous peroxidase activity. For Ki-67 and pHis-H3 detection, an antigen-retrieval step in sodium citrate buffer for 1h at 65°C was added following the H2O2 step. Sections were washed in 1x TBS and blocked in 3–10% normal serum (species as appropriate; 0.1% Triton X-100 in 1x TBS) for 30 min prior to overnight or 2 day incubation in primary antibody at 4°C. BrdU (1:6 series) was detected following methods modified from Kuhn (1996) as reported in Nixon and Crews (2002). Following the H2O2 step, sections were washed in 1x TBS, incubated in 50% formamide (prepared in sodium citrate buffer) for 2h at 65°C, washed 2 × 5 min in sodium citrate buffer, and then incubated in 2N HCl for 1h at 37°C. Sections were neutralized in 0.1M boric acid (pH 8.5), washed in 1x TBS, and then blocked in 3% normal horse serum for 30 min. After blocking, sections were rinsed in 0.02% MgCl, incubated in 100U/mL bovine pancreatic DNase I (Sigma-Aldrich, St. Louis, MO) for 1h, rinsed with blocking buffer, and then incubated overnight in mouse anti-BrdU (Nixon and Crews, 2002).
After the primary antibody step, sections were washed 3 × 10 min in blocking buffer and incubated in the appropriate biotinylated secondary antibody for 1h (Vector Laboratories, Burlingame, CA). This was followed by 3 × 10 min TBS washes and incubation in avidin-biotin-peroxidase complex for 1h (ABC Elite Kit, Vector Laboratories). Immunoreactivity was visualized using nickel-enhanced diaminobenzidine (0.5% 3,3-diaminobenzidine tetrahydrochloride containing 0.2mM CoCl2, 0.005% nickel ammonium sulfate, and 0.006% H2O2; Polysciences, Waltham, MA). Sections were mounted to glass slides and counterstained and/or coverslipped in Cytoseal® (Richard-Allen Scientific, Kalamazoo, MI). Ki-67 and BrdU-stained tissue was lightly counterstained with neutral red or cresyl violet, respectively. Slides were coded to keep the experimenter blind to treatment conditions during quantification. Representative images were collected with a DP70 digital camera. Images were converted to grayscale and figures compiled using Photoshop CS2 software (Adobe Systems Inc., San Jose, CA). Images were cropped and resized to include only the area of interest. Calibration lines placed on figure images were obtained in Photoshop by drawing a rectangle covering the desired distance on a micrometer image taken at the appropriate magnification. Brightness/Contrast were not altered in any image.
Fluoro-Jade B
Tissue (1:12) was processed for Fluoro-Jade B (FJB) staining identical to published methods (Schmued and Hopkins, 2000; Leasure and Nixon, 2010). Sections were mounted onto Superfrost® Plus microscope slides (Fisher Scientific, Pittsburgh, PA), dried overnight, and then sequentially incubated in 1% NaOH/80% ethanol (5 min), 70% ethanol (2 min), DI H2O (2 min), 0.06% potassium permanganate (10 min), DI H2O (2 min), 0.004% FJB (20 min in the dark; Millipore, Bellerica, MA), and DI H2O (3 × 1 min). Slides were dried and coverslipped in Cytoseal® (Richard-Allen Scientific, Kalamazoo, MI). Slides were coded to keep the experimenter blind to treatment conditions during quantification.
Stereology
Design-based stereology employing the optical fractionator method was used to quantify the number of immunoreactive cells within the hippocampal SGZ for each cell cycle marker. Equipment consisted of an Olympus BX51 microscope equipped with a ProScan II motorized stage, microcator, and DP70 digital camera (Olympus, Center Valley, PA) coupled to newCAST™ Stereology System software (Visiopharm, Hoersholm, Denmark) that was installed on a Dell Precision 380 workstation. The SGZ was defined as an approximate 30μm thick ribbon of tissue between the granular layer and hilus. Because the previous studies and effects were described in the dorsal hippocampus, only sections (1:12 series for Sox-2, Ki-67, and pHis-3; 1:6 series for BrdU) containing the dorsal hippocampus were quantified (Bregma −1.8 to −5.52; Paxinos and Watson, 2009). The SGZ was traced at 100x and section thickness was assessed at 600x using a 60x oil immersion lens (Olympus PlanAPoN, numerical aperature = 1.42) and was averaged from three measurements taken at different locations along the SGZ. For Sox-2, random sampling was conducted using a 20μm × 20μm counting frame with a 120μm ×,y step length. After tissue processing, section thickness was approximately 20μm; therefore, a dissector height of 14μm with 3μm guard zones was used. All cell counts were made from live images at 1200x projected onto the computer monitor showing the overlaying counting frame. The left and bottom borders of the counting frame were defined as the exclusion lines. Total Sox-2+ cells were calculated with the following equation (West et al., 1991):
where Q is the number of cells counted, asf is the area sampling fraction (the counting frame: x,y step ratio), tsf is the thickness sampling fraction (dissector height:section thickness ratio), and ssf is the section sampling fraction (the fraction of sections examined e.g. 1:6 or 1:12; West et al., 1991). For Ki-67, BrdU, and pHis-H3-stained tissue, a 100% asf was chosen due to the limited number and non-homogenous distribution of the immunoreactive cells. For all stereological quantification, coefficient of error ranged from 0.013 to 0.020 (Table 2; Gundersen et al., 1999). Additionally, complete antibody penetration of the tissue was confirmed by comparing profile counts in the top 1/3 and middle 1/3 of the section (Supplemental figure 1).
Table 2.
Stereology Coefficient of Error
| Marker | Control | Ethanol |
|---|---|---|
| Experiment 1 | ||
| Ki-67 | 0.014 ± 0.001 | 0.016 ± 0.001 |
| pHis-H3 | 0.020 ± 0.002 | 0.020 ± 0.004 |
| BrdU | 0.016 ± 0.002 | 0.013 ± 0.001 |
| Experiment 2 | ||
| BrdU | 0.013 ± 0.001 | 0.014 ± 0.001 |
| Ki-67 | 0.013 ± 0.001 | 0.014 ± 0.001 |
| Sox-2 | 0.018 ± 0.001 | 0.018 ± 0.001 |
Experiment 1: Cell Cycle Distribution
The goal of the first experiment was to determine the effect of binge ethanol exposure on the distribution of dividing NPCs within the different stages of the cell cycle. To calculate the distribution of NPCs within G1, S, and G2/M phases of the cell cycle, a combination of cell cycle markers was measured (Figure 1A). In this experiment, 14 rats were administered a saturating dose of BrdU (300mg/kg, i.p., Fluka, Buchs, Switzerland; Cameron and McKay, 2001) immediately following the last ethanol or control dose and then were euthanized 2h later (Figure 1B). One control and two ethanol rats were omitted from analysis due to complete absence of BrdU staining in the subgranular and subventricular zones. Ki-67, expressed during all stages of the cell cycle, was measured to determine the number of actively dividing NPCs in the SGZ, while BrdU, which is incorporated into the DNA during DNA synthesis, was used to quantify cells in S-phase (Nowakowski et al., 1989; Scholzen and Gerdes, 2000). pHis-H3 was measured to quantify the number of cells in G2 and M, since histone H3 becomes highly phosphorylated during these two phases of the cell cycle (Prigent and Dimitrov, 2003). Data from these 3 cell cycle markers were used to estimate the population of dividing NPCs in G1 by subtracting the total number of pHis-H3+ and BrdU+ cells from the number of Ki-67+ cells.
Figure 1.
Cell Cycle Markers and Experimental Timeline. A) Cell cycle diagram showing the stages of the cell cycle labeled by Ki-67, BrdU, and pHis-H3. BrdU labels cells in S-phase, pHis-H3 labels cells in G2 and M phase, and Ki-67 labels actively dividing cells regardless of the stage they are in. From these markers, the size of the G1 population can be calculated by subtracting total BrdU and pHis-H3 cells from Ki-67 cells. B) Time line for cell cycle distribution study (experiment 1). Adolescent rats were subjected to 4-day ethanol binge. Rats were injected with BrdU 90 min after last ethanol or control dose and then sacrificed 2h later. The distribution of actively dividing NPCs within the cell cycle phases were then determined by measuring Ki-67, BrdU, and pHis-H3. C) Time line for cell cycle kinetics study (experiment 2). Adolescent rats were subjected to 4-day ethanol binge. Cumulative BrdU injections were made every 2h for 4h beginning 1.5h after the last ethanol dose, with a control and ethanol group sacrificed 0.5h after each injection. BrdU = Bromo-deoxy-uridine, pHis-H3 = phospho-histone H3.
Experiment 2: Cell Cycle Kinetics
Forty-six rats were used for experiment 2 to measure the effect of adolescent binge ethanol exposure on cell cycle length and S-phase duration. This was accomplished by combining a cumulative BrdU injection method with Ki-67 and Sox-2 quantification (Nowakowski et al., 1989; Mooney and Miller, 2007; 2010). The first BrdU injection was made 90 min after the last ethanol gavage to ensure that rats remained intoxicated throughout the entire BrdU injection time course. BrdU was injected once every 2h for a total of 4h, with an ethanol and control group euthanized 30 minutes after each injection; thus, animals are injected at 0, 2, and 4h and groups are sacrificed at 0.5, 2.5, and 4.5h (Figure 1C; n = 6–8 per group). A saturating dose of BrdU (300mg/kg, i.p.) was used for each injection. In addition, Ki-67 was measured to determine the number of dividing cells, and the NPC marker Sox-2 was quantified to determine the size of the NPC pool.
From Ki-67, Sox-2, and cumulative BrdU stereological data, cell cycle length (Tc) and S-phase duration (Ts) were calculated (Nowakowski et al., 1989; Mooney and Miller, 2007; 2010). To do this, the growth fraction is calculated which is equal to the Ki-67 labeling index (Ki-67/Sox-2 = growth fraction). Similarly, the S-phase fraction is calculated which is equal to the BrdU labeling index (BrdU/Sox-2 = S-phase fraction). The S-phase fraction approaches the growth fraction over time as more dividing cells enter S-phase allowing Tc and Ts to be calculated using a linear regression model (Nowakowski et al., 1989). Tc and Ts were calculated with the following equations (Nowakowski et al., 1989; Mooney and Miller, 2007; 2010):
To determine if cell cycle data was influenced by ethanol-induced degeneration of hippocampal NPCs, cell death following 4-day binge ethanol exposure was measured with FJB staining. FJB+ cells within the dorsal dentate gyrus were examined in the 4.5 h group using an Olympus BX1 microscope equipped with a 488λ epifluorescent cube. FJB stained cells within the SGZ and granule cell layers were counted on sections spanning the dorsal dentate gyrus. This method was chosen over unbiased stereology due to the low number of cells within each section that are FJB+ and the low background staining inherent with this method that makes it difficult to accurately assess section thickness. Furthermore, we have shown previously that our profile counting methodology generates the same percent difference between experimental groups as stereological methodologies (Crews et al., 2004).
Statistical Analysis
Statistical tests were performed using GraphPad Prism version 5.02 for Windows (GraphPad Prism, San Diego, CA). Blood ethanol concentrations and daily ethanol intake were compared using one-way ANOVA followed by Tukey’s posthoc test. Intoxication scores were analyzed with Kruskall-Wallis non-parametric analysis of variance. All comparisons in experiment 1 were analyzed by t-test. Sox-2 stereology and BrdU labeling index data was compared using two-way ANOVA with time and diet serving as the dependent variables. FJB data was analyzed with the nonparametric Mann-Whitney test due to unequal variance between groups (verified by significant F-test). For linear regression analysis, control and ethanol regression line slope and intercepts were compared. All other two group comparisons were made with two-tailed, unpaired t-tests. Data is presented as mean ± standard error of the mean and differences were considered significant at p < 0.05.
RESULTS
Experiment 1: Cell Cycle Distribution
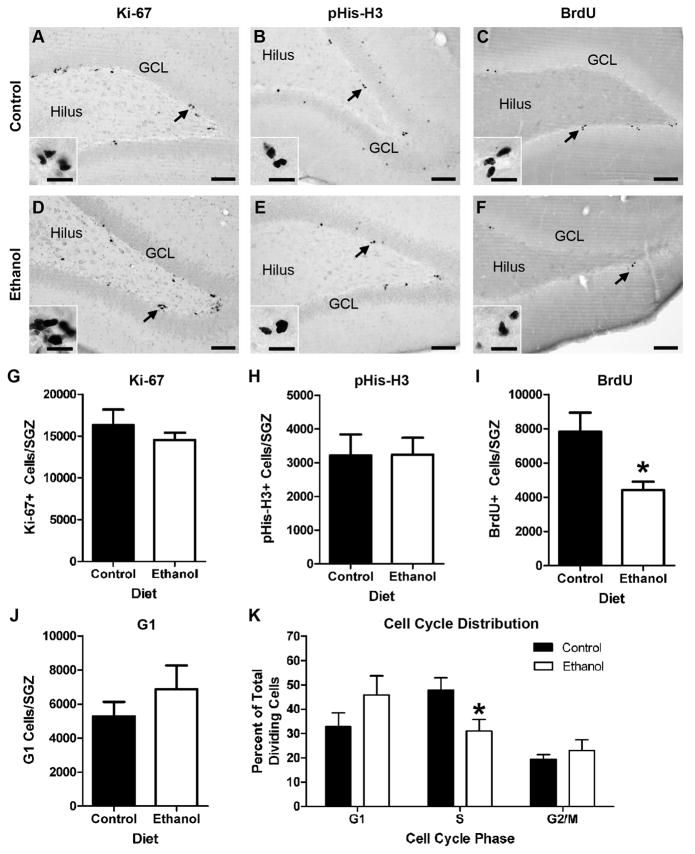
The effect of adolescent binge ethanol exposure on the distribution of hippocampal NPCs across the cell cycle was examined. In this experiment, the ethanol group had a mean intoxication score of 0.9 ± 0.1, was administered 12.0 ± 0.2 g/kg/d of ethanol, and had a mean blood ethanol concentration of 297 ± 20.3 mg/dL. In both treatment groups, Ki-67+, pHis-H3+, and BrdU+ NPCs were easily identified within cell clusters located along the SGZ of the dentate gyrus (Figure 2A–F). Rarely, immunoreactive endothelial cells were observed around blood vessels supplying the SGZ, while occasional cell clusters were observed within the hilus and granule neuron/molecular layer border; however, these cells were omitted from analysis. Ki-67, which labels cells in all active phases of the cell cycle, was unaltered by binge ethanol treatment (p = 0.41; Figure 2G). Similarly, the number of pHis-H3+ NPCs was not affected by ethanol (p = 0.98; Figure 2H). In contrast, binge ethanol exposure reduced the number of BrdU+ NPCs by 44% (p = 0.03; Figure 2I). As shown in Figure 2J, the size of the dividing NPC population residing in G1 was calculated by subtracting the total number of BrdU+ and pHis-H3+ cells from the number of Ki-67+ cells, which revealed that ethanol had no effect on G1 cell number (p = 0.36). The proportion of actively cycling hippocampal NPCs within G1, S, and G2/M was calculated to determine the effect of alcohol on NPC cell cycle distribution. This demonstrated that in adolescent rats, binge ethanol exposure preferentially reduces the proportion of hippocampal NPCs within S-phase (p = 0.04; Figure 2K), with no observed effect on the proportion of NPCs in G1 or G2/M.
Figure 2.
Binge ethanol exposure during adolescence alters the cell cycle distribution of SGZ NPCs. A–F) Representative images from sections stained for Ki-67, BrdU, and pHis-H3. In each case, immunoreactive cells were found in clusters concentrated along the SGZ. Arrows denote area represented in the inset. Scale bars = 100μm; inset 20μm. G) Ki-67 stereology data shows that 4-day binge ethanol exposure has no effect on the number of actively dividing cells in the SGZ. H) pHis-H3 stereology data shows that the number of dividing cells in G2 and M phases of the cell cycle is not altered by binge ethanol exposure. I) BrdU stereology data demonstrates that 4-day binge ethanol exposure significantly decreases the number of dividing cells in S-phase of the cell cycle. J) Calculated number of cells in G1 obtained by subtracting the number of BrdU+ and pHis-H3+ cells from the number of Ki-67+ cells. 4-day binge alcohol exposure had no effect on the number of dividing cells in G1 phase. K) Calculated distribution of dividing NPCs within each phase of the cell cycle showing that binge ethanol exposure specifically reduces the proportion of cells in S-phase. BrdU = Bromo-deoxy-uridine, GCL = granule cell layer, pHis-H3 = phosho-histone H3.
Experiment 2: Cell Cycle Kinetics
Due to the observed changes in NPC cell cycle distribution, the effect of ethanol on NPC cell cycle kinetics was examined. This required the measurement of BrdU accumulation over 4.5h following 3 BrdU injections; Sox-2 and Ki-67 were also quantified to provide an estimate of the size of the NPC population and the number of actively dividing cells. The number of Sox-2+ cells was used to calculate the BrdU and Ki-67 labeling index, allowing Tc and Ts to be calculated. There were no differences in behavioral intoxication, daily ethanol dose, or blood ethanol concentrations between the three ethanol groups used in this study (Table 3). The effect of time and diet on BrdU labeling index was analyzed by two-way ANOVA (Figure 3A). As expected, the population of NPCs labeled with BrdU increased significantly with time as cells entering S-phase were labeled following subsequent BrdU injections (F(2, 40) = 8.1, p = 0.001). In addition, binge ethanol exposure decreased the BrdU labeling index (F(1,40) = 13.4, p < 0.001); however, there was no time by diet interaction (F(2,40) = 0.32, p = 0.73), although post-hoc tests showed that BrdU labeling was significantly reduced by ethanol at the 0.5h (p = 0.01) and 2.5h (p = 0.01) time points, where labeling was decreased by 38.1% and 37.6%, respectively. In agreement with the previous cell cycle distribution study, Ki-67 labeling index (measured at 4.5h), which represents the NPC growth fraction, was not affected by 4-day binge ethanol treatment (p = 0.60; Figure 3B).
Table 3.
Binge Data-Cell Cycle Kinetics Experiment
| Time Point | Intoxication Score | EtOH Dose (g/kg/d) | BEC (mg/dL) |
|---|---|---|---|
| 0.5h | 0.9 ± 0.1 | 12.4 ± 0.29 | 316 ± 13.5 |
| 2.5h | 1.1 ± 0.08 | 11.7 ± 0.25 | 378 ± 17.6 |
| 4.5h | 1.2 ± 0.08 | 11.4 ± 0.25 | 348 ± 19.4 |
EtOH = Ethanol; BEC = Blood Ethanol Concentration
Figure 3.
BrdU and Ki-67 Labeling Indices. A) BrdU labeling index represents the S-phase fraction and was calculated as 100*(BrdU+cells/Sox-2+ cells). For both ethanol and control groups, BrdU labeling index increased with time; however, binge ethanol treatment reduced the BrdU labeling index compared to the control group, matching earlier results obtained with BrdU stereology. B) Ki-67 labeling index represents the growth fraction calculated as 100*(Ki-67+ cells/Sox-2+ cells). As expected and reported previously, binge ethanol treatment had no effect of the Ki-67 labeling index (Morris et al., 2010a). BrdU = Bromo-deoxy-uridine, Con = control, EtOH = ethanol.
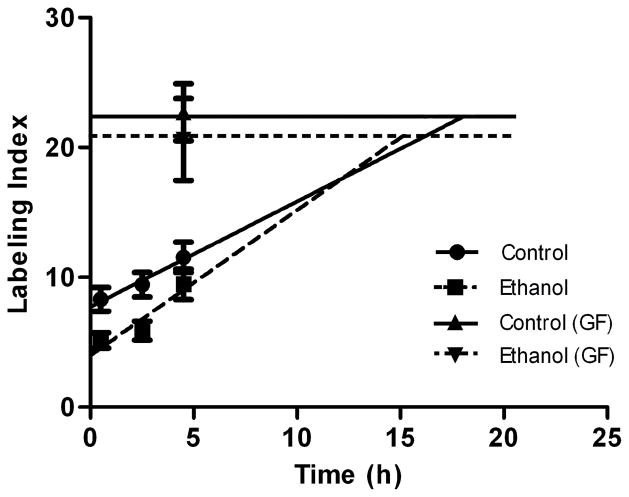
Linear regression analysis of BrdU labeling index combined with the growth fraction was used to calculate Tc and Ts as described in the experimental procedures. Binge ethanol exposure had no effect on the regression slope (F(1,42) = 0.39, p = 0.54); however, ethanol significantly decreased the y-intercept (F(1.43), p < 0.001), indicating that the lines are different (Figure 4). Control Tc (28.0 ± 2.6h) and Ts (9.6 ± 0.34h) were comparable to previously reported studies for juvenile to young rats (Cameron and Mckay, 2001; Olariu et al., 2007; Varodayan et al., 2009). Binge ethanol exposure shortened Tc by 36% to 17.9 ± 1.9h (p = 0.01). A large portion of this decrease was due to a 62% reduction in S-phase duration, in which Ts was shortened to 3.6 ± 0.36h (p < 0.001).
Figure 4.
The effect of adolescent binge alcohol exposure on SGZ NPC cell cycle kinetics. Shown are the linear regression lines for the control (solid line) and ethanol (dashed line) groups obtained from BrdU labeling index data using a least squares fit model. The regression lines were extrapolated to the point where they intersect the horizontal line that corresponds to the growth fraction, which represents the time needed to reach the maximum BrdU labeling index. The slopes of the two regression lines were not different, but binge ethanol significantly reduced the y-intercept. From the regression equations, Tc and Ts were calculated as described in the methods. Binge ethanol exposure during adolescence significantly shortened cell cycle length by decreasing the length of time spent in S-phase. GF = growth fraction.
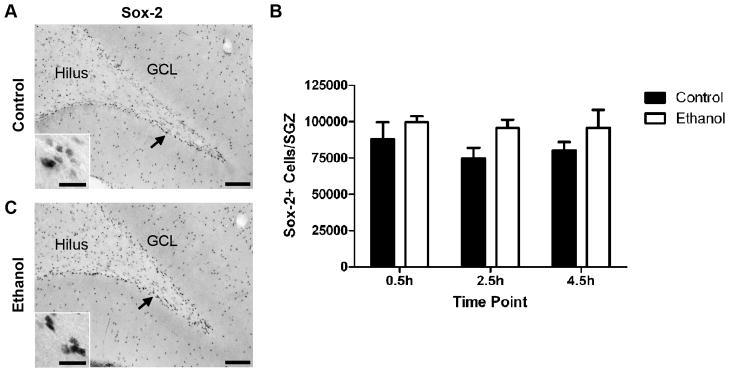
The abbreviated Tc and Ts suggest that binge ethanol exposure in adolescent rats accelerates progression through the cell cycle leading to an overall increase in NPC proliferation. This was examined further by analyzing the Sox-2 stereology data using two-way ANOVA. A dense population of Sox-2+ cells was observed within the SGZ of the dentate gyrus, although Sox-2+ cells were found scattered throughout the hilus, molecular layer, and even the granule neuron layer (Figure 5B). There was no main effect of time (F(2, 40) = 0.49, p = 0.61), which was not surprising given the short duration time course used for the cell cycle kinetics measurements. However, in support for an ethanol-induced increase in NPC proliferation, there was a significant main effect of diet (F(1,40) = 5.1, p = 0.03), in which 4-day binge ethanol exposure increased the number of Sox-2+ cells in the SGZ by 19% (Figure 5C). There was no time by diet interaction (F(2,40) = 0.14, p = 0.87).
Figure 5.
The effect of binge ethanol exposure during adolescence on the number of SGZ NPCs. A–B) Representative images from sections stained for Sox-2. Sox-2+ cells are found throughout the dentate gyrus, hippocampus, and other cortical regions; however, there is a large concentration of Sox-2+ cells located specifically within the SGZ. Arrows denote area represented in the inset. Scale bars = 100μm; inset 20μm. C) Sox-2 stereology data. Two-way ANOVA demonstrated that there was no effect of time; however, a main effect of diet was detected indicating that the 4-day binge paradigm used in this study slightly, but significantly increased the number of Sox-2+ cells in the SGZ.
To rule out the possibility that ethanol-induced degeneration of hippocampal NPCs is responsible for ethanol’s measured effects on cell cycle distribution and kinetics, FJB staining within the granule cell layer and along the SGZ was analyzed. Consistent with previous studies on alcohol-induced degeneration in this model (Crews et al., 2000; Leasure and Nixon, 2010; Morris et al., 2010a), binge ethanol exposed rats had only a few FJB+ cells in the dorsal dentate gyrus, while only a single FJB+ cell was found in one control rat (t = 1.8, p = 0.03; control = 0.125 ± 0.125 cells/dentate gyrus; ethanol = 2.6 ± 1.4 cells/dentate gyrus). The relatively low level of FJB staining confirms that cell death is not likely responsible for the large decrease seen in BrdU staining or changes in cell cycle kinetics following binge ethanol exposure.
DISCUSSION
Alcohol intoxication, whether during development or in adulthood, detrimentally impacts neurogenesis. Most recently, we have extended this to adolescence where four-day binge exposure results in decreased adult neurogenesis primarily through effects on cell proliferation (Morris et al., 2010a). Although we initially hypothesized that the apparent disconnect between ethanol’s effects on Ki-67 and BrdU were due to cell cycle arrest (Morris et al., 2010a), the current data provides strong evidence that binge ethanol exposure during adolescence accelerates rather than arrests cell cycle progression. First, we demonstrated that ethanol alters the distribution of NPCs within the phases of the cell cycle by specifically reducing the proportion of NPCs in S-phase without altering the proportion in G1 or G2/M phase. This result led us to assess cell cycle kinetics using the cumulative BrdU labeling method (Mooney and Miller, 2010) which indicated that the ethanol-induced decrease in the proportion of cells in S-phase following a single BrdU pulse results from a significant reduction in the length of S-phase and total cell cycle length. Thus, after four-day binge ethanol exposure, cell cycle length is shorter by 10 hours (36% decrease) versus control rats. NPC cycle acceleration implies that binge ethanol exposure results in an overall increase in NPC proliferation during intoxication. In support of this effect, ethanol slightly, but significantly (main effect of diet) increased the number of Sox-2 expressing NPCs within the adolescent SGZ.
This study emphasizes the importance of using multiple cell cycle markers to examine cell proliferation associated with hippocampal neurogenesis. Binge ethanol exposure significantly decreased BrdU incorporation from a single i.p. injection, an effect that could have been interpreted in many different ways unless additional cell cycle markers were studied. Decreased BrdU incorporation can be caused by cell cycle exit, cell death, or accelerated progression through S-phase (Salomoni and Calegari, 2010). Although binge ethanol exposure decreased BrdU labeling, there was no effect on Ki-67 which indicates that ethanol does not decrease the size of the dividing NPC population or result in NPC cell cycle exit. It is also unlikely that cells specifically within S-phase undergo cell death. This conclusion is supported by several lines of evidence. First, ethanol had no effect on the number of Ki-67+ or pHis-H3+ cells. If cells in S-phase were targeted for cell death, we would have anticipated a corresponding decrease in these two cell cycle markers. Second, the low amount of cell death in the dorsal dentate gyrus observed with FJB here as well as in our previous report (Morris et al., 2010a) suggest that effects on cell death are not sufficient to account for the 44% reduction of NPCs in S-phase. FJB staining showed that binge ethanol exposure results in no more than a few dozen dead cells per dorsal dentate gyrus, while BrdU staining demonstrated that the ethanol-induced effect on S-phase involves thousands of cells. Thus, the ethanol-induced decrease in BrdU combined with the lack of an effect on Ki-67 or evidence of cell death in the proliferating cell population provides additional strong support for an important effect of ethanol on NPC cell cycle kinetics.
To our knowledge, this is the first study to examine hippocampal NPC cell cycle kinetics in adolescent rats following binge ethanol exposure. At first glance, it may seem that an accelerated cell cycle or increased NPC proliferation would not be consistent with alcohol-induced decrease in neurogenesis (Morris et al., 2010a); however, the assumption that an increase in proliferation leads to an increase in neurogenesis ignores potentially important effects on other aspects of neurogenesis such as regulation of immature neuron survival, differentiation, and new cell incorporation into the existing hippocampal circuitry. In fact, effects on hippocampal proliferation do not always predict overall effect on neurogenesis. For example, binge 3,4-methylenedioxymethamphetamine (MDMA) exposure in adult rats has no effect on hippocampal NPC proliferation, but significantly decreases the survival of newborn neurons (Hernandez-Rabaza et al., 2006). Interestingly, intermittent MDMA exposure in adolescent rats increased hippocampal NPC proliferation but an overall decrease in neurogenesis was found due to MDMA’s detrimental effects on newborn neuron survival (Catlow et al., 2010).
There are two potential mechanisms that could explain how binge ethanol-mediated cell cycle acceleration results in decreased hippocampal neurogenesis. First, cell cycle acceleration could result in significant damage to daughter cells, triggering activation of apoptotic or other cell death pathways, leading to decreased new neuron survival. Similar results have been described for other drugs of abuse. For example, chronic morphine administration in adult mice increased caspase-3 activation and reduced BrdU incorporation within the dentate gyrus SGZ, an effect correlated with accelerated cell cycle progression from S-phase through G2/M that resulted in premature mitosis (Mandyam et al., 2004; Arguello et al., 2008). Whether a link exists between alcohol-induced cell cycle acceleration and survival of newborn neurons remains to be determined; however, the low level of cell death detected within the adolescent dorsal dentate gyrus following 4-day binge exposure is not sufficient to account for alcohol’s inhibition of neurogenesis (see results and Morris et al., 2010a).
A second potential mechanism that could explain how alcohol-induced NPC cell cycle acceleration leads to decreased neurogenesis in adolescents involves effects on cell fate. Hippocampal NPCs are multipotent, meaning they can differentiate into multiple cell types, including astrocytes. Cell phenotype analysis measured 4 weeks after binge alcohol exposure resulted in no change in the proportion of newborn cells that differentiated into neurons or astrocytes, indicating that cell cycle acceleration likely does not affect cell type once the cell has committed to differentiate (Morris et al., 2010a). However, this does not preclude effects on a newborn cell’s decision to differentiate or remain undifferentiated. Alcohol-mediated cell cycle acceleration could promote NPC population expansion and maintenance of the undifferentiated state, an effect supported by our observation of increased Sox-2+ cells and supported in models of fetal alcohol syndrome (Santillano et al., 2005). Several studies have shown that cell cycle length regulates a cell’s decision to differentiate with longer cell cycle times associated with differentiation and shorter cell cycle times linked to maintenance of the undifferentiated state (Salomoni and Calegari, 2010). For example, G1 length appears particularly important in regulating differentiation of mouse cortical progenitors, since accelerating G1 progression with overexpression of cdk4/cyclinD1 inhibited neurogenesis, while cdk4/cyclinD1 RNA interference lengthened G1 and promoted early differentiation (Lange et al., 2009). Recent work with human embryonic stem cells also demonstrated that inhibition of Notch signaling, an important pathway for stem cell maintenance, delayed G1 to S-phase transition, which was associated with increased neuronal differentiation (Borghese et al., 2010). Others have found an important role for S-phase in differentiation showing that guanosine triphosphate treatment lengthened S-phase in SH-SY5Y cells, which corresponded to enhanced neurite formation and expression of neuronal differentiation markers (Guarnieri et al., 2009). Based on these studies, ethanol-mediated shortening of hippocampal NPC cell cycle in adolescent rats may promote NPC population expansion and maintenance of the undifferentiated state. Such a mechanism would result in a short-term reduction in the generation of new granule cells during binge intoxication, matching our earlier observations that binge ethanol exposure inhibits hippocampal neurogenesis (Morris et al., 2010a). These data also suggest that the shortened cell cycle time and emerging increase in the progenitor pool reflect a compensatory response to repeated alcohol inhibition of neurogenesis or to alcohol-induced dentate gyrus degeneration. Either mechanism could cause sufficient degeneration of the dentate gyrus to initiate a reactive neurogenic response such as that described in adult rats following a 4-day binge (Nixon and Crews, 2004). Other models of degeneration show a similar response. For example, SGZ progenitors have a 20% shorter cell cycle within hours following seizure in juvenile rats, and subventricular zone progenitor cell cycle length drastically shortens two days after middle cerebral artery occlusion (Varodayan et al., 2009; Zhang et al., 2008). Consistent with other reports on NPC cell cycle following brain insult, the shorter cell cycle was associated with retention of NPCs within the cell cycle and NPC population expansion (Zhang et al., 2008). Importantly, these effects preceded increased neurogenesis observed weeks after the insult (Varodayan et al., 2009; Zhang et al., 2008). Based on these studies, the decrease in cell cycle time in adolescent rats could have significant consequences on neurogenesis after binge ethanol-induced damage and may represent the early stages of compensatory neurogenesis. Indeed, unpublished observations from the same adolescent AUD model revealed a burst in the number of proliferating cells (Ki-67+) along the SGZ 7 days after binge ethanol exposure, an effect that resulted in increased granule cell neurogenesis (Morris, McClain, and Nixon, personal communication).
Similar to many of ethanol’s teratogenic effects, the effect of ethanol on adult NPC cell cycle appears to be dependent on the NPC type as well as the dose, duration and developmental timing of ethanol exposure (Goodlett et al., 2005). For example, the results of the current study differ somewhat from those recently reported in a non-human primate model of adolescent alcohol consumption (Taffe et al., 2010). Our results agree with those of Taffe and colleagues (2010) in that ethanol decreases BrdU labeling along the dentate gyrus SGZ; however, in the non-human primate model, Ki-67 labeling was also decreased suggesting that ethanol suppresses NPC proliferation by reducing the number of NPCs that are actively dividing. Notably, this decrease in proliferation was measured 2 months after a much longer duration of binge like exposure, 11 months, and was due to a loss of progenitors in this model. In a fetal alcohol spectrum disorder (FASD) model, fetal alcohol exposure shortens cell cycle length and S-phase duration of NPCs responsible for postnatal generation of ventrobasal thalamic neurons (Mooney and Miller, 2010), while fetal cortical progenitors in the ventricular zone lengthen their cell cycle in response to ethanol (Miller and Nowakowski, 1991; Miller and Kuhn, 1995; Siegenthaler and Miller, 2005).
Many studies have investigated the effects of addictive drugs on hippocampal granule cell neurogenesis (Eisch, 2000; Domínguez-Escribà et al., 2006; Arguello et al., 2008; Mandyam et al., 2008); however, most work has focused on exposure in adulthood, rather than in adolescence, the period during which experimentation with drugs of abuse is most often initiated. Given that granule cell neurogenesis occurs on a greater scale during adolescence compared to adulthood (Altman and Das, 1965; He and Crews, 2007), the effects of drugs, such as alcohol, on this process may have more profound consequences when introduced to the vulnerable adolescent hippocampus. In addition to the effects already described, a recent study demonstrated that repeated exposure to 3,4-methylenedioxymethamphetamine (MDMA) during adolescence dose-dependently increased NPC proliferation, but inhibited survival of differentiating neurons 2-weeks after cell birth (Catlow et al., 2010). Importantly, in both cases, alcohol and MDMA’s effects on neurogenesis differ in adolescent rodents compared to adults. In adult rats, binge alcohol exposure reduces the number of proliferating NPCs and significantly inhibits survival of newborn neurons (Crews et al., 2006a; Nixon and Crews, 2002). While alcohol attenuates newborn neuron survival in adolescents, alcohol’s effects on NPC proliferation involve no change in dividing NPC number (Morris et al., 2010a) and an accelerated NPC cell cycle, as shown here. MDMA exposure in adult rats reduces neuronal survival, but, unlike with adolescents, MDMA has no effect on NPC proliferation (Hernández-Rabaza et al., 2006). Results from our adolescent binge alcohol model combined with those described for chronic morphine exposure (Mandyam et al., 2004) and FASD (Mooney and Miller, 2010) indicate that hippocampal NPC cell cycle regulation is a significant target of action for drugs of abuse. Furthermore, these differences underscore the important need for understanding how drugs of abuse interact with the developing adolescent brain.
Elucidating the mechanisms by which alcohol interferes with hippocampal structure may hold important clues as to how alcohol-mediated cognitive and behavioral deficits arise and contribute to the downward spiral towards the development of AUDs (Koob and Moal, 1997; Crews, 1999). Indeed, reduced hippocampal adult neurogenesis has recently been reported as a vulnerability factor for cocaine addiction, though the role of adult neurogenesis in alcohol context memory or relapse to alcohol seeking has not been described (Noonan et al., 2010). However, hippocampal degeneration has been much more widely observed in adolescent AUDs as opposed to other drugs of abuse (De Bellis et al., 2000; Nagel et al., 2005) and our work in animal models continues to support that this degeneration is due primarily to alcohol-inhibition of adult neurogenesis (Morris et al., 2010a). Here, we demonstrate that binge ethanol exposure during this vulnerable period of development targets S-phase of the cell cycle resulting in accelerated cell cycle progression, adding to the growing list of effects of ethanol on hippocampal granule cell neurogenesis. The long-term neurogenic consequences of accelerated cell cycle progression of hippocampal NPCs are unknown, but may drive a compensatory burst in neurogenesis that is often observed after brain insult. Given the important role of neurogenesis in hippocampal learning and memory function, further studies are needed to determine if these changes in neurogenesis contribute to ethanol-induced hippocampal volume loss and cognitive deficits in adolescents with AUDs (De Bellis et al., 2000; Nagel et al., 2005; Schulteis et al., 2008).
Supplementary Material
Acknowledgments
Support: This work was supported by NIAAA grants R21 AA0160307 and R01 AA016959.
We gratefully acknowledge the technical assistance of M. Ayumi Deeny in these studies. Research supported by the NIAAA (R21AA0160307, R01AA016959).
LITERATURE CITED
- Ackman JB, Ramos RL, Sarkisian MR, LoTurco JJ. Citron kinase is required for postnatal neurogenesis in the hippocampus. Dev Neurosci. 2007;29:113–123. doi: 10.1159/000096216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Arguello AA, Harburg GC, Schonborn JR, Mandyam CD, Yamaguchi M, Eisch AJ. Time course of morphine’s effects on adult hippocampal subgranular zone reveals preferential inhibition of cells in S phase of the cell cycle and a subpopulation of immature neurons. Neuroscience. 2008;157:70–79. doi: 10.1016/j.neuroscience.2008.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bava S, Thayer R, Jacobus J, Ward M, Jernigan TL, Tapert SF. Longitudinal characterization of white matter maturation during adolescence. Brain Res. 2010;1327:38–46. doi: 10.1016/j.brainres.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese L, Dolezalova D, Opitz T, Haupt S, Leinhaas A, Steinfarz B, Koch P, Edenhofer F, Hampl A, Brüstle O. Inhibition of notch signaling in human embryonic stem cell-derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells. 2010;28:955–964. doi: 10.1002/stem.408. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RDG. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Catlow BJ, Badanich KA, Sponaugle AE, Rowe AR, Song S, Rafalovich I, Sava V, Kirstein CL, Sanchez-Ramos J. Effects of MDMA (“ecstasy”) during adolescence on place conditioning and hippocampal neurogenesis. Eur J Pharmacol. 2010;628:96–103. doi: 10.1016/j.ejphar.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Crews F, Nixon K, Kim D, Joseph J, Shukitt-Hale B, Qin L, Zou J. BHT blocks NF-kappaB activation and ethanol-induced brain damage. Alcohol Clin Exp Res. 2006a;30:1938–1949. doi: 10.1111/j.1530-0277.2006.00239.x. [DOI] [PubMed] [Google Scholar]
- Crews FT. Alcohol and neurodegeneration. CNS Drug Rev. 1999;5:379–394. [Google Scholar]
- Crews FT, Braun CJ, Hoplight B, Switzer RC, Knapp DJ. Binge ethanol consumption causes differential brain damage in young adolescent rats compared with adult rats. Alcohol Clin Exp Res. 2000;24:1712–1723. [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006b;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Crews FT, Nixon K. Mechanisms of neurodegeneration and regeneration in alcoholism. Alcohol Alcohol. 2009;44:115–127. doi: 10.1093/alcalc/agn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crews FT, Nixon K, Wilkie ME. Exercise reverses ethanol inhibitionof neural stem cell proliferation. Alcohol. 2004;33:63–71. doi: 10.1016/j.alcohol.2004.04.005. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, Kersh A, Keshavan MS. Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry. 2000;157:737–744. doi: 10.1176/appi.ajp.157.5.737. [DOI] [PubMed] [Google Scholar]
- Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11:339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domínguez-Escribà L, Hernández-Rabaza V, Soriano-Navarro M, Barcia JA, Romero FJ, García-Verdugo JM, Canales JJ. Chronic cocaine exposure impairs progenitor proliferation but spares survival and maturation of neural precursors in adult rat dentate gyrus. Eur J Neurosci. 2006;24:586–594. doi: 10.1111/j.1460-9568.2006.04924.x. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming G-L, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Barrot M, Schad CA, Self DW, Nestler EJ. Opiates inhibit neurogenesis in the adult rat hippocampus. Proc Natl Acad Sci U S A. 2000;97:7579–7584. doi: 10.1073/pnas.120552597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Horn KH, Zhou FC. Alcohol teratogenesis: mechanisms of damage and strategies for intervention. Exp Biol Med. 2005;230:394–406. doi: 10.1177/15353702-0323006-07. [DOI] [PubMed] [Google Scholar]
- Gould TJ. Ethanol disrupts fear conditioning in C57BL/6 mice. J Psychopharmacol. 2003;17:77–81. doi: 10.1177/0269881103017001702. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the national longitudinal alcohol epidemiologic survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Guarnieri S, Pilla R, Morabito C, Sacchetti S, Mancinelli R, Fanò G, Mariggiò MA. Extracellular guanosine and GTP promote expression of differentiation markers and induce S-phase cell-cycle arrest in human SH-SY5Y neuroblastoma cells. Int J Dev Neurosci. 2009;27:135–147. doi: 10.1016/j.ijdevneu.2008.11.007. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EBV, Kieu K, Nielsen J. The efficiency of the systematic sampling in stereology–reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psych. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- He J, Crews FT. Neurogenesis decreases during brain maturation from adolescence to adulthood. Pharmacol Biochem Behav. 2007;86:327–333. doi: 10.1016/j.pbb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Hernández-Rabaza V, Domínguez-Escribà L, Barcia JA, Rosel JF, Romero FJ, García-Verdugo JM, Canales JJ. Binge administration of 3,4-methylenedioxymethamphetamine (“ecstasy”) impairs the survival of neural precursors in adult rat dentate gyrus. Neuropharmacology. 2006;51:967–973. doi: 10.1016/j.neuropharm.2006.06.019. [DOI] [PubMed] [Google Scholar]
- Hunt PS, Levillain ME, Spector BM, Kostelnik LA. Post-training ethanol disrupts trace conditioned fear in rats: effects of timing of ethanol, dose and trace interval duration. Neurobiol Learn Mem. 2009;91:73–80. doi: 10.1016/j.nlm.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Bachman JG, Schulenberg JE. Secondary school students. NIH Publication No 07–6205. I. Bethesda, MD: National Institute on Drug Abuse; 2007. Monitoring the Future Nation Survey Results on Drug Use, 1975–2006. [Google Scholar]
- Key G, Becker MH, Baron B, Duchrow M, Schluter C, Flad HD, Gerdes J. New Ki-67-equivalent murine monoclonal antibodies (MIB 1–3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest. 1993;68:629–636. [PubMed] [Google Scholar]
- Komitova M, Zhu X, Serwanski DR, Nishiyama A. NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J Comp Neurol. 2009;512:702–716. doi: 10.1002/cne.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science. 1997;278:52–58. doi: 10.1126/science.278.5335.52. [DOI] [PubMed] [Google Scholar]
- Kuhn H, Dickinson-Anson H, Gage F. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Huttner WB, Calegari F. Cdk4/CyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5:320–331. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- Leasure JL, Nixon K. Exercise neuroprotection in a rat model of binge alcohol consumption. Alcohol Clin Exp Res. 2010;34:404–414. doi: 10.1111/j.1530-0277.2009.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little PJ, Kuhn CM, Wilson WA, Swartzwelder HS. Differential effects of ethanol in adolescent and adult rats. Alcohol Clin Exp Res. 1996;20:1346–1351. doi: 10.1111/j.1530-0277.1996.tb01133.x. [DOI] [PubMed] [Google Scholar]
- Majchrowicz E. Induction of physical dependence upon ethanol and the associated behavioral changes in rats. Psychopharmacology. 1975;43:245–254. doi: 10.1007/BF00429258. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Norris RD, Eisch AJ. Chronic morphine induces premature mitosis of proliferating cells in the adult mouse subgranular zone. J Neurosci Res. 2004;76:783–794. doi: 10.1002/jnr.20090. [DOI] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Crawford EF, Eisch AJ, Richardson HN, Koob GF. Varied access to intravenous methamphetamine self-administration differentially alters adult hippocampal neurogenesis. Biol Psychiatry. 2008;64:958–965. doi: 10.1016/j.biopsych.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwiese BJ, Acheson SK, Levin ED, Wilson WA, Swartzwelder HS. Differential effects of ethanol on memory in adolescent and adult rats. Alcohol Clin Exp Res. 1998;22:416–421. [PubMed] [Google Scholar]
- Mathews EA, Morgenstern NA, Piatti VC, Zhao C, Jessberger S, Schinder AF, Gage FH. A distinctive layering pattern of mouse dentate granule cells is generated by developmental and adult neurogenesis. J Comp Neurol. 2010;518:4479–4490. doi: 10.1002/cne.22489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina KL, Schweinsburg AD, Cohen-Zion M, Nagel BJ, Tapert SF. Effects of alcohol and combined marijuana and alcohol use during adolescence on hippocampal volume and asymmetry. Neurotoxicol and Teratol. 2007;29:141–152. doi: 10.1016/j.ntt.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW. Generation of neurons in the rat dentate gyrus and hippocampus: effects of prenatal and postnatal treatment with ethanol. Alcohol Clin Exp Res. 1995;19:1500–1509. doi: 10.1111/j.1530-0277.1995.tb01014.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Kuhn PE. Cell cycle kinetics in fetal rat cerebral cortex: effects of prenatal treatment with ethanol assessed by a cumulative labeling technique with flow cytometry. Alcohol Clin Exp Res. 1995;19:233–237. doi: 10.1111/j.1530-0277.1995.tb01497.x. [DOI] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Effect of prenatal exposure to ethanol on the cell cycle kinetics and growth fraction in the proliferative zones of fetal rat Cerebral cortex. Alcohol Clin Exp Res. 1991;15:229–232. doi: 10.1111/j.1530-0277.1991.tb01861.x. [DOI] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Postnatal generation of neurons in the ventrobasal nucleus of the rat thalamus. J Neurosci. 2007;27:5023–5032. doi: 10.1523/JNEUROSCI.1194-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney SM, Miller MW. Prenatal exposure to ethanol affects postnatal neurogenesis in thalamus. Exp Neurol. 2010;223:566–573. doi: 10.1016/j.expneurol.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Eaves DW, Smith AR, Nixon K. Alcohol inhibition of neurogenesis: a mechanism of hippocampal neurodegeneration in an adolescent alcohol abuse model. Hippocampus. 2010a;20:596–607. doi: 10.1002/hipo.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SA, Kelso ML, Liput DJ, Marshall SA, Nixon K. Similar withdrawal severity in adolescents and adults in a rat model of alcohol dependence. Alcohol. 2010b;44:89–98. doi: 10.1016/j.alcohol.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel BJ, Schweinsburg AD, Phan V, Tapert SF. Reduced hippocampal volume among adolescents with alcohol use disorders without psychiatric comorbidity. Psychiatry Res. 2005;139:181–190. doi: 10.1016/j.pscychresns.2005.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K. Alcohol and adult neurogenesis: roles in neurodegeneration and recovery in chronic alcoholism. Hippocampus. 2006;16:287–295. doi: 10.1002/hipo.20162. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Binge ethanol exposure decreases neurogenesis in adult rat hippocampus. J Neurochem. 2002;83:1087–1093. doi: 10.1046/j.1471-4159.2002.01214.x. [DOI] [PubMed] [Google Scholar]
- Nixon K, Crews FT. Temporally specific burst in cell proliferation increases hippocampal neurogenesis in protracted abstinence from alcohol. J Neurosci. 2004;24:9714–9722. doi: 10.1523/JNEUROSCI.3063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan MA, Bulin SE, Fuller DC, Eisch AJ. Reduction of adult hippocampal neurogenesis confers vulnerability in an animal model of cocaine addiction. J Neurosci. 2010;30:304–315. doi: 10.1523/JNEUROSCI.4256-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowski RS, Lewin SB, Miller MW. Bromodeoxyuridine immunohistochemical determination of the lengths of the cell cycle and the DNA-synthetic phase for an anatomically defined population. J Neurocytol. 1989;18:311–318. doi: 10.1007/BF01190834. [DOI] [PubMed] [Google Scholar]
- NRC. Guide for the Care and Use of Laboratory Animals. Washington, D.C: The National Academies Press; 1996. [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Paxinos GW, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 2009. [Google Scholar]
- Prigent C, Dimitrov S. Phosphorylation of serine 10 in histone H3, what for? J Cell Sci. 2003;116:3677–3685. doi: 10.1242/jcs.00735. [DOI] [PubMed] [Google Scholar]
- Salomoni P, Calegari F. Cell cycle control of mammalian neural stem cells: putting a speed limit on G1. Trends Cell Biol. 2010;20:233–243. doi: 10.1016/j.tcb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- Santillano DR, Kumar LS, Prock TL, Camarillo C, Tingling JD, Miranda RC. Ethanol induces cell-cycle activity and reduces stem cell diversity to alter both regenerative capacity and differentiation potential of cerebral cortical neuroepithelial precursors. BMC Neurosci. 2005;6:59. doi: 10.1186/1471-2202-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmued LC, Hopkins KJ. Fluoro-Jade B: a high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000;874:123–130. doi: 10.1016/s0006-8993(00)02513-0. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Archer C, Tapert SF, Frank LR. Intermittent binge alcohol exposure during the periadolescent period induces spatial working memory deficits in young adult rats. Alcohol. 2008;42:459–467. doi: 10.1016/j.alcohol.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, McQueeny T, Nagel BJ, Eyler LT, Tapert SF. A preliminary study of functional magnetic resonance imaging response during verbal encoding among adolescent binge drinkers. Alcohol. 2010;44:111–117. doi: 10.1016/j.alcohol.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegenthaler JA, Miller MW. Ethanol disrupts cell cycle regulation in developing rat cortex interaction with transforming growth factor β1. J Neurochem. 2005;95:902–912. doi: 10.1111/j.1471-4159.2005.03461.x. [DOI] [PubMed] [Google Scholar]
- Sircar R, Basak AK, Sircar D. Repeated ethanol exposure affects the acquisition of spatial memory in adolescent female rats. Behav Brain Res. 2009;202:225–231. doi: 10.1016/j.bbr.2009.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar R, Sircar D. Adolescent rats exposed to repeated ethanol treatment show lingering behavioral impairments. Alcohol Clin Exp Res. 2005;29:1402–1410. doi: 10.1097/01.alc.0000175012.77756.d9. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taffe MA, Kotzebue RW, Crean RD, Crawford EF, Edwards S, Mandyam CD. Long-lasting reduction in hippocampal neurogenesis by alcohol consumption in adolescent nonhuman primates. Proc Natl Acad Sci U S A. 2010;107:11104–11109. doi: 10.1073/pnas.0912810107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varodayan FP, Zhu XJ, Cui XN, Porter BE. Seizures increase cell proliferation in the dentate gyrus by shortening progenitor cell-cycle length. Epilepsia. 2009;50:2638–2647. doi: 10.1111/j.1528-1167.2009.02244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitemier AZ, Ryabinin AE. Alcohol-induced memory impairment in trace fear conditioning: a hippocampus-specific effect. Hippocampus. 2003;13:305–315. doi: 10.1002/hipo.10063. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gunderen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Hippocampal function during adolescence: a unique target of ethanol effects. Ann NY Acad Sci. 2004;1021:206–220. doi: 10.1196/annals.1308.026. [DOI] [PubMed] [Google Scholar]
- White AM, Swartzwelder HS. Age-related effects of alcohol on memory and memory-related brain function in adolescents and adults. Recent Dev Alcohol. 2005;17:161–176. doi: 10.1007/0-306-48626-1_8. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Roberts C, LeTourneau Y, Lu M, Zhang L, Wang Y, Chopp M. Lengthening the G(1) phase of neural progenitor cells is concurrent with an increase of symmetric neuron generating division after stroke. J Cereb Blood Flow Metab. 2008;28:602–611. doi: 10.1038/sj.jcbfm.9600556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.