A 25-year-old female with a history of Ewing sarcoma presented with leukopenia (920/μL), neutropenia (180/μL), thrombocytopenia (147,000/μL) and macrocytic anemia (hemoglobin 12.2 g/dL, mean corpuscular volume (MCV) 90.3 fL). Metastatic Ewing sarcoma of the right gluteal muscle with pulmonary metastases had been diagnosed 7 years previously. Prior treatment had included chemotherapy, radiation therapy, and allogeneic hematopoietic stem cell transplantation (alloHSCT).
In any patient previously treated with chemotherapy, radiation, and/or stem cell transplant, cytopenias and/or a rising MCV should prompt further investigation with myelodysplastic syndrome (MDS) as a consideration in the differential diagnosis. The incidence of secondary hematologic malignancies in pediatric cancers treated with standard therapies has risen with the risk linked to the intensity of therapy. (3) Specifically, there is a 2–9% cumulative incidence over 5 years of treatment-related MDS (t-MDS) or acute myelogenous leukemia (AML) after treatment of Ewing sarcoma. (4, 5)
The patient had been treated in a clinical trial at the National Institutes of Health (NIH) for the treatment of high-risk pediatric sarcomas with non-myeloablative alloHSCT. (6) Pre-transplant conditioning consisted of fludarabine, cyclophosphamide, and melphalan. The donor was an HLA-matched sister and the graft source was filgrastim-mobilized peripheral blood stem cells. Graft-versus-host disease (GVHD) prophylaxis was with cyclosporine, which was discontinued on post-transplant day (Day +) 65. 100% donor engraftment was demonstrated on Day +14. The patient developed late-acute GVHD of the skin and the gastrointestinal tract, followed by chronic GVHD with involvement of the liver, skin, mouth, vagina and eyes. Subsequently, the patient sustained multiple recurrences of Ewing sarcoma beginning on Day +100, treated with chemotherapy, radiation, surgery, investigational monoclonal antibody against the insulin-like growth factor receptor-1 (CP751871, figitumumab), and an investigational tumor lysate dendritic cell vaccine.
Secondary malignancies, such as solid tumors and carcinomas, are relatively common after alloHSCT with a reported incidence of 2–20%. (7–9) The risk of therapy-related acute myelogenous leukemia (t-AML) and t-MDS after high-dose therapy with autologous stem cell rescue is also significant with a reported incidence ranging from 1–24%. (10, 11) In contrast, the risk of t-MDS/AML and other hematologic malignancies after alloHSCT is extremely rare with only 4 cases of t-AML observed in 4,749 patients. (12)
Cumulative doses of post-transplant therapy were etoposide 900 mg/m2, vincristine 7 mg/m2, cyclophosphamide 3,375 mg/m2, doxorubicin 525 mg/m2, irinotecan 1,500 mg/m2, temozolomide 4,000 mg/m2, and radiation 3,500 cGy. Cytopenias and macrocytosis developed at 71 months post-transplant.
The most commonly implicated agents in the etiology of t-MDS/AML include etoposide and alkylators (e.g., cyclophosphamide, ifosfamide) with a recently recognized association of temozolomide with MDS. (13–15) The cumulative dose thresholds that significantly predispose to t-MDS have been estimated to be 2,000 mg/m2 for etoposide, 8,000–10,000 mg/m2 for cyclophosphamide and 18,000–20,000 mg/m2 for temozolamide. The latency periods to t-MDS/AML development range from 1–10 years depending on the specific drug and exposure. (15) Although the total doses of these agents used after transplant in the presented case did not reach these single-dose thresholds, there may be cumulative impact of multiple agents. Additionally, a case of temozolomide-induced MDS has been reported in a child who received only 2,100 mg/m2 cumulative dose, suggesting that lower doses may be leukemogenic. (13) Recent reports have also suggested an increased rate of t-MDS following fludarabine-based regimens. (16)
Given the laboratory abnormalities, further evaluation with bone marrow aspirate and biopsy was performed revealing 16% blasts, hypocellularity (20–40%), and multilineage dysplasia leading to the diagnosis of MDS. Dysplastic changes were most prominent in the myeloid lineage (hyposegmented pseudopelgeroid neutrophils, hypogranular forms, and maturation asynchrony) and megakaryocyte lineage (small hypolobated megakaryocytes). Flow cytometry of the marrow aspirate revealed abnormal myeloid blasts as well as dysplastic granulocytic and monocytic lineages. Myeloid blasts were arrested with homogeneous CD13, CD34 and CD45 expression, and abnormal partial CD11b, bright CD33, partial dim CD38, dim to negative HLA-DR and dim to negative CD117 expression. Granulocytes were left shifted with decreased side light scatter consistent with hypogranularity, abnormal dim CD11b, and aberrant CD14 expression. Monocytes demonstrated abnormal bright CD15 and dim HLA-DR. A diagnosis of refractory anemia with excess blasts-2 (RAEB-2) was assigned.
Patients with RAEB (5%–19% marrow blasts) generally have a relatively poor prognosis, with a median survival of 5 to 12 months. In contrast, patients with refractory anemia (RA) (< 5% blasts) or those with refractory anemia with ringed sideroblasts (RARS) (< 5% blasts plus > 15% ringed sideroblasts) have a median survival of 3 to 6 years. The proportion of individuals whose disease transforms to AML ranges from 5% to 15% in the low-risk RA/RARS group to 40% to 50% in the RAEB groups. (17, 18) The prognosis and likelihood of progression to AML for t-MDS has not been validated using this classification system.
In suspected cases of post-transplant MDS, bone marrow aspirate and biopsy should be performed for diagnostic confirmation. (19) (20) (21) In cases without blast increase, a suspicion for MDS after alloHSCT may be less obvious than in the de novo setting, due in part to the common occurrence and/or persistence of post-transplant cytopenias, macrocytosis, and bone marrow hypocellularity. For example, common medication-associated abnormalities may confound the diagnosis (e.g., trimethoprim-sulfamethoxazole associated erythroid macrocytosis and myelosuppression). Van Marion et al. reviewed bone marrow histopathology in the post-transplant period and noted frequent dyshematopoiesis with cytoplasmic and nuclear abnormalities in the first months after transplantation. (22) Therefore, additional techniques are required to assess the diagnosis of MDS in the post-transplant period.
Cytogenetics revealed a normal 46, XX female karyotype in 20 of 20 metaphases analyzed, which was confirmed on two subsequent bone marrow aspirates performed over a 24-week period.
Chromosome banding analysis should be performed whenever MDS is suspected to help to confirm the diagnosis and differentiate between de novo and therapy-related disease. (23) Complex and hypodiploid karyotypes are seen more frequently in t-MDS/AML in comparison to de novo disease, as are specific aberrations such as 5q-, monosomy 7, and 11q23/MLL abnormalities. (24) Conventional metaphase cytogenetic analysis detects aberrations in less than 50% of patients with de novo MDS. (25, 26) In a recent study by Tiu et al., the addition of single nucleotide polymorphism arrays led to detection of chromosomal defects in 74% in patients with MDS (versus 44% with conventional cytogenetics alone). (27) Fluorescence in-situ hybridization (FISH) may be used to monitor specific cytogenetic alterations that were present prior to transplantation or for confirmation of results of chromosome banding analysis. (28) In the event that the mitotic index is insufficient for that analysis, interphase FISH can be used to identify previously known cytogenetic alterations. Multiparameter flow cytometry can be helpful in the diagnosis of MDS (29) and in the detection of minimal residual disease (MRD). (30) Nonetheless, monitoring for MDS after alloHSCT is often limited by the lack of specific markers, (31) although this is expected to improve with the increasing panel of molecular markers in MDS. (32) (33)
Peripheral blood chimerism studies utilizing short-tandem repeat (STR)-PCR analysis revealed 100% donor origin of both the T cell (CD3) and the myeloid (CD33/CD66b) components. These findings were consistent with a donor-cell myelodysplastic syndrome, presumed related to the extensive post-transplant therapy.
Donor cell leukemia (DCL) and donor cell myelodysplastic syndrome (donor-cell MDS) represent hematologic malignancies that arise in donor cells following alloHSCT. Detection of donor-cell MDS requires both a high index of suspicion as well as a way to distinguish between cells derived from recipient versus donor origin. This is especially important when trying to differentiate from relapse of the primary disease, which has implications in regard to treatment. Suspicion for donor-cell MDS is often first raised when there is a discrepancy between the detection of pathologic morphologic findings, such as blast increase or dysplasia, in the setting of full donor engraftment without evidence for recipient cells or when the new abnormalities differ from the original diagnosis for which the recipient was transplanted. Importantly in this regard, in 72% (18 of 25) of reported cases of donor-cell MDS or DCL with likely antecedent donor-cell MDS, transplantation was performed for diagnoses other than AML or MDS. (34, 35) Chimerism analysis serves as the primary method by which donor-cell MDS can be distinguished from relapse of host origin. The various methods and indications for use include XY-FISH (in case of sex-mismatched HSCT) and different molecular methods including PCR for variable number tandem repeats (VNTRs: repeats of 10–100 base pairs) or short tandem repeats (STRs) (repeats of 2–6 base pairs), real-time PCR for donor/recipient specific polymorphisms, or restriction fragment length polymorphisms (RFLP). (36–38) Given the inherent genetic instability found within leukemic cells and limited sensitivity in detection, multiple methods may need to be applied in the evaluation of donor-cell MDS. In comparison to relapsed disease, which almost always occurs before 2 years post transplant, DCL is commonly diagnosed later, with a median time to DCL of 31 months (range: 2–312 months). (35)
Discussion
Myelodysplastic syndrome that develops after alloHSCT can represent relapse or de novo disease, the latter of which can be of recipient or donor origin. Relapse rates of MDS after alloHSCT range from 5–60% depending on the stage of disease at presentation and the intensity of conditioning. (39, 40) Combined, 84 cases of DCL and donor-cell MDS have been reported. (34, 35, 38, 41–47) Donor-cell MDS appears to be less common than DCL, with only 21 cases previously reported (34, 35, 44, 45, 47–57) with several additional cases of DCL arising from an antecedent MDS phase. (35, 44, 50, 58–64) Prior cases of donor-cell MDS have been reported after alloHSCT for hematologic malignancies and bone marrow failure syndromes. However, this represents the first known case of donor-cell MDS after alloHSCT for a solid tumor and it (50, 53)likely represents therapy-related MDS (t-MDS) induced by exposure of donor cells to the toxic effects of chemotherapy and/or radiation administered after transplantation.
Pathobiologic Considerations
In patients previously transplanted for AML/MDS, relapse of the original disease is far more likely than MDS arising in donor cells. In contrast, MDS is more likely to be of donor origin in patients transplanted for other conditions, although recipient-derived t-MDS may be related to pre-transplant exposure to chemotherapy and/or radiation. Proposed mechanisms leading to donor-cell MDS include occult leukemia in the donor, abnormal or defective stroma and/or bone marrow microenvironment, genetic susceptibility, immune-mediated phenomenon, infection, transmission of an oncogene from the host’s original disease into donor cells, toxicity of post-transplant therapies, and possibly GVHD. (7, 35, 38, 43, 45, 50, 53, 54, 59, 65–69) In this reported case of MDS developing in a patient transplanted for a solid tumor, exposure of donor cells to the effects of the chemotherapy and radiation used to manage recurrent Ewing sarcoma is the most plausible etiology of donor-cell MDS. Consistent with this, 36 of 74 (49%) of previously reported cases of DCL had cytogenetic abnormalities typical of therapy-related disease, including abnormalities involving 11q23, 21q22, and chromosomes 5, 7, 8 and 21, and three of these cases were known to have had a history of post-transplant radiation or pre-transplant exposure to chemotherapy in the donor. (35, 47)
Management Considerations
The optimal management of MDS that recurs or develops after stem cell transplantation is not well defined. The options to be considered are usually extrapolated from the treatment of MDS in patients who have not undergone alloHSCT. The recommended approach after transplant varies depending on case-specific details (Figure 1, Table 1) and the rapidity of progression to AML.
Figure 1. Algorithm for the evaluation and treatment of MDS after allogeneic hematopoietic stem cell transplantation.
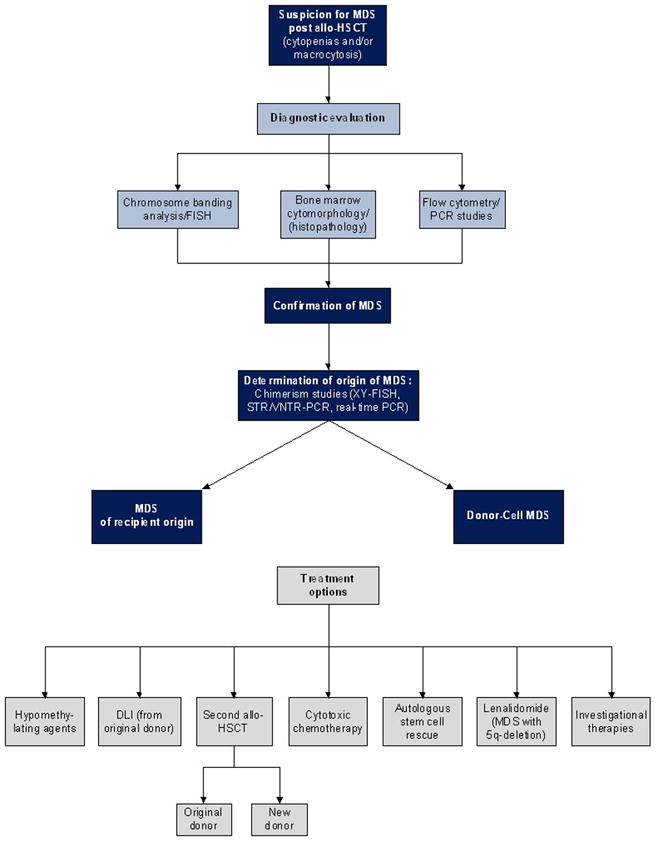
This algorithm provides general guidelines for the evaluation and treatment of MDS after allogeneic hematopoietic stem cell transplantation.
MDS should be considered in the differential diagnosis of persistent cytopenias and/or macrocytosis that are not otherwise explained by medication effect or post-transplant complications. Diagnostic bone marrow aspirate and biopsy should be performed for morphologic evaluation and extra aspirate samples collected for possible additional studies as detailed below. If dysplasia, atypia and/or increased blasts are detected, samples should be sent for cytogenetic evaluation by means of chromosome banding analysis and/or FISH, flow cytometry, PCR studies specific to previously documented molecular markers of the underlying disease, and/or chimerism analysis. The origin of the MDS as either recipient- or donor- derived should be determined. In patients previously transplanted for AML/MDS, relapse is more likely than de novo donor-cell MDS. In patients transplanted for other conditions, donor-cell MDS is most likely. Methods for determining the origin of MDS include STR/VNTR PCR, real-time PCR, and XY-FISH (in sex mismatched transplants). Treatment options for MDS after alloHSCT include hypomethylating agents, DLI, second alloHSCT (from the original or a new donor), cytotoxic chemotherapy, high-dose therapy with autologous stem cell rescue, and lenalidomide (for those with 5q-deletion). Enrollment in clinical trials should be considered. The decision regarding the best treatment for an individual patient is dependent upon the origin of the MDS (recipient versus donor), the rapidity of disease progression to AML, cytogenetic abnormalities, and co-morbidities. Treatment options should be weighed carefully and individualized with close considerations of such factors. Specifically, the origin of MDS plays an important role in determining the best therapy. For example, DLI may be a reasonable first choice to manage relapsed MDS of recipient origin, whereas a therapeutic GVL effect would not be expected in donor-derived MDS and thus an alternative treatment would be preferred in that setting. Although some treatment options have curative potential (e.g., second alloHSCT), these may be associated with high risk of treatment-related mortality. In contrast, other approaches are less likely to be curative, however, they may offer reduced toxicity and higher likelihood of improved short-term quality of life.
Table 1.
Treatment options for relapsed MDS and donor cell MDS after allogeneic hematopoietic stem cell transplantation
| Treatment Option | Primary Indication | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| Hypomethylating agents Azacitidine Decitabine | De novo MDS |
|
|
(70–72, 95) |
| Donor Lymphocyte Infusion | Relapsed primary disease |
|
|
(45, 81) |
| Second Allogeneic Stem Cell Transplant | Relapsed disease or graft failure |
|
|
(35, 94, 96) |
| Chemotherapy | De novo or t-MDS |
|
|
(22, 23, (84) |
| Autologous Stem Cell Rescue | De novo or t-MDS |
|
|
(87, 88) |
| Lenalidomide | MDS with 5q-deletion |
|
|
(90, 91) |
Abbreviations: AML: acute myelogenous leukemia; DCL: donor cell leukemia; GVHD: graft-versus-host disease; GVL: graft-versus-leukemia; alloHSCT: allogeneic hematopoietic stem cell transplantation; TRM: treatment-related mortality
Hypomethylating agents
The hypomethylating agents azacitidine and decitabine have demonstrated efficacy in the treatment of MDS, but there are only limited data in the post-transplant setting.(70–72) These agents have associated toxicities, most notably cytopenias that limit the dose that can be safely administered after alloHSCT. (73) Maintenance therapy with low-dose azaciditine to prevent post-transplant recurrence is being tested in clinical trials. (74)
Allogeneic stem cell transplantation and donor lymphocyte infusion
AlloHSCT is the treatment modality with the highest curative potential for patients with newly diagnosed MDS and high-risk features. (75) Consequently, second stem cell transplant is considered for patients with MDS after alloHSCT, although data are somewhat limited and outcomes are guarded with second transplants in general. The Center for International Blood and Marrow Transplant Research (CIBMTR) conducted a retrospective study of alloHSCT in 868 patients with t-MDS/AML. Although none of these patients had undergone prior alloHSCT, 17% had undergone prior autologous stem cell rescue. (76) Other smaller studies have included patients with prior allogeneic transplantation. (77–79) Second alloHSCT for relapsed disease after reduced intensity conditioning was utilized for 10 patients with relapsed MDS with 17% OS, 21% relapse, and 23% TRM rates at one year. (80) Donor lymphocyte infusion (DLI) has been successfully employed, albeit with relatively limited efficacy and poor long-term survival. One series reported 3 complete remissions in 14 evaluable patients, but with the consequence of extensive chronic GVHD. (81)
Other therapeutic options
AML-type chemotherapy is commonly employed for patients with high-risk subtypes of MDS, although in general the results are poor.(75, 82–85) Furthermore, the expected toxicity of AML-type chemotherapy prohibits use after alloHSCT in most cases. (86) Thus, the use of chemotherapy for MDS after transplant is commonly limited to low-dose palliation. High-dose therapy with autologous stem cell rescue has been used in the treatment of newly diagnosed MDS with reduced TRM and no risk of GVHD in comparison to alloHSCT, but without the benefit of a graft-versus-leukemia (GVL) effect and with an associated increased risk of relapse. (87) (88) However, there is no known experience using “autologous” stem cell rescue in the management of MDS after alloHSCT. Other therapeutic approaches that have been utilized in the management of newly diagnosed MDS might be considered. For example, hematopoietic growth factors and transfusion support would be expected to be well tolerated and might be of palliative benefit. (89) Lenalidomide is active in the subgroup of patients with MDS with deletion of 5q, although the utility of this agent in t-MDS is unclear especially in the absence of deletion 5q. (90, 91). Finally, antithymocyte globulin (ATG) and cyclosporine have been associated with hematologic responses in some patients. (92, 93)
Specific considerations in the treatment of donor-cell MDS
Treatment of donor-cell MDS is challenging and carries a poor prognosis. In general, the treatment options to be considered are similar to those detailed above. In a recent analysis of 64 patients with DCL/donor-cell MDS, 34 patients had died at a median of 5.5 months after the diagnosis of DCL/donor-cell MDS was made. Median OS for treated patients was estimated at 32.8 months (95% confidence interval 22.5–43.1 months). Re-induction chemotherapy with curative intent was attempted in 47 of 52 patients who received any therapy with a complete response achieved in 27 and a sustained response in 16 of these. (35) These data suggest that complete remission can be attained with re-induction chemotherapy in the setting of DCL. Second transplant is also an option for the treatment of donor-cell MDS and includes the possibility of using either the original donor or a new donor. Utilizing the original donor in the setting of 100% donor engraftment may reduce TRM and GVHD in comparison to a transplant from another donor and this approach has been successfully employed in a few cases of DCL. (35, 94) However, there would be no expectation of a therapeutic GVL effect given that the MDS is of the same donor origin. Transplantation from a different donor would have the potential to induce a therapeutic GVL effect, although this would carry additional risks (e.g., graft rejection, GVHD). In a recent review of DCL, 17 patients were identified as having undergone a second alloHSCT and 7 (41%) remained alive at a median of 29 months from the diagnosis of DCL. Five of these patients were known to have received a second transplant from the original donor, and 3 were reported with durable responses. (35, 50) As noted above, DLI has potential efficacy in the treatment of relapsed MDS after transplant. (81) Although unlikely to be curative, DLI in the setting of donor cell MDS has been reported to be associated with improvements in hematologic parameters. (45)
Additional case-specific considerations
The treatment of MDS in this reported case was complicated by two major factors: residual post-transplant toxicities including chronic GVHD and organ dysfunction; and continued recurrences of Ewing sarcoma. In the setting of progressive clinically symptomatic Ewing sarcoma that was treated with chemotherapy and radiation, the ability to administer any specific therapy for MDS was compromised. Only supportive care was administered for the MDS that consisted of intermittent filgrastim as needed to reverse severe neutropenia and antibiotics to treat and prevent infection. The patient remained transfusion independent and serial bone marrow biopsies revealed stable MDS with blast counts below 10% until her death from progressive Ewing sarcoma 13 months from the diagnosis of donor-cell MDS.
Conclusions
The development of a novel hematologic malignancy after alloHSCT is rare. In the setting of post-transplant MDS, it is important to determine the origin of the disease as this has implications for treatment. Treatment of post-transplant MDS is challenging and therapeutic options should be considered in relation to patient- and transplant-specific characteristics, as well as the origin of the MDS.
Acknowledgments
Research support: This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and the Warren Grant Magnuson Clinical Center.
We thank the patient and her family and medical care team, including the staff of the NCI and NIH Clinical Center.
Footnotes
Financial Disclosure Statement: No authors have any financial disclosures or conflicts of interest to report.
References
- 1.Bishop MR, Alyea EP, 3rd, Cairo MS, Falkenburg JH, June CH, Kroger N, Little RF, Miller JS, Pavletic SZ, Porter D, Riddell SR, van Besien K, Wayne AS, Weisdorf DJ, Wu R, Giralt S. Introduction to the reports from the National Cancer Institute First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:563–564. doi: 10.1016/j.bbmt.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bishop MR, Alyea EP, 3rd, Cairo MS, Falkenburg JH, June CH, Kroger N, Little RF, Miller JS, Pavletic SZ, Porter DL, Riddell SR, van Besien K, Wayne AS, Weisdorf DJ, Wu RS, Giralt S. National Cancer Institute’s First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: summary and recommendations from the organizing committee. Biol Blood Marrow Transplant. 2011;17:443–454. doi: 10.1016/j.bbmt.2010.12.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rihani R, Bazzeh F, Faqih N, Sultan I. Secondary hematopoietic malignancies in survivors of childhood cancer: an analysis of 111 cases from the Surveillance, Epidemiology, and End Result-9 registry. Cancer. 2010;116:4385–4394. doi: 10.1002/cncr.25313. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia S, Krailo MD, Chen Z, Burden L, Askin FB, Dickman PS, Grier HE, Link MP, Meyers PA, Perlman EJ, Rausen AR, Robison LL, Vietti TJ, Miser JS. Therapy-related myelodysplasia and acute myeloid leukemia after Ewing sarcoma and primitive neuroectodermal tumor of bone: A report from the Children’s Oncology Group. Blood. 2007;109:46–51. doi: 10.1182/blood-2006-01-023101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miser JS, Goldsby RE, Chen Z, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers PA, Donaldson SS, Moore SG, Rausen AR, Vietti TJ, Grier HE. Treatment of metastatic Ewing sarcoma/primitive neuroectodermal tumor of bone: evaluation of increasing the dose intensity of chemotherapy--a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2007;49:894–900. doi: 10.1002/pbc.21233. [DOI] [PubMed] [Google Scholar]
- 6.Baird K, Fry TJ, Steinberg SM, Bishop MR, Fowler DH, Delbrook CP, Humphrey JL, Rager A, Richards K, Wayne AS, Mackall CL. Reduced Intensity Allogeneic Stem Cell Transplantation in Children and Young Adults with Ultra-High Risk Pediatric Sarcomas. Biol Blood Marrow Transplant. 2011 doi: 10.1016/j.bbmt.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Socie G, Curtis RE, Deeg HJ, Sobocinski KA, Filipovich AH, Travis LB, Sullivan KM, Rowlings PA, Kingma DW, Banks PM, Travis WD, Witherspoon RP, Sanders J, Jaffe ES, Horowitz MM. New malignant diseases after allogeneic marrow transplantation for childhood acute leukemia. J Clin Oncol. 2000;18:348–357. doi: 10.1200/JCO.2000.18.2.348. [DOI] [PubMed] [Google Scholar]
- 8.Rizzo JD, Curtis RE, Socie G, Sobocinski KA, Gilbert E, Landgren O, Travis LB, Travis WD, Flowers ME, Friedman DL, Horowitz MM, Wingard JR, Deeg HJ. Solid cancers after allogeneic hematopoietic cell transplantation. Blood. 2009;113:1175–1183. doi: 10.1182/blood-2008-05-158782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen A, Rovelli A, Merlo DF, van Lint MT, Lanino E, Bresters D, Ceppi M, Bocchini V, Tichelli A, Socie G. Risk for secondary thyroid carcinoma after hematopoietic stem-cell transplantation: an EBMT Late Effects Working Party Study. J Clin Oncol. 2007;25:2449–2454. doi: 10.1200/JCO.2006.08.9276. [DOI] [PubMed] [Google Scholar]
- 10.Baker KS, DeFor TE, Burns LJ, Ramsay NK, Neglia JP, Robison LL. New malignancies after blood or marrow stem-cell transplantation in children and adults: incidence and risk factors. J Clin Oncol. 2003;21:1352–1358. doi: 10.1200/JCO.2003.05.108. [DOI] [PubMed] [Google Scholar]
- 11.Pedersen-Bjergaard J, Andersen MK, Christiansen DH. Therapy-related acute myeloid leukemia and myelodysplasia after high-dose chemotherapy and autologous stem cell transplantation. Blood. 2000;95:3273–3279. [PubMed] [Google Scholar]
- 12.Kollmannsberger C, Hartmann JT, Kanz L, Bokemeyer C. Risk of secondary myeloid leukemia and myelodysplastic syndrome following standard-dose chemotherapy or high-dose chemotherapy with stem cell support in patients with potentially curable malignancies. J Cancer Res Clin Oncol. 1998;124:207–214. doi: 10.1007/s004320050156. [DOI] [PubMed] [Google Scholar]
- 13.Dufour C, Da Costa L, Auger N, Jullien M, Bhangoo R, Grill J. Treatment-related myelodysplastic syndrome after temozolomide use in a child: first report. J Pediatr Hematol Oncol. 2008;30:857–859. doi: 10.1097/MPH.0b013e318182e74f. [DOI] [PubMed] [Google Scholar]
- 14.Noronha V, Berliner N, Ballen KK, Lacy J, Kracher J, Baehring J, Henson JW. Treatment-related myelodysplasia/AML in a patient with a history of breast cancer and an oligodendroglioma treated with temozolomide: case study and review of the literature. Neuro Oncol. 2006;8:280–283. doi: 10.1215/15228517-2006-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natelson EA, Pyatt D. Temozolomide-induced myelodysplasia. Adv Hematol. 2010;2010:760402. doi: 10.1155/2010/760402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carney DA, Westerman DA, Tam CS, Milner A, Prince HM, Kenealy M, Wolf M, Januszewicz EH, Ritchie D, Came N, Seymour JF. Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy. Leukemia. 2010;24:2056–2062. doi: 10.1038/leu.2010.218. [DOI] [PubMed] [Google Scholar]
- 17.Germing U, Strupp C, Kuendgen A, Isa S, Knipp S, Hildebrandt B, Giagounidis A, Aul C, Gattermann N, Haas R. Prospective validation of the WHO proposals for the classification of myelodysplastic syndromes. Haematologica. 2006;91:1596–1604. [PubMed] [Google Scholar]
- 18.Greenberg PL, Attar E, Bennett JM, Bloomfield CD, De Castro CM, Deeg HJ, Foran JM, Gaensler K, Garcia-Manero G, Gore SD, Head D, Komrokji R, Maness LJ, Millenson M, Nimer SD, O’Donnell MR, Schroeder MA, Shami PJ, Stone RM, Thompson JE, Westervelt P. Myelodysplastic syndromes. J Natl Compr Canc Netw. 2011;9:30–56. doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. World Health Organization; 2008. Cancer TIAfRo. [Google Scholar]
- 20.Hasle H, Niemeyer CM, Chessells JM, Baumann I, Bennett JM, Kerndrup G, Head DR. A pediatric approach to the WHO classification of myelodysplastic and myeloproliferative diseases. Leukemia. 2003;17:277–282. doi: 10.1038/sj.leu.2402765. [DOI] [PubMed] [Google Scholar]
- 21.Rizzo JD, Wingard JR, Tichelli A, Lee SJ, Van Lint MT, Burns LJ, Davies SM, Ferrara JL, Socie G. Recommended screening and preventive practices for long-term survivors after hematopoietic cell transplantation: joint recommendations of the European Group for Blood and Marrow Transplantation, the Center for International Blood and Marrow Transplant Research, and the American Society of Blood and Marrow Transplantation. Biol Blood Marrow Transplant. 2006;12:138–151. doi: 10.1016/j.bbmt.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 22.van Marion AM, Thiele J, Kvasnicka HM, van den Tweel JG. Morphology of the bone marrow after stem cell transplantation. Histopathology. 2006;48:329–342. doi: 10.1111/j.1365-2559.2006.02332.x. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Manero G. Myelodysplastic syndromes: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011;86:490–498. doi: 10.1002/ajh.22047. [DOI] [PubMed] [Google Scholar]
- 24.Mauritzson N, Albin M, Rylander L, Billstrom R, Ahlgren T, Mikoczy Z, Bjork J, Stromberg U, Nilsson PG, Mitelman F, Hagmar L, Johansson B. Pooled analysis of clinical and cytogenetic features in treatment-related and de novo adult acute myeloid leukemia and myelodysplastic syndromes based on a consecutive series of 761 patients analyzed 1976–1993 and on 5098 unselected cases reported in the literature 1974–2001. Leukemia. 2002;16:2366–2378. doi: 10.1038/sj.leu.2402713. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen-Bjergaard J, Andersen MK, Andersen MT, Christiansen DH. Genetics of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2008;22:240–248. doi: 10.1038/sj.leu.2405078. [DOI] [PubMed] [Google Scholar]
- 26.Qian Z, Joslin JM, Tennant TR, Reshmi SC, Young DJ, Stoddart A, Larson RA, Le Beau MM. Cytogenetic and genetic pathways in therapy-related acute myeloid leukemia. Chem Biol Interact. 2010;184:50–57. doi: 10.1016/j.cbi.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiu RV, Gondek LP, O’Keefe CL, Elson P, Huh J, Mohamedali A, Kulasekararaj A, Advani AS, Paquette R, List AF, Sekeres MA, McDevitt MA, Mufti GJ, Maciejewski JP. Prognostic impact of SNP array karyotyping in myelodysplastic syndromes and related myeloid malignancies. Blood. 2011;117:4552–4560. doi: 10.1182/blood-2010-07-295857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuehrer M, Gerusel-Bleck M, Konstantopoulos N, Bender-Goetze C, Walther JU. FISH analysis of native smears from bone marrow and blood for the monitoring of chimerism and clonal markers after stem cell transplantation in children. Int J Mol Med. 2005;15:291–297. [PubMed] [Google Scholar]
- 29.Valent P, Horny HP, Bennett JM, Fonatsch C, Germing U, Greenberg P, Haferlach T, Haase D, Kolb HJ, Krieger O, Loken M, van de Loosdrecht A, Ogata K, Orfao A, Pfeilstocker M, Ruter B, Sperr WR, Stauder R, Wells DA. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: Consensus statements and report from a working conference. Leuk Res. 2007;31:727–736. doi: 10.1016/j.leukres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 30.Diez-Campelo M, Perez-Simon JA, Perez J, Alcoceba M, Richtmon J, Vidriales B, San Miguel J. Minimal residual disease monitoring after allogeneic transplantation may help to individualize post-transplant therapeutic strategies in acute myeloid malignancies. Am J Hematol. 2009;84:149–152. doi: 10.1002/ajh.21340. [DOI] [PubMed] [Google Scholar]
- 31.Kroger N, Bacher U, Bader P, Bottcher S, Borowitz MJ, Dreger P, Khouri I, Macapinlac HA, Olavarria E, Radich J, Stock W, Vose JM, Weisdorf D, Willasch A, Giralt S, Bishop MR, Wayne AS. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on Disease-Specific Methods and Strategies for Monitoring Relapse following Allogeneic Stem Cell Transplantation. Part I: Methods, acute leukemias, and myelodysplastic syndromes. Biol Blood Marrow Transplant. 2010;16:1187–1211. doi: 10.1016/j.bbmt.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langemeijer SM, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, Stevens-Linders E, van Hoogen P, van Kessel AG, Raymakers RA, Kamping EJ, Verhoef GE, Verburgh E, Hagemeijer A, Vandenberghe P, de Witte T, van der Reijden BA, Jansen JH. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet. 2009;41:838–842. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]
- 33.Dicker F, Haferlach C, Sundermann J, Wendland N, Weiss T, Kern W, Haferlach T, Schnittger S. Mutation analysis for RUNX1, MLL-PTD, FLT3-ITD, NPM1 and NRAS in 269 patients with MDS or secondary AML. Leukemia. 2010;24:1528–1532. doi: 10.1038/leu.2010.124. [DOI] [PubMed] [Google Scholar]
- 34.Abeliovich D, Yehuda O, Nagler A, Lerer I, Ben-Neriah S, Amar A, Or R. Predominant 45, X,--Y karyotype in donor cells after allogeneic BMT: cytogenetic and molecular analysis. Cancer Genet Cytogenet. 1996;86:1–7. doi: 10.1016/0165-4608(95)00170-0. [DOI] [PubMed] [Google Scholar]
- 35.Wiseman DH. Donor cell leukemia: a review. Biol Blood Marrow Transplant. 2011;17:771–789. doi: 10.1016/j.bbmt.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Thiede C. Diagnostic chimerism analysis after allogeneic stem cell transplantation: new methods and markers. Am J Pharmacogenomics. 2004;4:177–187. doi: 10.2165/00129785-200404030-00005. [DOI] [PubMed] [Google Scholar]
- 37.Khan F, Agarwal A, Agrawal S. Significance of chimerism in hematopoietic stem cell transplantation: new variations on an old theme. Bone Marrow Transplant. 2004;34:1–12. doi: 10.1038/sj.bmt.1704525. [DOI] [PubMed] [Google Scholar]
- 38.Ruiz-Arguelles GJ, Ruiz-Arguelles A, Garces-Eisele J. Donor cell leukemia: a critical review. Leuk Lymphoma. 2007;48:25–38. doi: 10.1080/10428190601003462. [DOI] [PubMed] [Google Scholar]
- 39.Pavletic SZ, Kumar S, Mohty M, de Lima M, Foran JM, Pasquini M, Zhang MJ, Giralt S, Bishop MR, Weisdorf D. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:871–890. doi: 10.1016/j.bbmt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luger SM, Ringden O, Zhang MJ, Perez WS, Bishop MR, Bornhauser M, Bredeson CN, Cairo MS, Copelan EA, Gale RP, Giralt SA, Gulbas Z, Gupta V, Hale GA, Lazarus HM, Lewis VA, Lill MC, McCarthy PL, Weisdorf DJ, Pulsipher MA. Similar outcomes using myeloablative vs reduced-intensity allogeneic transplant preparative regimens for AML or MDS. Bone Marrow Transplant. 2011 doi: 10.1038/bmt.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crow J, Youens K, Michalowski S, Perrine G, Emhart C, Johnson F, Gerling A, Kurtzberg J, Goodman BK, Sebastian S, Rehder CW, Datto MB. Donor cell leukemia in umbilical cord blood transplant patients: a case study and literature review highlighting the importance of molecular engraftment analysis. J Mol Diagn. 2010;12:530–537. doi: 10.2353/jmoldx.2010.090215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cooley LD, Sears DA, Udden MM, Harrison WR, Baker KR. Donor cell leukemia: report of a case occurring 11 years after allogeneic bone marrow transplantation and review of the literature. Am J Hematol. 2000;63:46–53. doi: 10.1002/(sici)1096-8652(200001)63:1<46::aid-ajh11>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 43.Reichard KK, Zhang QY, Sanchez L, Hozier J, Viswanatha D, Foucar K. Acute myeloid leukemia of donor origin after allogeneic bone marrow transplantation for precursor T-cell acute lymphoblastic leukemia: case report and review of the literature. Am J Hematol. 2006;81:178–185. doi: 10.1002/ajh.20389. [DOI] [PubMed] [Google Scholar]
- 44.Hertenstein B, Hambach L, Bacigalupo A, Schmitz N, McCann S, Slavin S, Gratwohl A, Ferrant A, Elmaagacli A, Schwertfeger R, Locasciulli A, Zander A, Bornhauser M, Niederwieser D, Ruutu T. Development of leukemia in donor cells after allogeneic stem cell transplantation--a survey of the European Group for Blood and Marrow Transplantation (EBMT) Haematologica. 2005;90:969–975. [PubMed] [Google Scholar]
- 45.Komrokji R, Ifthikharuddin JJ, Felgar RE, Abboud CN, Wedow LA, Connaughton A, Bennett JM. Donor cell myelodysplastic syndrome after allogeneic stem cell transplantation responding to donor lymphocyte infusion: case report and literature review. Am J Hematol. 2004;76:389–394. doi: 10.1002/ajh.20111. [DOI] [PubMed] [Google Scholar]
- 46.Sala Torra O, Loeb KR. Donor cell-derived leukemia and myelodysplastic neoplasm: unique forms of leukemia. Am J Clin Pathol. 2011;135:501–504. doi: 10.1309/AJCPXW8DKEG5QMTB. [DOI] [PubMed] [Google Scholar]
- 47.Wang E, Hutchinson CB, Huang Q, Lu CM, Crow J, Wang FF, Sebastian S, Rehder C, Lagoo A, Horwitz M, Rizzieri D, Yu J, Goodman B, Datto M, Buckley P. Donor cell-derived leukemias/myelodysplastic neoplasms in allogeneic hematopoietic stem cell transplant recipients: a clinicopathologic study of 10 cases and a comprehensive review of the literature. Am J Clin Pathol. 2011;135:525–540. doi: 10.1309/AJCPPJUQ9DNR1GHP. [DOI] [PubMed] [Google Scholar]
- 48.Haltrich I, Muller J, Szabo J, Kovacs G, Koos R, Poros A, Dobos M, Fekete G. Donor-cell myelodysplastic syndrome developing 13 years after marrow grafting for aplastic anemia. Cancer Genet Cytogenet. 2003;142:124–128. doi: 10.1016/s0165-4608(02)00804-x. [DOI] [PubMed] [Google Scholar]
- 49.Shekhter-Levin S, Bloom EJ, Swerdlow SH, Sherer ME, Wald N, Gollin SM. Acquired monosomy 7 in donor cells in a patient treated for acute lymphoblastic leukemia with bone marrow transplantation. Cancer Genet Cytogenet. 1997;95:190–197. doi: 10.1016/s0165-4608(96)00263-4. [DOI] [PubMed] [Google Scholar]
- 50.Komeno Y, Kanda Y, Kandabashi K, Kawazu M, Goyama S, Takeshita M, Nannya Y, Niino M, Nakamoto T, Kurokawa M, Tsujino S, Ogawa S, Aoki K, Chiba S, Motokura T, Hirai H. Reduced-intensity bone marrow transplantation from an alternative unrelated donor for myelodysplastic syndrome of first-donor origin. Am J Hematol. 2003;72:220–222. doi: 10.1002/ajh.10286. [DOI] [PubMed] [Google Scholar]
- 51.Lang Z, Dinndorf P, Ladisch S, Bayever E, Reaman G. Chromosomal transformation in donor cells following allogeneic bone marrow transplantation. Bone Marrow Transplant. 2004;33:1253–1256. doi: 10.1038/sj.bmt.1704450. [DOI] [PubMed] [Google Scholar]
- 52.Orciuolo E, Azzara A, Bandini G, Galimberti S, Bonifazi F, Fazzi R, Petrini M. Contemporaneous appearance, 18 years after allogeneic bone marrow transplantation, of myelodysplastic syndrome in the patient and the donor. Bone Marrow Transplant. 2004;33:859–861. doi: 10.1038/sj.bmt.1704417. [DOI] [PubMed] [Google Scholar]
- 53.Boulton-Jones R, Parker A, Hepburn M, Bowen DT. Secondary myelodysplasia of donor cell origin following nonmyeloablative sibling allogeneic stem cell transplantation. Bone Marrow Transplant. 2005;36:471–472. doi: 10.1038/sj.bmt.1705086. [DOI] [PubMed] [Google Scholar]
- 54.Kraut L, Trebeljahr G, Thiele B, Fauser AA. Donor cell-derived myelodysplastic syndrome in a patient 7 years after unrelated allogeneic HLA-mismatched transplantation for Ph+ chronic myeloid leukemia. Bone Marrow Transplant. 2005;36:737. doi: 10.1038/sj.bmt.1705125. [DOI] [PubMed] [Google Scholar]
- 55.Beck C, Humpe A, Harder S, Schmid M, Horst HA. Myelodysplastic syndrome of donor origin subsequent to successful treatment of myeloid/NK-cell precursor leukaemia with allogeneic PBSCT: two very rare conditions in one patient. Ann Hematol. 2005;84:616–618. doi: 10.1007/s00277-005-1032-6. [DOI] [PubMed] [Google Scholar]
- 56.Sala-Torra O, Hanna C, Loken MR, Flowers ME, Maris M, Ladne PA, Mason JR, Senitzer D, Rodriguez R, Forman SJ, Deeg HJ, Radich JP. Evidence of donor-derived hematologic malignancies after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2006;12:511–517. doi: 10.1016/j.bbmt.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 57.Hashino S, Fujisawa F, Kondo T, Imamura M, Sato K, Torimoto Y, Kohgo Y, Kimura K, Furukawa H, Todo S, Asaka M. Donor-type myelodysplastic syndrome with t(2;3) and monosomy 7 after allogeneic peripheral blood stem cell transplantation and liver transplantation in a patient with severe-type aplastic anemia. Int J Hematol. 2006;84:363–366. doi: 10.1532/IJH97.06057. [DOI] [PubMed] [Google Scholar]
- 58.Fraser CJ, Hirsch BA, Dayton V, Creer MH, Neglia JP, Wagner JE, Baker KS. First report of donor cell-derived acute leukemia as a complication of umbilical cord blood transplantation. Blood. 2005;106:4377–4380. doi: 10.1182/blood-2005-06-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brunstein CG, Hirsch BA, Hammerschmidt D, McGlennen RC, Nguyen PL, Verfaillie CM. Leukemia in donor cells after allogeneic hematopoietic stem cell transplant. Bone Marrow Transplant. 2002;29:999–1003. doi: 10.1038/sj.bmt.1703577. [DOI] [PubMed] [Google Scholar]
- 60.Lowsky R, Fyles G, Minden M, Lipton J, Meharchand J, Tejpar I, Zipursky A, Messner H. Detection of donor cell derived acute myelogenous leukaemia in a patient transplanted for chronic myelogenous leukaemia using fluorescence in situ hybridization. Br J Haematol. 1996;93:163–165. doi: 10.1046/j.1365-2141.1996.454991.x. [DOI] [PubMed] [Google Scholar]
- 61.Gopcsa L, Barta A, Banyai A, Konya M, Pajor L, Foldi J, Paloczi K. Acute myeloid leukaemia of donor cell origin developing 5 years after allogeneic bone marrow transplantation for chronic myeloid leukaemia. Bone Marrow Transplant. 2002;29:449–452. doi: 10.1038/sj.bmt.1703378. [DOI] [PubMed] [Google Scholar]
- 62.Bielorai B, Deeg HJ, Weintraub M, Neumann Y, Rosner E, Amariglio N, Rechavi G, Toren A. B-cell lymphoma developing in the donor 9 years after donor-origin acute myeloid leukemia post bone marrow transplantation. Bone Marrow Transplant. 2003;31:931–934. doi: 10.1038/sj.bmt.1703953. [DOI] [PubMed] [Google Scholar]
- 63.Daly AS, Kamel-Reid S, Lipton JH, Messner HA, Kiss TL, Chun K, Busque L, Chang H. Acute leukemia of donor origin arising after stem cell transplantation for acute promyelocytic leukemia. Leuk Res. 2004;28:1107–1111. doi: 10.1016/j.leukres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 64.Stevens JM, Syndercombe-Court D, Oakervee HE, McCloskey D, Jenner MJ, Gribben JG, Cavenagh JD. Development of original donor cell leukemia after successful engraftment from a second donor. Blood. 2007;110:4621–4622. doi: 10.1182/blood-2007-05-091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aizawa S, Nakano M, Iwase O, Yaguchi M, Hiramoto M, Hoshi H, Nabeshima R, Shima D, Handa H, Toyama K. Bone marrow stroma from refractory anemia of myelodysplastic syndrome is defective in its ability to support normal CD34-positive cell proliferation and differentiation in vitro. Leuk Res. 1999;23:239–246. doi: 10.1016/s0145-2126(98)00163-5. [DOI] [PubMed] [Google Scholar]
- 66.Deeg HJ. Marrow stroma in MDS: culprit or bystander? Leuk Res. 2002;26:687–688. doi: 10.1016/s0145-2126(02)00015-2. [DOI] [PubMed] [Google Scholar]
- 67.Raaijmakers MH, Mukherjee S, Guo S, Zhang S, Kobayashi T, Schoonmaker JA, Ebert BL, Al-Shahrour F, Hasserjian RP, Scadden EO, Aung Z, Matza M, Merkenschlager M, Lin C, Rommens JM, Scadden DT. Bone progenitor dysfunction induces myelodysplasia and secondary leukaemia. Nature. 2010;464:852–857. doi: 10.1038/nature08851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leguit RJ, van den Berg H, Buijs A, Mellink C, Bierings M. Donor-cell MDS in a 12-year-old girl 3 years after allogeneic hematopoietic SCT for MDS, both with a t(3;3)(q21; q26) cytogenetic aberration. Bone Marrow Transplant. 2010 doi: 10.1038/bmt.2010.214. [DOI] [PubMed] [Google Scholar]
- 69.Menes M, Vakiani E, Keller CE, Ho EK, Colovai A, Nichols G, Diuguid D, Mears JG, Murty VV, Alobeid B, Bhagat G. The spectrum of myelodysplastic syndromes post-solid organ transplantation: a single institutional experience. Leuk Res. 2007;31:59–65. doi: 10.1016/j.leukres.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 70.Fenaux P, Mufti GJ, Hellstrom-Lindberg E, Santini V, Finelli C, Giagounidis A, Schoch R, Gattermann N, Sanz G, List A, Gore SD, Seymour JF, Bennett JM, Byrd J, Backstrom J, Zimmerman L, McKenzie D, Beach C, Silverman LR. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: arandomised, open-label, phase III study. Lancet Oncol. 2009;10:223–232. doi: 10.1016/S1470-2045(09)70003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Itzykson R, Thepot S, Quesnel B, Dreyfus F, Beyne-Rauzy O, Turlure P, Vey N, Recher C, Dartigeas C, Legros L, Delaunay J, Salanoubat C, Visanica S, Stamatoullas A, Isnard F, Marfaing-Koka A, de Botton S, Chelghoum Y, Taksin AL, Plantier I, Ame S, Boehrer S, Gardin C, Beach CL, Ades L, Fenaux P. Prognostic factors of response and overall survival in 282 higher-risk myelodysplastic syndromes treated with azacitidine. Blood. 2010 doi: 10.1182/blood-2010-06-289280. [DOI] [PubMed] [Google Scholar]
- 72.Steensma DP, Stone RM. Practical recommendations for hypomethylating agent therapy of patients with myelodysplastic syndromes. Hematol Oncol Clin North Am. 2010;24:389–406. doi: 10.1016/j.hoc.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 73.Jabbour E, Giralt S, Kantarjian H, Garcia-Manero G, Jagasia M, Kebriaei P, de Padua L, Shpall EJ, Champlin R, de Lima M. Low-dose azacitidine after allogeneic stem cell transplantation for acute leukemia. Cancer. 2009;115:1899–1905. doi: 10.1002/cncr.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, Braun TM, Nguyen HQ, Champlin R, Garcia-Manero G. Maintenance therapy with low-dose azacitidine after allogeneic hematopoietic stem cell transplantation for recurrent acute myelogenous leukemia or myelodysplastic syndrome: a dose and schedule finding study. Cancer. 2010;116:5420–5431. doi: 10.1002/cncr.25500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bartenstein M, Deeg HJ. Hematopoietic stem cell transplantation for MDS. Hematol Oncol Clin North Am. 2010;24:407–422. doi: 10.1016/j.hoc.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Litzow MR, Tarima S, Perez WS, Bolwell BJ, Cairo MS, Camitta BM, Cutler CS, de Lima M, Dipersio JF, Gale RP, Keating A, Lazarus HM, Luger S, Marks DI, Maziarz RT, McCarthy PL, Pasquini MC, Phillips GL, Rizzo JD, Sierra J, Tallman MS, Weisdorf DJ. Allogeneic transplantation for therapy-related myelodysplastic syndrome and acute myeloid leukemia. Blood. 2010;115:1850–1857. doi: 10.1182/blood-2009-10-249128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ballen KK, Gilliland DG, Guinan EC, Hsieh CC, Parsons SK, Rimm IJ, Ferrara JL, Bierer BE, Weinstein HJ, Antin JH. Bone marrow transplantation for therapy-related myelodysplasia: comparison with primary myelodysplasia. Bone Marrow Transplant. 1997;20:737–743. doi: 10.1038/sj.bmt.1700971. [DOI] [PubMed] [Google Scholar]
- 78.Hale GA, Heslop HE, Bowman LC, Rochester RA, Pui CH, Brenner MK, Krance RA. Bone marrow transplantation for therapy-induced acute myeloid leukemia in children with previous lymphoid malignancies. Bone Marrow Transplant. 1999;24:735–739. doi: 10.1038/sj.bmt.1701962. [DOI] [PubMed] [Google Scholar]
- 79.Kroger N, Brand R, van Biezen A, Zander A, Dierlamm J, Niederwieser D, Devergie A, Ruutu T, Cornish J, Ljungman P, Gratwohl A, Cordonnier C, Beelen D, Deconinck E, Symeonidis A, de Witte T. Risk factors for therapy-related myelodysplastic syndrome and acute myeloid leukemia treated with allogeneic stem cell transplantation. Haematologica. 2009;94:542–549. doi: 10.3324/haematol.2008.000927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shaw BE, Mufti GJ, Mackinnon S, Cavenagh JD, Pearce RM, Towlson KE, Apperley JF, Chakraverty R, Craddock CF, Kazmi MA, Littlewood TJ, Milligan DW, Pagliuca A, Thomson KJ, Marks DI, Russell NH. Outcome of second allogeneic transplants using reduced-intensity conditioning following relapse of haematological malignancy after an initial allogeneic transplant. Bone Marrow Transplant. 2008;42:783–789. doi: 10.1038/bmt.2008.255. [DOI] [PubMed] [Google Scholar]
- 81.Campregher PV, Gooley T, Scott BL, Moravec C, Sandmaier B, Martin PJ, Deeg HJ, Warren EH, Flowers ME. Results of donor lymphocyte infusions for relapsed myelodysplastic syndrome after hematopoietic cell transplantation. Bone Marrow Transplant. 2007;40:965–971. doi: 10.1038/sj.bmt.1705840. [DOI] [PubMed] [Google Scholar]
- 82.Kantarjian H, Beran M, Cortes J, O’Brien S, Giles F, Pierce S, Shan J, Plunkett W, Keating M, Estey E. Long-term follow-up results of the combination of topotecan and cytarabine and other intensive chemotherapy regimens in myelodysplastic syndrome. Cancer. 2006;106:1099–1109. doi: 10.1002/cncr.21699. [DOI] [PubMed] [Google Scholar]
- 83.Barnard DR, Lange B, Alonzo TA, Buckley J, Kobrinsky JN, Gold S, Neudorf S, Sanders J, Burden L, Woods WG. Acute myeloid leukemia and myelodysplastic syndrome in children treated for cancer: comparison with primary presentation. Blood. 2002;100:427–434. doi: 10.1182/blood.v100.2.427. [DOI] [PubMed] [Google Scholar]
- 84.Hasle H, Niemeyer CM. Advances in the prognostication and management of advanced MDS in children. Br J Haematol. 2011 doi: 10.1111/j.1365-2141.2011.08724.x. [DOI] [PubMed] [Google Scholar]
- 85.Beran M. Intensive chemotherapy for patients with high-risk myelodysplastic syndrome. Int J Hematol. 2000;72:139–150. [PubMed] [Google Scholar]
- 86.Porter DL, Alyea EP, Antin JH, DeLima M, Estey E, Falkenburg JH, Hardy N, Kroeger N, Leis J, Levine J, Maloney DG, Peggs K, Rowe JM, Wayne AS, Giralt S, Bishop MR, van Besien K. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:1467–1503. doi: 10.1016/j.bbmt.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kroger N, Brand R, van Biezen A, Cahn JY, Slavin S, Blaise D, Sierra J, Zander A, Niederwieser D, de Witte T. Autologous stem cell transplantation for therapy-related acute myeloid leukemia and myelodysplastic syndrome. Bone Marrow Transplant. 2006;37:183–189. doi: 10.1038/sj.bmt.1705226. [DOI] [PubMed] [Google Scholar]
- 88.de Witte T, Oosterveld M, Muus P. Autologous and allogeneic stem cell transplantation for myelodysplastic syndrome. Blood Rev. 2007;21:49–59. doi: 10.1016/j.blre.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Steensma DP. Hematopoietic growth factors in myelodysplastic syndromes. Semin Oncol. 2011;38:635–647. doi: 10.1053/j.seminoncol.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 90.List A, Dewald G, Bennett J, Giagounidis A, Raza A, Feldman E, Powell B, Greenberg P, Thomas D, Stone R, Reeder C, Wride K, Patin J, Schmidt M, Zeldis J, Knight R. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 91.Ades L, Boehrer S, Prebet T, Beyne-Rauzy O, Legros L, Ravoet C, Dreyfus F, Stamatoullas A, Chaury MP, Delaunay J, Laurent G, Vey N, Burcheri S, Mbida RM, Hoarau N, Gardin C, Fenaux P. Efficacy and safety of lenalidomide in intermediate-2 or high-risk myelodysplastic syndromes with 5q deletion: results of a phase 2 study. Blood. 2009;113:3947–3952. doi: 10.1182/blood-2008-08-175778. [DOI] [PubMed] [Google Scholar]
- 92.Sloand EM, Wu CO, Greenberg P, Young N, Barrett J. Factors affecting response and survival in patients with myelodysplasia treated with immunosuppressive therapy. J Clin Oncol. 2008;26:2505–2511. doi: 10.1200/JCO.2007.11.9214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Passweg JR, Giagounidis AA, Simcock M, Aul C, Dobbelstein C, Stadler M, Ossenkoppele G, Hofmann WK, Schilling K, Tichelli A, Ganser A. Immunosuppressive therapy for patients with myelodysplastic syndrome: a prospective randomized multicenter phase III trial comparing antithymocyte globulin plus cyclosporine with best supportive care--SAKK 33/99. J Clin Oncol. 2011;29:303–309. doi: 10.1200/JCO.2010.31.2686. [DOI] [PubMed] [Google Scholar]
- 94.Jacobs JF, Brons PP, Simons A, van der Reijden BA, Hoogerbrugge PM. Therapy-related, donor-derived AML responding to a second allogeneic BMT. Bone Marrow Transplant. 2007;40:499–500. doi: 10.1038/sj.bmt.1705750. [DOI] [PubMed] [Google Scholar]
- 95.Graef T, Kuendgen A, Fenk R, Zohren F, Haas R, Kobbe G. Successful treatment of relapsed AML after allogeneic stem cell transplantation with azacitidine. Leuk Res. 2007;31:257–259. doi: 10.1016/j.leukres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 96.Reis JP, von Muhlen D, Michos ED, Miller ER, 3rd, Appel LJ, Araneta MR, Barrett-Connor E. Serum vitamin D, parathyroid hormone levels, and carotid atherosclerosis. Atherosclerosis. 2009;207:585–590. doi: 10.1016/j.atherosclerosis.2009.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]


