Abstract
Glutamate transporters play a crucial role in physiological glutamate homeostasis and neurotoxicity. Recently, we have shown that downregulation of glutamate transporters after chronic morphine exposure contributed to the development of morphine tolerance. In the present study, we examined whether regulation of the glutamate transporter expression with the proposed proteasome inhibitor MG-132 would contribute to the development of tolerance to repeated intrathecal (twice daily × 7 days) morphine administration in rats. The results showed that MG-132 (5 nmol) given intrathecally blocked morphine-induced glutamate transporter downregulation and the decrease in glutamate uptake activity within the spinal cord dorsal horn. Co-administration of morphine (15 nmol) with MG-132 (vehicle = 1 < 2.5 < 5 = 10 nmol) also dose-dependently prevented the development of morphine tolerance in rats. These findings suggest that prevention of spinal glutamate transporter downregulation may regulate the glutamatergic function that has been implicated in the development of morphine tolerance. The possible relationship between MG-132-mediated regulation of glutamate transporters, ubiquitin-proteasome system, and the cellular mechanisms of morphine tolerance is discussed in light of these findings.
Keywords: Glutamate transporter, EAAC1, GLAST, GLT-1, Morphine tolerance, MG-132
1. Introduction
Glutamate transporters play a crucial role in physiological glutamate homeostasis, neurotoxicity, and glutamatergic regulation of opioid tolerance [12,15,16,26,28]. However, how the glutamate transport degradation is regulated remains unclear. The ubiquitin-proteasome system (UPS) is a major non-lysosomal proteolytic pathway that degrades cellular proteins including those with important roles in the regulation of cell growth and function [4,7,21]. In addition, activation of UPS has been shown to regulate the PSD-95 degradation and AMPA receptor surface expression [3], suggesting a possible relationship between UPS and glutamatergic activities.
Ubiquitination is a process involving three enzymes: E1 (ubiquitin-activating enzyme), E2 (ubiquitin-conjugating enzyme), and E3 (ubiquitin ligase) [8,29]. Interactions between an E3 ligase and its target molecule are considered a key step in determining the selectivity of UPS for a target molecule and its subsequent proteasomal degradation, a process that is subject to intracellular modulation by various upstream regulators [29]. Antinociceptive tolerance induced by chronic morphine has been shown to be mediated, at least in part, through a central glutamatergic mechanism including an altered glutamate transporter expression [14,15,23,25,27]. Inhibition of glutamate transporter activity directly contributes to a heightened activity of N-methyl-D-aspartate receptors (NMDAR) and the development of opioid tolerance [24]. These previous studies suggest that regulation of the glutamate transporter turnover may contribute to the mechanisms of opioid tolerance.
Utilizing a rat model of morphine tolerance induced by chronic intrathecal morphine exposure [15], we examined whether the proposed proteasome inhibitor MG-132 would 1) regulate the downregulation of spinal glutamate transporter and glutamate uptake activity induced by chronic morphine exposure and 2) modulate the development of morphine tolerance in rats. We find that intrathecal administration of MG-132 prevented spinal glutamate transporter downregulation, the decreased glutamate uptake activity, and morphine tolerance. The possible role of proteasome-mediated glutamate transporter degradation in morphine tolerance is discussed.
2. Methods
2.1. Experimental animals
Adult male Sprague Dawley rats (Charles River Laboratories, Wilmington, MA) weighing 300 –350 gm were used. Rats were housed in individual cages with water and food pellets available ad libitum. Animal room was artificially illuminated from 7:00 A.M. to 7:00 P.M. The protocol was approved through our Institutional Animal Care and Use Committee.
2.2. Intrathecal drug delivery
A polyethylene (PE) 10 intrathecal catheter was implanted in each rat according to a previously described method [30]. Rats exhibiting neurological deficits after the implantation were excluded. The experiment began at 24 hr after the intrathecal catheter implantation. Morphine (15 nmol) plus vehicle, MG-132 (5 nmol in 10 μl of saline with 0.4 % DMSO) alone, morphine plus MG-132 (1, 2.5, 5, 10 nmol, respectively), or vehicle (saline with 0.4 % DMSO) alone was administrated (n= 6 rats/group) twice daily for seven days through the intrathecal catheter in a total volume of 20 μl followed by 10 μl of saline flush to purge the residual from the catheter dead space. MG-132 was chosen because it has been used in previous in vivo studies that examined the role of the ubiquitin-proteasome system in the cellular mechanisms of neuropathic pain and morphine-induced hyperalgesia [18,20].
2.3. Behavioral paradigm and tail-flick test
The tail flick test [1] was used to examine the nociceptive response in the presence or absence of a treatment. On day 1, the baseline nociceptive response was first tested in each group of rats. Each group of rats was then treated with morphine alone, morphine plus MG-132, MG-132 alone, or vehicle alone and tested at 30 min after each treatment. Each group then received twice daily the assigned treatment over the next seven days and the same behavioral test was repeated on day 3, 5, 7. After the behavioral test on day 7, the cumulative morphine dose-response curve was generated for each group and compared [5]. Rats were also observed for their gait to examine the possible adverse effect of MG-132 on the motor function.
2.4. Statistical analysis for behavioral data
The percentage of maximal possible antinociceptive effect (%MPAE) was calculated by using the equation (BL-baseline; TL-after treatment): %MPAE = [(TL − BL)/(cutoff-BL)] × 100. For dose-response experiments, cumulative dose–response curves were used for the calculation of ED50 doses and 95% confidence intervals [5]. Repeated measure one-way ANOVA followed by Tukey-Kramer test was used to analyze the behavioral data. Values were expressed as mean± SEM.
2.5. Western blotting
Spinal cord tissue samples (L4–L5 spinal cord segments) were collected on day 7. The dorsal part of these spinal cord segments was then used for the assay. Western blotting was carried out according to the previously published method [2,10,15,31]. The following antibodies were used: GLAST and GLT-1 (Chemicon International, Inc., Temecula, CA, 1:1000); EAAC1 (ADI, 1:500); monoclonal α-tubulin (Sigma Saint Louis, MO, 1:1000). Spinal cord dorsal horn samples (30 μg of protein) were subjected to standard SDS-PAGE and then transferred to a nitrocellulose membrane. After being blocked at room temperature for 1 hr in a blocking buffer [PBS(−)−5% skim milk−0.1% Tween 20], the membranes were incubated with antibodies diluted (as described above) in blocking buffer at room temperature for 2 hr or at 4 °C overnight. This was followed by three washes in washing buffer [PBS(−)−0.1% Tween 20] and incubation with HRP-linked anti-mouse IgG (1:5000), HRP-linked anti-rabbit IgG (1:10000) (Amersham Biosciences UK Limited, Little Chalfont Buckinghamshire, England), or HRP-linked anti-guinea pig IgG (1:3000) (SIGMA, Saint Louis, MO) at room temperature for 1 hr. After three washes, the bands were visualized by the ECL plus Western Blotting Detection System (Amersham Biosciences UK Limited, Little Chalfont Buckinghamshire, England). One-way ANOVA followed by Tukey-Kramer test was used to analyze the data. A calibration curve was generated for each protein to ensure that the optical density values obtained for these samples were within a linear range of detection [6]. The intensity of each protein level was analyzed using ImageJ 1.34s software (NIH, Bethesda, MD).
2.6. In vitro glutamate uptake assay
An in vitro preparation was used to assess glutamate uptake activity according to a previously published method [15,17,23]. In brief, spinal cord dorsal horn samples were homogenized in 500 μl of ice-cold homogenization buffer [0.32 M sucrose, 2 mM EDTA, 2 mM EGTA, and 20 mM HEPES, pH 7.2, plus 1 mM PMSF and protease inhibitor cocktail tablet (Roche Diagnostics GmbH, Mannheim, Germany) and centrifuged at 1500 rpm for 10 min at 4 °C, and the supernatant was collected. Pellets were re-suspended in the same homogenization buffer, re-centrifuged as above. Both supernatants were combined and again centrifuged at 13,000 rpm for 10 min at 4 °C. The so-obtained pellets were suspended in 1 ml of Locke's buffer (154 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 1.0 mM MgCl2, 3.6 mM NaHCO3, 5 mM glucose, 5 mM HEPES, pH 7.2, and saturated with 95% O2/5% CO2). Glutamate uptake activity was determined by incubating the preparation (100 μg of protein content) with 0.4 μCi of L-[3H] glutamic acid (Perkin Elmer Life Sciences, Boston, MA) in a total volume of 1 ml of Locke's buffer for 5 min at 37 °C. The reaction was terminated by filtering the pellets through a Whatman (Maidstone, UK) GF/C 2.4 cm filter presoaked in the Locke's buffer. The filter was then washed with 2 ml of ice-cold Locke's buffer three times, air-dried, and transferred into vials containing 10 ml of scintillation mixture (Fisher Scientific, Houston, TX). The radioactivity was measured by Liquid Scintillation Analyzer Tri-Carb 2900TR (PerkinElmer, Boston, MA). The basal uptake activity in counts per minute (cpm) was measured in the absence of any treatment. Fold of change in glutamate uptake activity was calculated with the following equation: [(basal cpm without treatment – cpm with treatment)/(basal cpm without treatment)].
2.7. RT-PCR
Total RNA was extracted from the rat's spinal cord dorsal horn. Isolations were performed using RNeasy Mini Kit (QIAGEN, Germantown, MD) according to the manufacture's protocol. RT-PCR reactions were carried out using Titan One Tube RT-PCR System (Roche Diagnostics Corporation, Indianapolis, IN) according to the manufacturer's manual. All primers for rat glutamate transporters, and a house-keeping gene were designed by Invitrogen OligoPerfect based on GeneBank sequences (EAAC1, NM 013032; GLT-1, NM 017215; GLAST, NM 019225, and glyceraldehyde 3-phosphate dehydrogenase, GAPDH, XO 02231 and synthesized by Invitrogen Life Technologies (Carlsbad, CA): for GLAST, forward: 5′-GATGGAAAGATTCCAGCAA-3′ and reverse: 5′-CGAAGCACATGGAGAAGACA-3′, amplifying 720 base pair products; for GLT-1, forward: 5′-CCTCCCTCTCATCATTTCCA-3′ and reverse: 5′-CACAGATCAAGCAGGCGATA-3′, amplifying 611 base pair products; for EAAC1, forward: 5′-TGGTTCGAGGACACAGTGAG-3′ and reverse: 5′-GATCAGTGGCAGCACTACGA-3′, amplifying 800 base pair products; for GAPDH, forward: 5′-GGTGATGCTGGTGCTGAGTA-3′ and reverse: 5′-GGATGCAGGGATGATGTTCT-3′, amplifying 369 base pair products.
RT-PCR was started with incubating the sample (20 ng of total RNA) at 50°C for 30 min for reverse transcription, followed by one cycle of denaturation at 94 °C for 2 min, 10 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 68 °C for 1 min and 25 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and elongation at 68 °C for 1 min with cycle elongation of 5 s for each cycle. A 7 min extension at 68 °C was carried out at the end of the final cycle. The samples were then cooled to 4 °C. Ten μl of the RT-PCR product was loaded onto one lane and subjected to electrophoresis at 50 V through 1.8% (w/v) agarose gel containing 0.4 μg /ml ethidium bromide. The RT-PCR product bands and a 100 bp ladder molecular weight marker (New England BioLabs, Ipswich, MA) were visualized under UV light and imaged using AlphaEaseFC system (Alpha Innotech Corporation, San Leandro, CA). The intensity of each transporter mRNA level was analyzed using ImageJ 1.34s software (NIH, Bethesda, MD). GAPDH level was used for the sample normalization.
2.8. Reagents
Morphine and MG-132 were purchased from Sigma-Aldrich (St. Louis, MO) and Calbiochem (La Jolla, CA), respectively.
3. Results
3.1. Effect of MG-132 on morphine tolerance
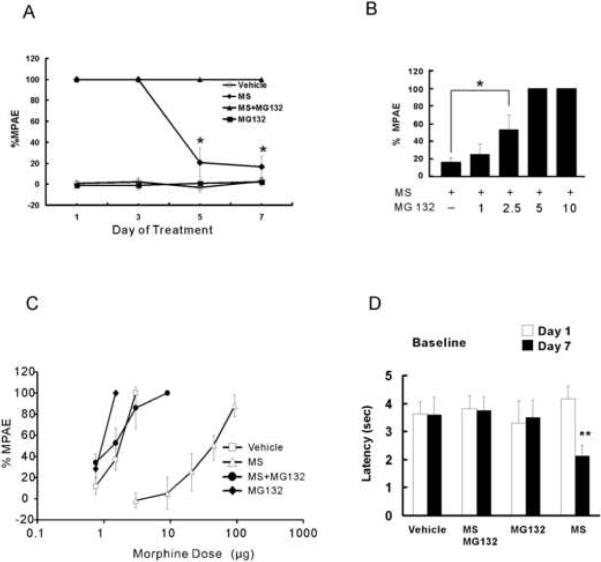
To examine whether MG-132 would influence the development of morphine tolerance, each group of rats (n=6) were administrated intrathecally (twice daily) with vehicle, morphine (15 nmol), morphine plus MG-132 (1, 2.5, 5 or 10 nmol) or MG132 alone (5 nmol) for seven consecutive days. The following results were obtained: 1) The time-course analysis showed that rats treated with morphine alone developed tolerance on day 5 of morphine exposure, which was prevented in those rats receiving the combined morphine and 5 nmol MG-132 (Fig. 1A, P< 0.05); 2) When examined on day 7, MG-132 dose-dependently (vehicle = 1 < 2.5 < 5 = 10 nmol) prevented the development of morphine tolerance (Fig. 1B, P< 0.01); 3) The rightward shift of the morphine antinociceptive dose-response curve examined on day 7 in the morphine alone group was completely blocked by co-administration of morphine with MG-132 (Fig. 1C). MG-132 alone did not change the baseline nociceptive response (Fig. 1D), nor did it shift the morphine dose-response curve when examine on day 7 (Fig. 1C). ED50 values and 95% confidence intervals (in μg) on day 7 are as follows: vehicle: 1.51 (0.37–1.57); morphine alone: 35.83 (29.97–42.74); morphine plus MG-132: 1.22 (1.04–1.42); and MG-132 alone: 0.98 (0.96 – 1.0); and 4) Rats treated with MG-132 did not show signs of motor deficits (e.g., abnormal gait or limb paralysis).
Fig. 1. The Proteasome inhibitor MG-132 prevented morphine tolerance.
Rats (n=6) were administrated intrathecally (twice daily) with vehicle, morphine (MS, 15 nmol), morphine plus MG-132 (1, 2.5, 5 or 10 nmol) or MG132 alone (5 nmol) for seven consecutive days. A, Morphine antinociceptive tolerance was developed on day 5 after the repeated treatment with morphine but not with morphine plus MG132 (5 nmol). B, Morphine antinociceptive tolerance was prevented in a dose-dependent manner by co-administration of morphine and MG-132 (day 7). C, The rightward shift of the dose-response curve from morphine exposure was prevented in these rats treated with morphine plus MG-132 (5 nmol). D, MG132 alone did not change the baseline latency. One-way ANOVA followed by Tukey-Kramer Test. * P< 0.05.
These results demonstrated that the development of morphine tolerance was dose-dependently prevented by the co-administration of morphine with MG-132, whereas MG-132 alone did not change baseline nociceptive responses or produce motor deficits.
3.2. Downregulation of spinal glutamate transporters after morphine exposure
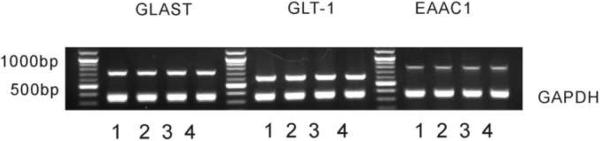
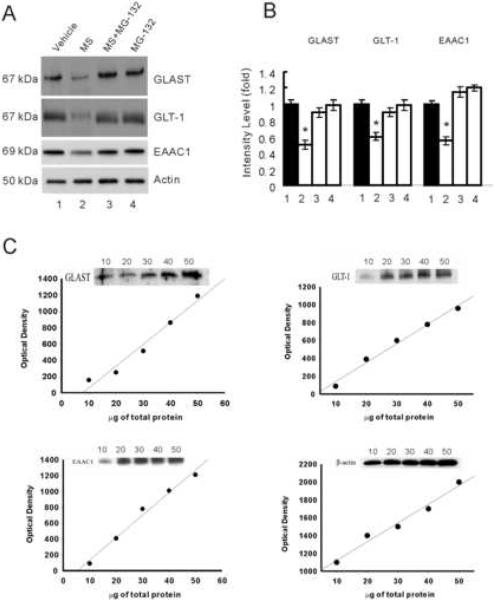
In our previous study [15], the expression of glutamate transporters was downregulated within the rat's spinal cord dorsal horn after chronic morphine exposure, as demonstrated by Western blot alone. In this study, we examined the expression of glutamate transporters using both RT-PCR and Western blot. Three glutamate transporters (EAAC1, GLSAT, GLT-1) in the spinal cord dorsal horn were examined after chronic exposure to morphine (15 nmol) or vehicle (n=6, intrathecal, twice daily × 7 days). Spinal cord tissue samples were taken at the end of the 7-day treatment. The data in Fig. 2 showed that the glutamate transporter expression was not downregulated at the mRNA level when examined on day 7, because there were no differences in the mRNA content of these glutamate transporters between the morphine and vehicle treatment group. In contrast, the expression of these glutamate transporters was significantly downregulated at the protein level in the morphine group as compared to the vehicle group (Fig. 3A, B, P< 0.05), when examined on day 7 of chronic morphine exposure. The optical density values obtained for these samples were within a linear range of detection as shown by individual calibration curve for each protein (Fig. 3C).
Fig. 2. Spinal glutamate transporter expression was not downregulated at the transcriptional level after morphine exposure.
Rats (n=6) were administrated intrathecally (twice daily) with vehicle, morphine (MS, 15 nmol), morphine plus MG-132 (1, 2.5, 5 or 10 nmol) or MG132 (5 nmol) alone for seven consecutive days. Independent samples from these rats were used to examine the glutamate transporter mRNA level using RT-PCR. Lane 1: vehicle, Lane 2: morphine (15 nmol), Lane 3: morphine plus MG-132 (5 nmol), Lane 4: MG-132 (5 nmol) alone. One-way ANOVA followed by Tukey-Kramer test was used to analyze the data. There were no differences among these groups. GAPDH: glyseraldehyde-3-phosphate dehydrogenase.
Fig. 3. The proteasome inhibitor MG-132 prevented spinal glutamate transporter downregulation.
Rats (n=6) were administrated intrathecally (twice daily) with vehicle, morphine (MS, 15 nmol), morphine plus MG-132 (1, 2.5, 5 or 10 nmol) or MG132 (5 nmol) alone for seven consecutive days. Independent samples from these rats were used for the Western blot assay. MG-132 effectively prevented morphine-induced downregulation of EAAC1, GLAST, and GLT-1. A, Western blotting of glutamate transporter EAAC1, GLAST, and GLT-1 within the spinal cord dorsal horn. B, Densitometric quantification of Western blotting results in A. The amount of glutamate transporter EAAC1, GLAST, and GLT-1 normalized to that of tubulin was plotted as fold change. One-way ANOVA followed by Tukey-Kramer test was used to analyze the data. * P< 0.05, as compared with vehicle, morphine plus MG-132, or MG-132 alone. Lane 1: vehicle, Lane 2: morphine (15 nmol), Lane 3: morphine (15 nmol) plus MG-132 (5 nmol), Lane 4: MG-132 (5 nmol) alone. Results are expressed as the group mean (± SEM) obtained from three independent extract preparations from the same rats in each group. Actin: loading control. C, A linear range of immunoreactivity for GLAST, GLT-1, EAAC1, and β-actin. Spinal cord dorsal horn samples containing 10, 20, 30, 40, and 50 μg of protein were analyzed by Western blotting. Densitometric assessment of bands showed a linear response between 10 and 50 μg of total protein loading.
3.3. Effect of MG-132 on the downregulation of glutamate transporters
To examine whether MG-132 would regulate the expression of EAAC1, GLAST, GLT-1, MG-132 (5 nmol) was co-administered intrathecally (twice daily) with morphine (15 nmol) for seven days (n=6) and independent samples from these rats were used for the Western blot assay. The combination of morphine and MG-132 effectively prevented the downregulation of EAAC1, GLAST, and GLT-1 within the spinal cord dorsal horn, that is, there were no differences in the Western blot analysis between the morphine alone group and the morphine plus MG-132 group (Fig. 3A, B, P> 0.05). MG-132 alone did not alter the baseline level of GLT-1, GLAST, or EAAC1 within the spinal cord dorsal horn (Fig. 3A, B). These results indicate that the proposed proteasome inhibitor MG-132 regulated the expression of glutamate transporters within the spinal cord dorsal horn following chronic morphine exposure.
3.4. Effect of MG-132 on spinal glutamate uptake activity after chronic morphine exposure
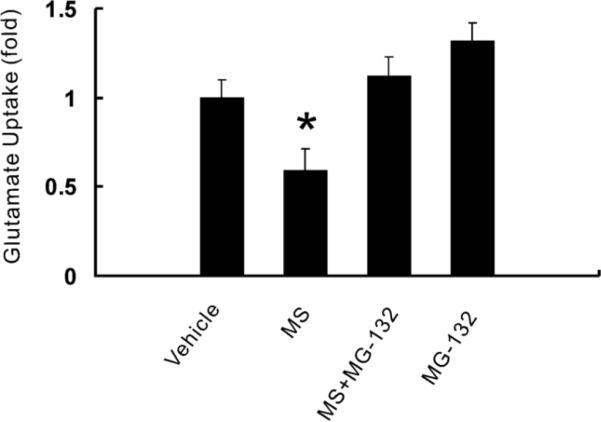
Consistent with the downregulation of glutamate transporters, chronic morphine exposure (15 nmol; intrathecal, twice daily, 7 days) also significantly reduced the spinal glutamate uptake activity (Fig. 4, P<0.05; n=6). To examine whether MG-132 would also regulate the spinal cord glutamate uptake activity, MG-132 (5 nmol) was co-administered intrathecally (twice daily) with morphine for seven days. The combination of morphine and MG-132 effectively prevented the decreased glutamate uptake activity (Fig. 4, P<0.05), indicating that the proposed proteasome inhibitor MG-132 also played a functional role in the regulation of regional glutamate uptake activity.
Fig. 4. The proteasome inhibitor MG-132 prevented the decrease of spinal glutamate uptake activity after chronic morphine exposure.
Rats (n=6) were administrated intrathecally (twice daily) with vehicle, morphine (MS, 15 nmol), morphine plus MG-132 (1, 2.5, 5 or 10 nmol) or MG132 (5 nmol) alone for seven consecutive days. Glutamate uptake activity within the spinal cord dorsal horn was examined using an in vitro glutamate uptake assay. Lane 1: vehicle, Lane 2: morphine (15 nmol), Lane 3: morphine (15 nmol) plus MG-132 (5 nmol), Lane 4: MG-132 (5 nmol) alone. One-way ANOVA followed by Tukey-Kramer test was used to analyze the data. * P< 0.05, as compared with each of the remaining groups. Results are the group mean (± SEM) obtained from the independent extract preparation from the same rats in each group.
4. Discussion
The present results demonstrate that the proposed proteasome inhibitor MG-132 prevented the development of morphine tolerance as well as the downregulation of spinal glutamate transporters and the decreased spinal glutamate uptake activity in rats. The data support the notion that modulation of the glutamate transporter expression may be an important cellular mechanism contributory to the glutamatergic regulation of morphine tolerance [15]. We demonstrated that three glutamate transporters (EAAC1, GLAST, GLT-1) were downregulated within the spinal cord dorsal following a 7-day intrathecal morphine regimen. This finding is consistent with our previous report using a similar experimental protocol [15]. Of interest is that there were no changes at the mRNA level of all three glutamate transporters on day 7 of chronic morphine exposure despite a significant downregulation of glutamate transporters at the protein level. These results suggest that the downregulation of glutamate transporters may have occurred at the post-transcriptional level. However, since spinal cord dorsal horn samples were taken on day 7, the possibility of glutamate transporter mRNA changes at other time points of morphine exposure could not be ruled out.
Activation of UPS is an important cellular mechanism of posttranslational regulation of the protein turnover. In the present study, rats were co-administered with morphine and the proposed proteasome inhibitor MG-132. This combined treatment regimen effectively prevented the glutamate transporter downregulation and reversed the decreased glutamate uptake activity within the spinal cord dorsal horn, suggesting a possible role of UPS in this process. However, it should be emphasized that, although MG-132 has been proposed as a proteasome inhibitor and used in previous in vivo studies on the role of UPS in neuropathic pain in rats [18,20], MG-132 also has an inhibitory effect on calpains [9,11]. In addition, the role of translation, RNA transport and protein trafficking in the glutamate transporter expression and their possible modulation by MG-132 remain to be determined. Thus, our in vivo data alone does not exclusively indicate the role of UPS activation in the downregulation of spinal glutamate transporters after chronic morphine exposure. Using a C6 glioma cell line, we recently observed that the downregulation of EAAC1 induced by chronic morphine exposure was inhibited by MG-132 but not the lysosomal inhibitor chloroquin (unpublished data). Nevertheless, future studies using both in vivo and in vitro preparations may further clarify the role of the ubiquitin-proteasome activity in the regulation of spinal glutamate transporters.
The exact mechanisms by which chronic morphine exposure leads to the UPS activation remain to be investigated. Two recent studies have shown that inhibition of proteasomal activity attenuated neuropathic pain behaviors in rats [18,20]. Moreover, inhibition of the UPS activity also prevented hyperalgesia induced by chronic morphine administration, a phenomenon mediated at least in part by the glutamatergic mechanisms [20]. Since the glutamatergic mechanism plays a critical role in the mechanisms of neuropathic pain, morphine tolerance, and neurotoxicity [13–15,15,19,23,25,27] and regulation of the glutamate transporter expression is contributory to this process [23], it would be of interest in future studies to determine how the glutamate transporter turnover might be regulated by UPS and whether such a regulatory process has a broad role in the regulation of glutamate homeostasis. In this regard, it has been shown that peripheral nerve injury induced changes in the expression of ubiquitin C-terminal hydrolase [18]. Moreover, proteasome inhibitors such as MG-132 have been shown to prevent capsaicin-induced release of calcitonin gene-related peptide and dynorphin A upregulation [20] but not protein kinase C translocation [18] in the spinal cord dorsal horn. These previous findings may shed light on the cellular mechanisms concerning a possible relationship between activation of UPS by chronic morphine exposure, downregulation of glutamate transporters, and morphine tolerance under in vivo conditions.
The present findings may have significant clinical implications. For example, using proteasome inhibitor and/or targeting key elements of the UPS-mediated glutamate transporter turnover may offer a new strategy for treating certain neurological disorders [12,15,16,22,23,26,28]. Another area of the potential clinical utility regarding the UPS-mediated glutamatergic system is to improve clinical opioid therapy for treatment of neuropathic pain and cancer-related pain by preventing opioid tolerance and opioid-induced hyperalgesia [15]. While modulating the glutamatergic system may not acutely reverse morphine tolerance [15, 27], it is encouraging to note that in both present and previous in vivo studies, proteasome inhibitors attenuated neuropathic pain behaviors, morphine-induced hyperalgesia, and morphine tolerance without affecting baseline nociceptive responses and motor function [18,20]. The potential clinical utility of proteasome inhibitors remains to be examined.
Acknowledgements
This work was supported by US PHS RO1 grants DE 18214, DE18538, and NS45681.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
With regard to the above manuscript, all authors declare no conflict of interest.
References
- [1].Akil H, Mayer DJ, Liebeskind JC. Antagonism of stimulation-produced analgesia by naloxone, a narcotic antagonist. Science. 1976;191:961–962. doi: 10.1126/science.1251210. http://www.ncbi.nlm.nih.gov/pubmed/1251210?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [2].Chen SR, Sweigart KL, Lakoski JM, Pan HL. Functional mu opioid receptors are reduced in the spinal cord dorsal horn of diabetic rats. Anesthesiology. 2002;97:1602–1608. doi: 10.1097/00000542-200212000-00037. http://www.ncbi.nlm.nih.gov/pubmed/12459691?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [3].Colledge M, Snyder EM, Crozier RA, Soderling JA, Jin Y, Langeberg LK, Lu H, Bear MF, Scott JD. Ubiquitination regulates PSD-95 degradation and AMPA receptor surface expression. Neuron. 2003;40:595–607. doi: 10.1016/s0896-6273(03)00687-1. http://www.ncbi.nlm.nih.gov/pubmed/14642282?dopt=Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Coux O, Tanaka K, Goldberg AL. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. http://www.ncbi.nlm.nih.gov/pubmed/8811196?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [5].Elliott K, Hynansky A, Inturrisi CE. Dextromethorphan attenuates and reverses analgesic tolerance to morphine. Pain. 1994;59:361–368. doi: 10.1016/0304-3959(94)90022-1. http://www.ncbi.nlm.nih.gov/pubmed/7708410?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [6].Halim ND, Weickert CS, McClintock BW, Hyde TM, Weinberger DR, Kleinman JE, Lipska BK. Presynaptic proteins in the prefrontal cortex of patients with schizophrenia and rats with abnormal prefrontal development. Mol Psychiatry. 2003;8:797–810. doi: 10.1038/sj.mp.4001319. http://www.ncbi.nlm.nih.gov/pubmed/12931207?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [7].Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. http://www.ncbi.nlm.nih.gov/pubmed/9759494?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [8].Joazeiro CA, Hunter T. Biochemistry. Ubiquitination--more than two to tango. Science. 2000;289:2061–2062. doi: 10.1126/science.289.5487.2061. http://www.ncbi.nlm.nih.gov/pubmed/11032556?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [9].Khoutorsky A, Spira ME. Calcium-activated proteases are critical for refilling depleted vesicle stores in cultured sensory-motor synapses of Aplysia. Learn Mem. 2005;12:414–422. doi: 10.1101/lm.92105. http://www.ncbi.nlm.nih.gov/pubmed/16077020?dopt=Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kren MC, Haller VL, Welch SP. The role of gonadal hormones on opioid receptor protein density in arthritic rats. Eur J Pharmacol. 2008;578:177–184. doi: 10.1016/j.ejphar.2007.08.036. http://www.ncbi.nlm.nih.gov/pubmed/18054782?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [11].Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. http://www.ncbi.nlm.nih.gov/pubmed/9789328?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [12].Lievens JC, Bernal F, Forni C, Mahy N, Kerkerian-Le Goff L. Characterization of striatal lesions produced by glutamate uptake alteration: cell death, reactive gliosis, and changes in GLT1 and GADD45 mRNA expression. Glia. 2000;29:222–232. doi: 10.1002/(sici)1098-1136(20000201)29:3<222::aid-glia4>3.0.co;2-0. http://www.ncbi.nlm.nih.gov/pubmed/10642749?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [13].Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217. doi: 10.1016/S0304-3959(02)00422-0. http://www.ncbi.nlm.nih.gov/pubmed/12467992?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [14].Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. http://www.ncbi.nlm.nih.gov/pubmed/7908958?dopt=Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–8323. doi: 10.1523/JNEUROSCI.22-18-08312.2002. http://www.ncbi.nlm.nih.gov/pubmed/12223586?dopt=Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mennerick S, Shen W, Xu W, Benz A, Tanaka K, Shimamoto K, Isenberg KE, Krause JE, Zorumski CF. Substrate turnover by transporters curtails synaptic glutamate transients. J Neurosci. 1999;19:9242–9251. doi: 10.1523/JNEUROSCI.19-21-09242.1999. http://www.ncbi.nlm.nih.gov/pubmed/10531428?dopt=Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mitrovic AD, Maddison JE, Johnston GA. Influence of the oestrous cycle on L-glutamate and L-aspartate transport in rat brain synaptosomes. Neurochem Int. 1999;34:101–108. doi: 10.1016/s0197-0186(98)00066-7. http://www.ncbi.nlm.nih.gov/pubmed/10213067?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [18].Moss A, Blackburn-Munro G, Garry EM, Blakemore JA, Dickinson T, Rosie R, Mitchell R, Fleetwood-Walker SM. A role of the ubiquitin-proteasome system in neuropathic pain. J Neurosci. 2002;22:1363–1372. doi: 10.1523/JNEUROSCI.22-04-01363.2002. http://www.ncbi.nlm.nih.gov/pubmed/11850463?dopt=Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Nakagawa T, Ozawa T, Shige K, Yamamoto R, Minami M, Satoh M. Inhibition of morphine tolerance and dependence by MS-153, a glutamate transporter activator. Eur J Pharmacol. 2001;419:39–45. doi: 10.1016/s0014-2999(01)00965-7. http://www.ncbi.nlm.nih.gov/pubmed/11348628?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [20].Ossipov MH, Bazov I, Gardell LR, Kowal J, Yakovleva T, Usynin I, Ekstrom TJ, Porreca F, Bakalkin G. Control of chronic pain by the ubiquitin proteasome system in the spinal cord. J Neurosci. 2007;27:8226–8237. doi: 10.1523/JNEUROSCI.5126-06.2007. http://www.ncbi.nlm.nih.gov/pubmed/17670969?dopt=Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Rolfe M, Chiu MI, Pagano M. The ubiquitin-mediated proteolytic pathway as a therapeutic area. J Mol Med. 1997;75:5–17. doi: 10.1007/s001090050081. http://www.ncbi.nlm.nih.gov/pubmed/9020379?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [22].Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes HM, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. http://www.ncbi.nlm.nih.gov/pubmed/15635412?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [23].Sung B, Lim G, Mao J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J Neurosci. 2003;23:2899–2910. doi: 10.1523/JNEUROSCI.23-07-02899.2003. http://www.ncbi.nlm.nih.gov/pubmed/12684477?dopt=Citation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Thomson LM, Zeng J, Terman GW. Differential effect of glutamate transporter inhibition on EPSCs in the morphine naive and morphine tolerant neonatal spinal cord slice. Neurosci Lett. 2006;407:64–69. doi: 10.1016/j.neulet.2006.08.004. http://www.ncbi.nlm.nih.gov/pubmed/16949209?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [25].Tiseo PJ, Inturrisi CE. Attenuation and reversal of morphine tolerance by the competitive N-methyl-D-aspartate receptor antagonist, LY274614. J Pharmacol Exp Ther. 1993;264:1090–1096. http://www.ncbi.nlm.nih.gov/pubmed/8450453?dopt=Citation. [PubMed] [Google Scholar]
- [26].Trotti D, Aoki M, Pasinelli P, Berger UV, Danbolt NC, Brown RH, Jr., Hediger MA. Amyotrophic lateral sclerosis-linked glutamate transporter mutant has impaired glutamate clearance capacity. J Biol Chem. 2001;276:576–582. doi: 10.1074/jbc.M003779200. http://www.ncbi.nlm.nih.gov/pubmed/11031254?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [27].Trujillo KA, Akil H. Inhibition of morphine tolerance and dependence by the NMDA receptor antagonist MK-801. Science. 1991;251:85–87. doi: 10.1126/science.1824728. http://www.ncbi.nlm.nih.gov/pubmed/1824728?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [28].Vorwerk CK, Naskar R, Schuettauf F, Quinto K, Zurakowski D, Gochenauer G, Robinson MB, Mackler SA, Dreyer EB. Depression of retinal glutamate transporter function leads to elevated intravitreal glutamate levels and ganglion cell death. Invest Ophthalmol Vis Sci. 2000;41:3615–3621. http://www.ncbi.nlm.nih.gov/pubmed/11006260?dopt=Citation. [PubMed] [Google Scholar]
- [29].Wojcik C, Di Napoli M. Ubiquitin-proteasome system and proteasome inhibition: new strategies in stroke therapy. Stroke. 2004;35:1506–1518. doi: 10.1161/01.STR.0000126891.93919.4e. http://www.ncbi.nlm.nih.gov/pubmed/15118168?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [30].Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. http://www.ncbi.nlm.nih.gov/pubmed/14677603?dopt=Citation. [DOI] [PubMed] [Google Scholar]
- [31].Zhang RX, Wang L, Liu B, Qiao JT, Ren K, Berman BM, Lao L. Mu opioid receptor-containing neurons mediate electroacupuncture-produced anti-hyperalgesia in rats with hind paw inflammation. Brain Res. 2005;1048:235–240. doi: 10.1016/j.brainres.2005.05.008. http://www.ncbi.nlm.nih.gov/pubmed/15922310?dopt=Citation. [DOI] [PubMed] [Google Scholar]