Abstract
Cancer cells are hypersensitive to nutrient limitation because oncogenes constitutively drive glycolytic and tricarboxylic acid (TCA) cycle intermediates into biosynthetic pathways. Because the anaplerotic reactions that replace these intermediates are fueled by imported nutrients, the cancer cell’s ability to generate ATP becomes compromised under nutrient-limiting conditions. In addition, most cancer cells have defects in autophagy, the catabolic process that provides nutrients from internal sources when external nutrients are unavailable. Normal cells, in contrast, can adapt to nutrient stress that kills cancer cells by becoming quiescent and catabolic. We show that FTY720, a water soluble sphingolipid drug that is effective in many animal cancer models, selectively starves cancer cells to death by down-regulating nutrient transporter proteins. Consistent with a bioenergetic mechanism of action, FTY720 induced homeostatic autophagy. Cells were protected from FTY720 by cell permeable nutrients or by reducing nutrient demand, but blocking apoptosis was ineffective. Importantly, AAL-149, an FTY720 analog that lacks FTY720’s dose limiting toxicity, also triggered transporter loss and killed patient-derived leukemias while sparing cells isolated from normal donors. Because they target the metabolic profile of cancer cells rather than specific oncogenic mutations, FTY720 analogs like AAL-149 should be effective against many different tumor types, particularly in combination with drugs that inhibit autophagy.
Keywords: bioenergetics, nutrient limitation, autophagy, FTY720, AAL-149
INTRODUCTION
While it was recognized almost a century ago that cancer cells are highly glycolytic, it has only recently become appreciated that the enhanced rate of glycolysis in tumor cells most likely reflects a need for accelerated biosynthesis. All rapidly proliferating cells use glycolytic and TCA cycle intermediates as building blocks for nucleotide, membrane, and protein synthesis [1, 2]. The intermediates consumed in these biosynthetic reactions are replenished by anaplerotic reactions that depend upon imported nutrients. All rapidly proliferating cells rely on these processes to support biosynthesis. However, the regulation of anabolic metabolism is different in normal and transformed cells. In normal cells, anabolism is driven by growth factors and sensitive to extracellular nutrient levels. When nutrients become limiting, normal cells make adaptive changes in their metabolism, undergoing cell cycle arrest and becoming quiescent and catabolic. Cancer cells, in contrast, continue biosynthesis despite nutrient deprivation because anabolism is driven by constitutively-active oncogenes and uncoupled from environmental cues by the deletion or inactivation of negative regulators of growth. Because glycolytic and TCA cycle intermediates continue to be utilized but cannot be replenished, ATP generation is eventually compromised in nutrient-restricted cancer cells [3, 4]. In addition to the problems associated with constitutive anabolism, cancer cells also have defects in autophagy, the catabolic process through which cells derive nutrients from self-digestion [5]. Together, constitutive anabolism and defective autophagy trigger a bioenergetic crisis in transformed cells under conditions that produce proliferative arrest and quiescence in normal cells. Consistent with this model, hyperactivation of growth promoting oncogenes such as the serine/threonine kinase Akt, the GTPase K-Ras, and the transcription factor Myc sensitizes cells to nutrient limitation [4, 6–8]. Inactivation of the tumor suppressor proteins that orchestrate quiescence and catabolism during nutrient stress also increase dependence on extracellular nutrients. For example, loss of the tuberous sclerosis complex that limits the activity of the mammalian target of rapamycin (mTOR) kinase, the 5′-AMP-activated kinase (AMPK) that coordinates the cellular response to energy stress, the serine/threonine kinase LKB1 that regulates AMPK and related kinases, or the transcription factor p53 sensitizes cells to starvation [9–13]. Because many different mutations that transform cells increase dependence on imported nutrients, limiting access to nutrients could be a means to selectively kill diverse types of cancer cells.
Some currently available cancer therapies work by limiting nutrient availability. Angiogenesis inhibitors restrict nutrient delivery to expanding tumors by limiting the growth of new blood vessels. However, these drugs have several significant drawbacks: 1) they select for resistant cancer cells that are more aggressive and invasive [14, 15], 2) leukemias are unlikely to be nutrient-limited by this approach as they reside in the bloodstream, and 3) because small tumors can obtain nutrients by diffusion, blocking angiogenesis would not eliminate all cancer cells. L-asparaginase, an enzyme that degrades the amino acid asparagine, is used to treat acute lymphocytic leukemia [16]. Leukemia cells cannot synthesize sufficient quantities of asparagine to meet their high demand while asparagine production can counterbalance the effect of L-asparaginase in normal cells. Pegylated arginine deiminase is effective in animal studies but against tumor cells with insufficient capacity to synthesize arginine [17]. While beneficial, these enzymes target individual nutrients and are only active against a limited spectrum of tumors. If therapies that restrict cellular access to multiple nutrients can be developed, they should be more broadly effective and might limit the ability of tumor cells to develop resistance by switching to alternate fuels. If these compounds block nutrient uptake rather than nutrient delivery, many of the limitations of angiogenesis inhibition could be avoided.
Our previous work demonstrated that multiple mammalian nutrient transporter proteins could be targeted for down-regulation by the sphingolipid ceramide [18]. Because ceramide is extremely hydrophobic and readily metabolized, we investigated whether sphingolipid-based drugs with superior pharmacological properties might selectively kill cancer cells through a similar mechanism. The water soluble, sphingosine analog FTY720 is a highly effective, non-toxic anti-cancer agent in a wide variety of animal model systems. FTY720 limits the growth of the primary tumor and metastatic nodules in breast cancer and hepatocellular carcinoma models [19, 20], inhibits the growth of bladder cancer and androgen-independent prostate cancer xenografts [21, 22], and protects mice from BCR-ABL-driven leukemia [23]. FTY720 is selectively toxic to transformed cells, and mice can be maintained on the anti-neoplastic dose for as long as 26 weeks with no ill effects [22–24]. While FTY720 is effective in animal cancer models, it cannot be used in human cancer patients because it causes a significant reduction in heart rate at the anti-neoplastic dose by activating sphingosine-1-phosphate (S1P) receptors. Because the mechanism behind FTY720’s anti-cancer activity was not understood and it was unclear whether the anti-neoplastic and toxic effects of FTY720 were separable, FTY720 derivatives have not been pursued as potential cancer therapies.
Here we report that FTY720 selectively kills cancer cells by down-regulating nutrient transporter proteins. Furthermore, we demonstrate that an FTY720 analog that lacks FTY720’s dose limiting toxicity retains its ability to trigger nutrient transporter loss and selectively kill cancer cells. These studies suggest that FTY720 analogs could be a novel, safe, and effective means to starve constitutively anabolic cancer cells to death in the midst of abundant extracellular nutrients.
EXPERIMENTAL
Materials
Chemicals were from Cayman, EMD, Biomol, or Sigma. Antibodies: murine 4F2hc, human CD4, B220 (CD45.2) (eBioscience); murine transferrin receptor and human 4F2hc (BD Biosciences); human glucose transporter 1 (GLUT1; Novus); murine GLUT1 (RDI; however, recent lots of antibody did not recognize mouse GLUT1); LC3 (MBL); all others were from Cell Signaling. The B-cell lymphoma 2 (Bcl-2) G154A plasmid was supplied by Stanley Korsmeyer via Addgene (#8751). The GFP-HTLV-2-RBD was generously provided by Marc Sitbon. Phosphorylated FTY720 and AAL-149 were generously supplied by Volker Brinkmann (Novartis).
Cell culture
The IL-3 dependent murine hematopoetic cell line FL5.12 was maintained at 250,000–700,000 cells/mlin RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 10% fetal calf serum (FCS) (Sigma-Aldrich), 500 pM recombinant mouse interleukin(IL)-3 (eBioscience),10 mM HEPES (Mediatech), 55 μM β-mercaptoethanol(Sigma-Aldrich), antibiotics, and L-glutamine (Mediatech). RPMI lacking glucose and amino acids was made from chemical components. For nutrient deprivation, cells were maintained in this medium supplemented with 10% dialyzed fetal calf serum (Invitrogen). To adapt cells to low nutrient conditions, amino acid and glucose free medium was supplemented with standard FCS. Oleic acid was conjugated to fatty acid-free BSA at a molar ratio of 3:1. AMPK−/− murine embryonic fibroblasts (MEFs) lacking both α subunits originally developed by Keith Laderoute and were kindly provided by Reuben Shaw. Atg5−/− MEFs were generously provided by Noboru Mizushima. Rab7+/− MEFs were generated from mice conditionally deficient in Rab7 developed in the Edinger lab. IL-3 dependent Bax−/−Bak−/− (Bcl-2-associated X protein and Bcl-2 homologous antagonist/killer, respectively) cells were kindly provided by Tullia Lindsten. Where Fumonisin B1 was used, cells were pre-treated for 1 h with twice the final concentration. Cells were pre-treated for 4 h with okadaic acid. Cells were spun-down and re-suspended in fresh media daily in experiments that lasted longer than 72 h. Methocult colony assays were performed as in [25]. Peripheral blood mononuclear cells isolated from three normal volunteers were plated in methylcellulose medium containing recombinant human IL-3 (30 ng/mL) and GM-CSF (500 ng/mL) at 1 × 106 cells/mL and granulocyte/macrophage colonies counted at 15 days.
Amino acid uptake
FL5.12 cells were suspended at low density in fresh, warm complete RPMI in the presence or absence of 5 μM FTY720. After 2 h, 1 μCi/ml 3H-arginine (MP Biomedical) was added and cells were incubated at 37°C, or on ice where indicated, for 3 h. To stop uptake, RPMI containing 100X unlabeled arginine was added on ice and cells were washed twice with ice cold RPMI containing 10X arginine (2 mg/ml) before lysis. 3H-arginine in cell lysates was measured by scintillation counting. Uptake was linear over the course of the experiment (R2=0.99).
Flow cytometry assays and microscopy
Analysis was restricted to viable cells; cells that excluded vital dyes (propidium iodide or 4′,6-diamidino-2-phenylindole (DAPI)) were deemed viable. Surface GLUT1 was measured using GFP-HTLV-2 RBD as previously described [26]. Cell accumulation was measured by flow cytometry by collecting for a fixed interval, generally 30–60 sec. For immunofluorescence, cells were fixed with 1% paraformaldehyde and permeabilized with 0.3% saponin. For electron microscopy, cells were fixed in 2.5% glutaraldehyde/2.5% formaldehyde in 0.1 M sodium cacodylate buffer (Electron Microscopy Systems) and stored at 4°C until embedding. Cells were post-fixedin 1% OsO4 on ice for 1 h and contrasted with 1% uranylacetate at room temperature for 1 h, dehydrated in ethanol, and embedded inEpon 812. Ultrathin sections (60 nm) were cut on a ReichertUltracut Ultramicrotome. Sections were stained again in 2% uranylacetate for 2 min, followed by Reynold’s lead citrate for 2min. Sections were examined on a Philips CM10 transmission electronmicroscope and micrographs were taken with a Gatan UltrascanUS1000 digital camera.
Mass spectrometry
Ceramide levels in Bax−/−Bak−/− cells were measured by LC/LC mass spectrometry performed in the Lipidomics Core Facility at the Medical University of South Carolina. For experiments using FL5.12 cells, flow injection ESI-MS-MS analysis was performed using a Waters Quattro Ultima electrospray tandem mass spectrometer. Detailed mass spectrometry methods for the experiments using FL5.12 cells are provided in the Supplementary Information for this article.
Mice
Sub-lethally irradiated (4.5 Gy) 6–9 week old female Balb/c mice were injected with 500,000 BCR-ABL p190-expressing FL5.12 cells and leukemia allowed to progress for 6–7 d (FL5.12 cells were originally derived from Balb/c mice [27]). Mice were then treated with FTY720 (10 mg/kg i.p. daily) or with vehicle (water) alone for 3 d at which point splenocytes were harvested and analyzed. A two-tailed t test was used to determine statistical significance. All animal protocols were approved by the Institutional Animal Care and Use Committee of University of California, Irvine.
RESULTS
FTY720 triggers nutrient transporter loss in mammalian cells
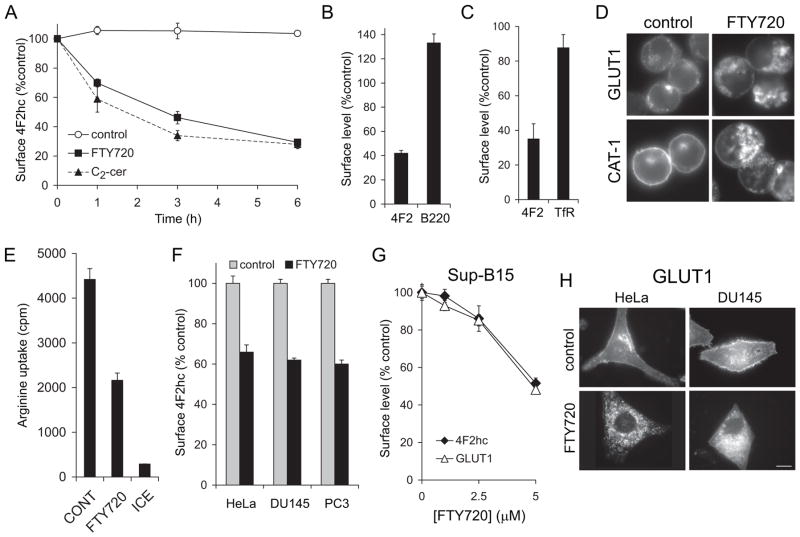
FTY720 is structurally similar to ceramide, a sphingolipid that we have previously shown produces nutrient transporter down-regulation [18]. Studies in yeast suggested that FTY720 might also limit nutrient permease expression [28]. We used FL5.12 murine hematopoetic cells to test whether FTY720 could induce transporter loss in mammalian cells because this cell line expresses high levels of nutrient transporters and is sensitive to ceramide-induced transporter loss [18]. In addition, FL5.12 cells are particularly useful for studies assessing the contribution of the cellular metabolic state to drug toxicity because they are immortalized but not transformed. FL5.12 cells are immortalized and will undergo unlimited proliferation in culture as long as they are supplied with IL-3. However, their rapid growth is driven by growth factors rather than oncogenes, and thus their anabolic rate can be reduced by gradually decreasing growth factor or nutrient levels. Thus, the response of genetically identical cells with different metabolic profiles can be compared. We first evaluated the effect of FTY720 on the 4F2 heavy chain (4F2hc), a type II membrane protein that associates with several different light chains to form heterodimeric amino acid transporter complexes [29, 30]. FTY720 rapidly decreased 4F2hc surface levels with kinetics similar to those previously described for ceramide (Figure 1A, [18]). The B cell marker protein B220 was, in contrast, not down-regulated (Figure 1B). The transferrin receptor which traffics via a distinct pathway from 4F2hc [31] was also minimally affected (Figure 1C). Like the 4F2 complex, the glucose transporter GLUT1 and the high affinity cationic amino acid transporter 1 (CAT-1), are found in lipid raft domains [31, 32]. These nutrient transporters were also down-regulated by FTY720 (Figure 1D). As expected from these results, amino acid uptake was markedly reduced by FTY720 (Figure 1E). FTY720 inhibits proliferation and triggers cell death in a wide variety of cancer cell types [19–24]. In keeping with these findings, FTY720 reduced surface 4F2hc and GLUT1 levels in HeLa cervical carcinoma cells, DU145 and PC3 prostate cancer cells, and in Sup-B15 human leukemia cells (Figure 1F–H). Thus, FTY720 coordinately down-regulated nutrient transporter proteins in diverse mammalian cell types.
Figure 1. FTY720 down-regulates nutrient transporters in mammalian cells.
A) Surface 4F2hc in FL5.12 cells maintained in control medium or treated with 2.5 μM FTY720 or 25 μM C2-ceramide were measured by flow cytometry at the indicated time points. B) Surface 4F2hc and B220 levels were measured as in (A) in FL5.12 cells treated with 5 μM FTY720 for 17 h. C) Surface 4F2hc or transferrin receptor (TfR) levels were measured in FL5.12 cells treated with 2.5 μM FTY720 for 18 h using flow cytometry (cells expressed Bcl-2 G145A to increase viability). D) FL5.12 cells were treated with 5 μM FTY720 for 7–9 h and stained for GLUT1 or HA-CAT1. E) Arginine uptake in FL5.12 cells treated with 5 μM FTY720. ICE, cells were incubated with tritiated arginine but maintained on ice for the course of the experiment. F) Surface 4F2hc levels were measured in cells treated with 5 μM FTY720 for 15 h (HeLa) or 12 h (DU145 and PC3). G) Surface 4F2hc or GLUT1 levels were measured by flow cytometry in Sup-B15 cells treated with FTY720 for 3 h. H) HeLa or DU145 cells were treated with 5 μM FTY720 for 15 h and stained for GLUT1. In all panels, n ≥ 3. Means +/− SD of triplicates from representative experiments are shown.
FTY720 induces starvation despite abundant extracellular nutrients
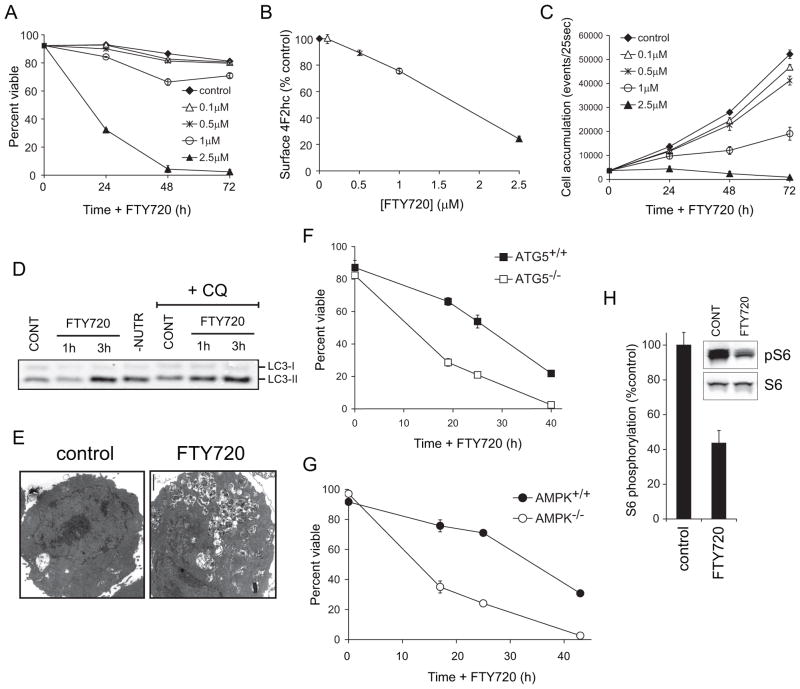
Given that FTY720 produced profound nutrient transporter loss (Figure 1), we hypothesized that FTY720 kills cells by restricting access to extracellular nutrients. The dose of FTY720 necessary for nutrient transporter loss and cell death was tightly correlated (Figure 2A–B). Reduction of 4F2hc levels by >30% resulted in the death of all the cells in the culture. When transporter levels remained above 70% of control levels, viability was minimally affected but proliferation was reduced (Figure 2B–C). Similar effects were observed in HeLa, DU145, PC3, and Sup-B15 cells (not shown). To determine whether transporter loss was sufficient to induce bioenergetic stress, we assessed whether FTY720 induced autophagy. Through autophagy, nutrient-limited cells encapsulate and degrade non-essential components to recycle intracellular nutrients [33]. Consistent with profound transporter loss, LC3-II, an autophagosome marker, accumulated to an equivalent degree following FTY720 treatment and extracellular nutrient limitation (Figure 2D). An increase in autophagosome number was also observed in FTY720-treated cells (Figure 2E and Supplementary Figure S1A). Autophagic flux was increased by FTY720 treatment as LC3-II accumulation was enhanced by chloroquine addition (Figure 2D). These results are consistent with autophagy induction in response to intracellular nutrient limitation.
Figure 2. FTY720-induced nutrient transporter loss causes bioenergetic stress.
A–C) Viability (A), 4F2hc surface levels at 24 h (B), or cell accumulation (C) was measured by flow cytometry in FL5.12 cells incubated with FTY720 under the indicated conditions. D) FL5.12 cells were treated with 5 μM FTY720 for 1 or 3 h or subjected to 3 h of nutrient restriction (-NUTR). Chloroquine (CQ, 10 μg/ml) was added where indicated to block autophagosome degradation. Western blots were performed on cell lysates to evaluate LC3-II levels. CONT, control. E) Electron micrographs of FL5.12 cells treated with FTY720 for 24 h. F–G) Atg5+/+ and Atg5−/− MEFs (F), or AMPK+/+ and AMPK−/− MEFs (G) were treated with 5 μM FTY720 for the indicated intervals and viability measured by vital dye exclusion and flow cytometry. H) S6 phosphorylation in FL5.12 cells treated with FTY720 was evaluated by Western blot and quantified using a LI-COR Odyssey imaging system. In all panels, n ≥ 3. Means +/− SD of triplicates from representative experiments are presented.
To confirm that FTY720-induced autophagy was a homeostatic response to starvation, we assessed the consequences of limiting autophagy. Atg5−/− MEFs that are unable to form autophagosomes were hypersensitive to FTY720-induced cell death (Figure 2F) demonstrating that autophagy is a protective response. Autophagy generates nutrients but also promotes cellular health by removing damaged organelles that consume ATP and generate free radicals. Encapsulating these organelles in autophagosomes may limit their access to substrates, sparing nutrients and relieving toxicity. If, however, the protective function of autophagy in FTY720-treated cells is nutrient generation, autophagosome degradation would be required. Rab7 is necessary for the degradation of autophagosomes in the lysosome but not for their formation [34]. Consistent with a model where autophagy is required to generate nutrients, Rab7+/− MEFs were hypersensitive to FTY720 (Supplementary Figure S1B). AMPK coordinates the cellular response to energy stress, increasing autophagy and inhibiting biosynthesis [35]. AMPK-deficient MEFs were also hypersensitive to FTY720 (Figure 2G). mTOR is also a nutrient sensor, exhibiting reduced activity when amino acids are limiting [36]. Phosphorylation of the downstream mTOR target, ribosomal protein S6, was also decreased by FTY720 (Figure 2H). In summary, FTY720-induced nutrient transporter loss is sufficient to induce starvation in the midst of abundant extracellular nutrients.
FTY720 kills cells by down-regulating nutrient transporter proteins
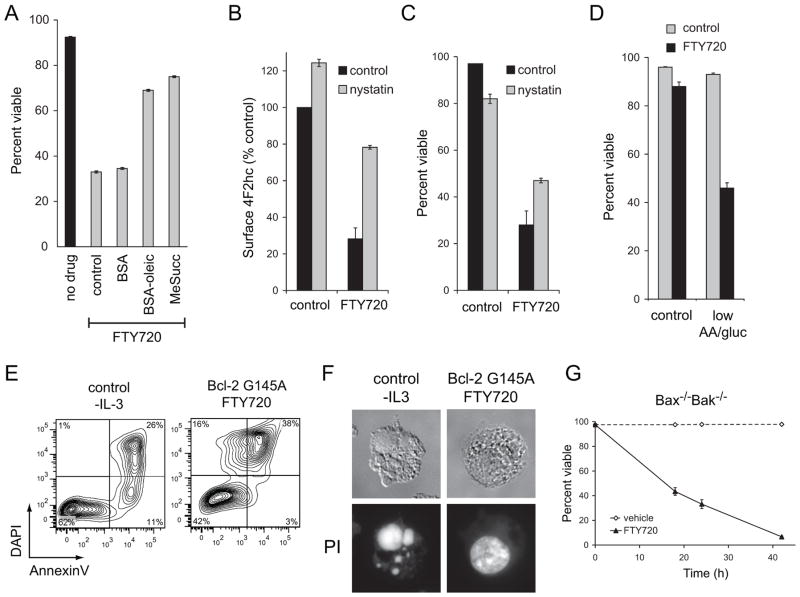
If FTY720 kills cells by causing nutrient transporter loss, membrane-permeable nutrients that do not require transporters to enter cells should be protective. Two cell-permeable nutrients, dimethyl succinate and oleic acid, limited FTY720-induced death without blocking nutrient transporter loss (Figure 3A and Supplementary Figure S2A). Interfering with transporter down-regulation should also rescue cells from FTY720. FTY720 affects nutrient transporter proteins that are internalized via a clathrin-independent, lipid raft-dependent pathway [31, 32, 37]. Consistent with this, the lipid raft-disrupting agent nystatin increased basal levels of surface 4F2hc and maintained 4F2hc levels in the presence of FTY720 (Figure 3B). Although nystatin is itself somewhat toxic, increased transporter levels led to a parallel increase in cell viability (Figure 3C). If transporters are limiting, FTY720-treated cells should be hypersensitive to extracellular nutrient limitation. In fact, under conditions where neither nutrient limitation nor FTY720 were toxic, striking synergy was observed when these treatments were combined (Figure 3D). Together, these results support a model where FTY720 kills cells by triggering transporter down-regulation.
Figure 3. Bioenergetic stress secondary to nutrient transporter loss is responsible for FTY720-induced cell death.
A) FL5.12 cells were treated with 2.5 μM FTY720 for 24 h in standard medium or in medium supplemented with bovine serum albumin, 10 μM oleic acid conjugated to bovine serum albumin, or 22 mM dimethyl succinate (MeSucc) and viability measured by vital dye exclusion and flow cytometry. B) Surface 4F2hc levels were measured using flow cytometry in FL5.12 cells treated with 5 μM FTY720 and/or 50 μg/ml nystatin for 4 h. C) The viability of the cells in (B) was measured at 24 h by flow cytometry. D) FL5.12 cells were acutely withdrawn from nutrients and/or treated with 5 μM FTY720 and viability measured by vital dye exclusion and flow cytometry at 9 h. E) FL5.12 cells withdrawn from IL-3 for 11 h or Bcl-2 G145A-expressing FL5.12 cells exposed to 5 μM FTY720 for 18 h were stained with Annexin V and DAPI and analyzed by flow cytometry. F) Wildtype FL5.12 cells were withdrawn from IL-3 for 18 h. FL5.12 cells expressing Bcl-2 G145A were treated with FTY720 for 24 h. Cells were then stained with propidium iodide and imaged. G) Bax−/−Bak−/− hematopoetic cells were treated with 5 μM FTY720 and viability measured over time by flow cytometry. In all panels, n ≥ 3. Means +/− SD of triplicates from representative experiments are shown.
Apoptosis will ensue if nutrient stress is prolonged or severe. However, bioenergetic stress will eventually lead to necrotic death if apoptosis is disabled [18, 38]. To determine whether FTY720-induced transporter loss led to sufficient bioenergetic stress to kill apoptosis-deficient cells, we used cells over-expressing Bcl-2 G145A, a mutant form of Bcl-2 that blocks apoptosis but does not interfere with autophagy [39]. Bcl-2 G145A expression abrogated cell death following the loss of growth factor signaling, a purely apoptotic stimulus (Supplementary Figure S2B). However, Bcl-2 G145A over-expression did not prevent FTY720-induced death (Figure 3E–F). FTY720 did not induce the DAPI-negative, Annexin V-positive population characteristic of apoptosis that is observed following growth factor withdrawal (Figure 3E). The nuclear condensation and fragmentation that is characteristic of apoptotic death was also not observed (Figure 3F). Furthermore, FTY720 rapidly killed Bax−/−Bak−/− cells that are highly resistant to apoptotic forms of death such as growth factor withdrawal and staurosporine treatment (Figure 3G, [40]). From these experiments, we conclude that FTY720 induces bioenergetic stress severe enough to trigger necrosis when apoptosis is blocked.
Bioenergetic state determines sensitivity to FTY720-induced transporter loss
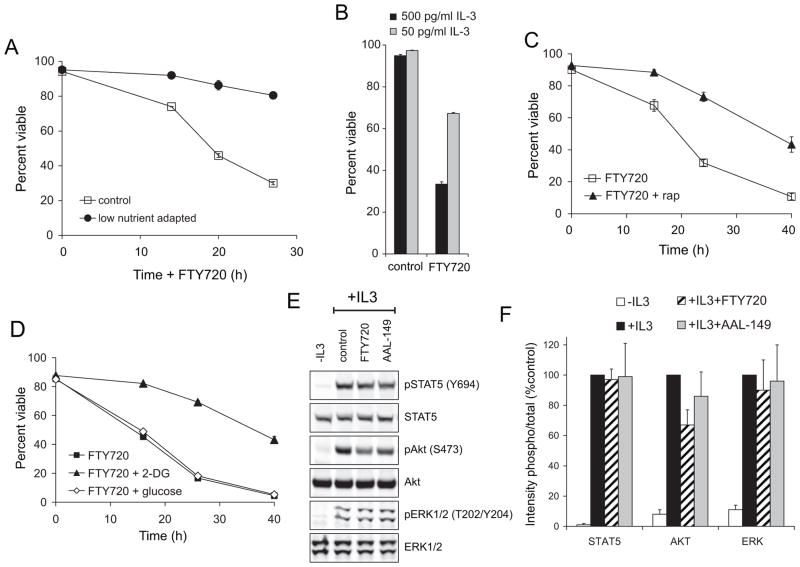
If FTY720 kills cells by limiting nutrient uptake, drug sensitivity should parallel bioenergetic demand. To test this hypothesis, we generated genetically identical cells with different metabolic programs. Because rapid growth is not oncogene-driven in FL5.12 cells, their bioenergetic program is flexible and can be altered by varying the culture conditions [41]. By gradually reducing the nutrients in the culture medium, we produced FL5.12 cells able to grow in only 5% of the normal level of amino acids and glucose. These cells exhibited stress response hormesis, a phenomenon where sub-lethal exposure to a stress triggers a protective response that confers resistance to lethal level of the same form of stress [42]. FL5.12 cells adapted to low nutrient medium were highly resistant to FTY720-induced death (Figure 4A), demonstrating that FTY720 and extracellular nutrient limitation induce similar forms of stress. As an alternate means to reduce metabolic demand, FL5.12 cells were adapted to grow in low levels of IL-3. Although acute IL-3 withdrawal triggers apoptosis in FL5.12 cells (Figure 3E–F), gradually reducing IL-3 concentration slows anabolism, reducing glycolysis and increasing cellular dependence on oxidative phosphorylation [41, 43]. FL5.12 cells adapted to low levels of IL-3 were resistant to FTY720-induced cell death (Figure 4B) consistent with a model where a high rate of anabolism sensitizes cells to the drug. This interpretation is supported by the fact that IL-3 is a critical survival factor in this cell line, yet reducing its concentration in the medium was protective. Anabolism was also reduced pharmacologically. The mTOR complex 1 inhibitor rapamycin reduces glycolysis and protein synthesis [36, 44]. Consistent with its ability to rescue cells from starvation [9, 10, 13], rapamycin afforded substantial protection from FTY720-induced cell death (Figure 4C). Weaning cells off glycolysis in advance of exposure to FTY720 by pre-treating with a low dose of the glycolysis inhibitor 2-deoxy-D-glucose (2-DG) was also protective (Figure 4D); as expected, pre-treating with D-glucose was not beneficial.
Figure 4. Sensitivity to FTY720 depends on cellular bioenergetic state.
A) FL5.12 cells were gradually adapted to grow in RPMI containing 5% the normal level of amino acids and glucose. Control cells were grown in standard medium. Cells were treated with 2.5 μM FTY720 for the indicated times and viability measured by vital dye exclusion and flow cytometry. B) FL5.12 cells were gradually adapted to grow at a low concentration of IL-3 (50 pg/ml). Control cells were maintained at the standard IL-3 concentration (500 pg/ml). Cells were treated with 2.5 μM FTY720 for 24 h and viability measured. C) FL5.12 cells were pre-treated with 20 nM rapamycin for 24 h prior to exposure to 2.5 μM FTY720. Viability was measured at the indicated times by vital dye exclusion and flow cytometry. D) As in (C), but pre-treatment was with 1 mM 2-DG or 1 mM D-glucose. E,F) FL5.12 cells expressing Bcl-2 G145A to block apoptosis were withdrawn from IL-3 overnight. Cells were then treated with FTY720 or AAL-149 as indicated (2.5 μM for 3 h), exposed to IL-3 for 10 min, and protein phosphorylation quantified by Western blotting using a LI-COR Odyssey imaging system. The average results from the quantification of at least 3 independent experiments are shown +/− SD in (F). In all panels, n ≥ 3. Means +/−SD of triplicates from representative experiments are shown in A–D.
Lipid rafts are signaling platforms, and it was possible that disruption of growth factor receptor signaling contributed to the toxicity of FTY720. However, IL-3 dependent phosphorylation of STAT5 and ERK1/2 was not disrupted by FTY720 and Akt phosphorylation was only marginally affected (Figure 4E–F). Moreover, slowing anabolism with rapamycin or 2-DG did not protect cells from the loss of growth factor receptor-dependent signal transduction (Supplementary Figure S3A–B). Taken together, these experiments show that FTY720 induces death secondary to starvation rather than by compromising growth factor receptor-dependent signaling.
The anti-neoplastic dose of FTY720 triggers nutrient transporter loss in vivo
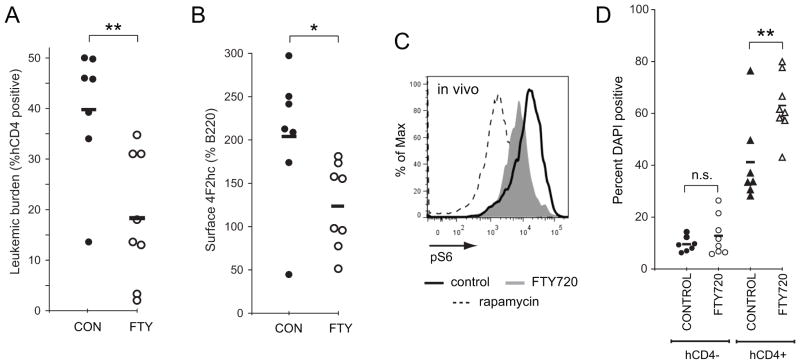
To test whether FTY720-induced nutrient transporter down-regulation might explain its activity in cancer models, we utilized a BCR-ABL driven leukemogenesis model in which FTY720 is effective [23]. This model system was selected because leukemia cells can be recovered and analyzed by flow cytometry with minimal processing. To track the leukemic cells, BCR-ABL p190 expression was coupled to human CD4 expression via an internal ribosome entry site. BCR-ABL p190 expression rendered FL5.12 cells IL-3 independent as expected but did not block FTY720-induced transporter loss and death in vitro (Supplementary Figure S4A–B). In vivo, FTY720 significantly decreased the average leukemic burden in the spleen from 40% to 18% (Figure 5A); mice were treated for only 4 days to ensure that sufficient numbers of leukemic cells would be recovered to allow further analysis. Importantly, FTY720 induced nutrient transporter down-regulation in vivo (Figure 5B). This decrease was detected both prior to and following normalization to B220, a molecule that is not down-regulated by FTY720 (Supplementary Figure S4B). FTY720 treatment led to reduced S6 phosphorylation (Figure 5C) suggesting that transporter loss induced nutrient stress in vivo. Finally, FTY720 selectively killed leukemic cells in vivo (Figure 5D). In summary, the established anti-neoplastic dosing schedule for FTY720 produces tissue concentrations of the drug that are sufficient to trigger nutrient transporter loss.
Figure 5. FTY720 triggers nutrient transporter loss in leukemia cells in vivo.
A) 500,000 FL5.12 cells expressing BCR-ABL p190 were introduced into the retro-orbital sinus of sub-lethally irradiated Balb/c mice. Seven days later, mice were treated daily with vehicle or 10 mg/kg FTY720 by intraperitoneal injection. After 4 d of treatment, mice were sacrificed and splenocytes harvested. The leukemic burden in the spleen was determined by measuring the percentage of human CD4-positive cells. B) 4F2hc surface expression on the BCR-ABL p190-expressing splenocytes from (A). 4F2hc staining was normalized to B220 to facilitate comparisons between animals. Similar results were obtained when 4F2hc staining intensity was plotted directly. C) Phospho-S6 levels in BCR-ABL p190 leukemia cells freshly isolated from vehicle or FTY720-treated mice were measured by flow cytometry. BCR-ABL p190-expressing FL5.12 cells treated with 100 nM rapamycin in vitro were included as a control. D) Cell death in normal splenocytes (human CD4-negative) and leukemia cells (human CD4-positive) freshly isolated from mice treated with vehicle or FTY720 was measured by vital dye exclusion and flow cytometry. Black bars represent means; * = p<0.05; ** = p<0.01 using a two-tailed t test. n.s., not significant (p>0.05).
FTY720-induced nutrient transporter loss does not require increased ceramide production
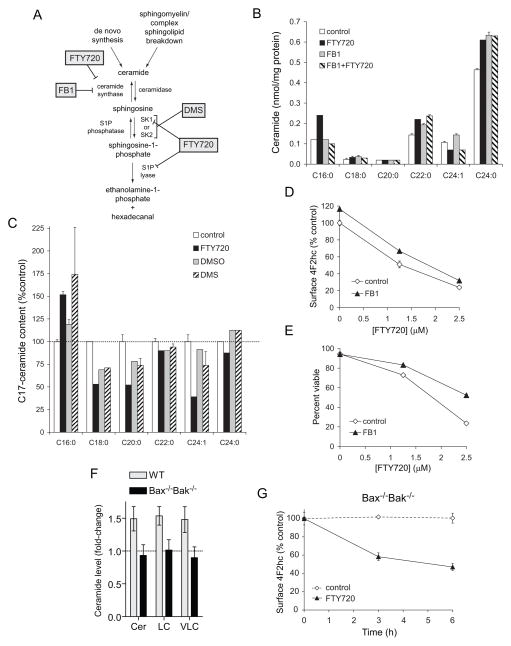
Ceramide down-regulates nutrient transporter proteins [18]. FTY720 inhibits both S1P lyase [45] and sphingosine kinase 1 [46], potentially increasing ceramide production from sphingosine via the salvage pathway (Figure 6A). We therefore hypothesized that FTY720 triggers nutrient transporter loss by increasing ceramide generation. Consistent with published studies [47], we found that FTY720 increased ceramide levels in intact cells (Supplementary Figure S5). Because FTY720 inhibits ceramide synthase (CerS) in vitro [47, 48], we tested whether FTY720 increased ceramide production through a CerS-dependent mechanism. The CerS inhibitor Fumonisin B1 blocked the production of C16:0 ceramide, but not C22:0 or C24:0 ceramide, in response to FTY720 (Figure 6B). To complement these inhibitor studies, we performed C17-sphingosine labeling experiments. Endogenous sphingosine is an 18-carbon amino alcohol. By supplementing the culture medium with low levels of sphingosine with a 17-carbon backbone, C17-sphingosine metabolism to ceramide can be followed by mass spectrometry due to the distinctive molecular mass of the products. This assay is an in situ CerS activity assay with the caveat that ceramidase running in reverse can also generate ceramide from the C17-sphingosine [49]. Consistent with the selective decrease in C16:0 ceramide with Fumonisin B1 (Figure 6B), FTY720 treatment increased the amount of C17-sphingosine converted into C16:0 ceramide but not C22:0 or C24:0 ceramide (Figure 6C). Thus, FTY720 increases ceramide levels via CerS-dependent and -independent mechanisms.
Figure 6. FTY720-induced nutrient transporter down-regulation does not require ceramide generation.
A) Effects of FTY720 and relevant inhibitors on sphingolipid metabolism. B) Ceramide production in FL5.12 cells treated with 5 μM FTY720 and/or 50 μM Fumonisin B1 (FB1) for 6 h was measured by mass spectrometry. C) FL5.12 cells were treated with 5 μM FTY720 or dimethylsphingosine (DMS, positive control) for 6 h. C17-sphingosine (100 nM) was added during the final 30 min of treatment. Ceramides containing the C17-sphingosine backbone were measured by mass spectrometry, expressed relative to total protein, and normalized to untreated controls. D,E) Surface levels of 4F2hc (D) or viability (E) was measured by flow cytometry after 24 h of treatment with FTY720 in the presence or absence of 50μM FB1. F) Ceramide levels were measured in Bax−/−Bak−/− and control hematopoetic cells incubated with 5 μM FTY720 for 6 h by mass spectrometry. Cer, total ceramides; LC, long-chain ceramides; VLC, very long-chain ceramides. G) Bax−/−Bak−/− hematopoetic cells were treated with 5 μM FTY720 and surface 4F2hc levels measured by flow cytometry at the indicated times. In all panels, n ≥ 3. Means +/− SD of triplicates from representative experiments are shown.
The effect of blocking ceramide production on FTY720-induced nutrient transporter loss and death was next determined. Fumonisin B1 provided limited protection from FTY720-induced transporter loss and cell death (Figure 6D–E) suggesting that C16:0 ceramide production contributes to but is not essential for these outcomes. To assess the contribution of C22:0 and C24:0 ceramide, we utilized Bax−/−Bak−/− cells. Although basal ceramide levels are within normal limits, cells lacking Bak fail to generate ceramide in response to acute stress [50]. Bax−/−Bak−/− cells did not generate ceramide in response to FTY720 treatment (Figure 6F). Despite the lack of ceramide production, FTY720 triggered transporter loss and death (Figures 3G & 6G). Nutrient transporter down-regulation was somewhat blunted in Bax−/−Bak−/− cells (Figures 1A vs. 6G). Thus, while not essential, ceramide generation may contribute to FTY720-induced nutrient transporter loss and cell death.
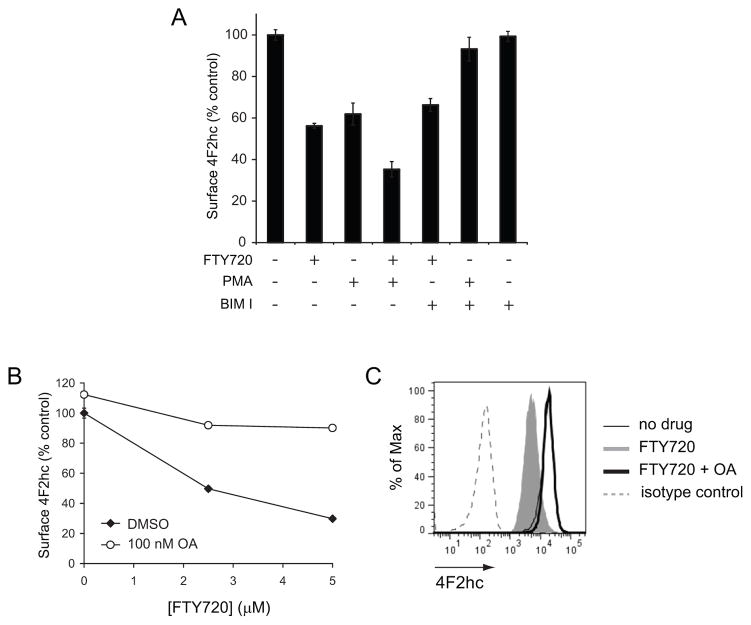
As ceramide generation could not completely explain the effects of FTY720 on transporters, we considered other mechanisms of action. The CAT-1 amino acid transporter is down-regulated in response to protein kinase C (PKC) activation by phorbol 12-myristate 13-acetate (PMA) [51, 52]. We therefore tested whether FTY720 regulated transporter expression through the same mechanism. PMA reduced surface 4F2hc levels to a similar degree as FTY720 (Figure 7A). However, the PKC inhibitor bisindolylmaleimide I blocked PMA- but not FTY720-induced 4F2hc down-regulation. These results suggest that FTY720 decreases transporter levels through a parallel pathway. Consistent with this model, the effects of FTY720 and PMA on transporters were additive (Figure 7A). Ceramide and FTY720 activate protein phosphatase 2A (PP2A) [23, 53]. PP2A activation has been proposed as part of the mechanism by which FTY720 kills cancer cells [23]. Consistent with an important role for PP2A in FTY720-induced death, the PP2A inhibitor okadaic acid blocked nutrient transporter loss (Figure 7B–C). Bax−/−Bak−/− cells that failed to generate ceramide in response to FTY720 were also protected from transporter loss by okadaic acid (data not shown). These results implicate PP2A and to a lesser degree ceramide in FTY720-induced transporter loss.
Figure 7. FTY720-induced nutrient transporter down-regulation does not depend on PKC activation but is sensitive to PP2A inhibition.
A) Surface 4F2hc levels were measured by flow cytometry in FL5.12 cells treated with 2.5 μM FTY720 and/or 100 nM PMA for 1 h. Where indicated, cells were pre-treated for 15 min with 1 μM bisindolylmaleimide I (BIM I). B) Surface 4F2hc levels were measured by flow cytometry in FL5.12 cells treated with the indicated concentration of FTY720 for 3 h in the presence or absence of 100 nM okadaic acid (OA). C) Representative histograms at 5 μM FTY720 from (B). In all panels, n ≥ 3. Means +/− SD of triplicates from representative experiments are shown.
FTY720-induced transporter loss is S1P receptor independent
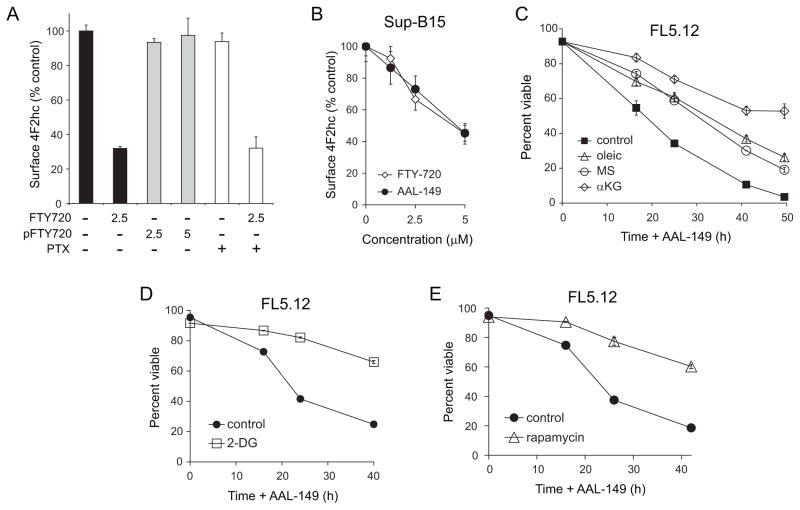
FTY720 cannot be used in human cancer patients because the anti-neoplastic dose slows heart rate by activating S1P receptors [54, 55]. However, the concentration of FTY720 required to trigger nutrient transporter loss is several logs above the EC50 for S1P receptors. We therefore predicted that nutrient transporter down-regulation was unrelated to and separable from S1P receptor activation. This idea was tested in several ways. FTY720 must be phosphorylated to activate S1P receptors [56]. However, phosphorylated FTY720 did not affect 4F2hc surface expression, slow proliferation, or kill cells (Figure 8A and data not shown). Similarly, pertussis toxin, which blocks a significant fraction of S1P receptor signaling [57], did not limit FTY720-induced transporter loss (Figure 8A). As a more definitive test, we evaluated AAL-149, an FTY720 analog that does not activate S1P receptors because it is not phosphorylated [58–60]. AAL-149 down-regulated nutrient transporter proteins with identical kinetics and potency to FTY720 (Figure 8B) without affecting growth factor receptor-dependent signal transduction (Figure 4E–F). Consistent with a model where death is secondary to nutrient transporter loss, the cell permeable nutrients oleic acid, dimethyl succinate, and dimethyl α-ketoglutarate reduced AAL-149-induced death in non-transformed FL5.12 cells (Figure 8C). In keeping with the idea that oncogenic mutations may lock cancer cells into particular metabolic pathways, only dimethyl α-ketoglutarate supplementation protected Sup-B15 leukemia cells (Supplementary Figure S6A). Also consistent with toxicity secondary to intracellular nutrient limitation and bioenergetic stress, AAL-149 killed apoptosis-deficient Bax−/−Bak−/− cells as efficiently as FTY720 in a cell permeable nutrient-sensitive manner (Supplementary Figure S6B). Stress response hormesis was also observed for AAL-149; pre-treatment with either 2-DG or rapamycin inhibited AAL-149-induced death (Figure 8D–E). These results with AAL-149 definitively demonstrate that the ability to induce nutrient transporter loss and cell death is separable from FTY720’s dose-limiting toxicity.
Figure 8. FTY720-induced nutrient transporter down-regulation is S1P receptor-independent.
A) Surface 4F2hc was measured by flow cytometry in FL5.12 cells treated with the indicated concentration of FTY720 (in μM) or phosphorylated FTY720 (pFTY720) for 4 h. Where indicated, 200 ng/ml pertussis toxin (PTX) was added. B) Surface 4F2hc was measured in human Sup-B15 leukemia cells after 3 h of treatment with the indicated concentrations of AAL-149 or FTY720. C) FL5.12 cells were treated with 2.5 μM AAL-149 in the presence or absence of 2 mM dimethyl-αketoglutarate (αKG), 10 μM BSA-conjugated oleic acid and 50 μM L-carnitine (oleic), or 11 mM dimethyl succinate (MS). D,E) FL5.12 cells were pre-treated with 1 mM 2-DG (D) or 100 nM rapamycin (E) for 24 h prior to exposure to 2.5 μM AAL-149. Viability was measured by vital dye exclusion and flow cytometry. In all panels, n ≥ 3. Means +/− SD of triplicates from representative experiments are shown.
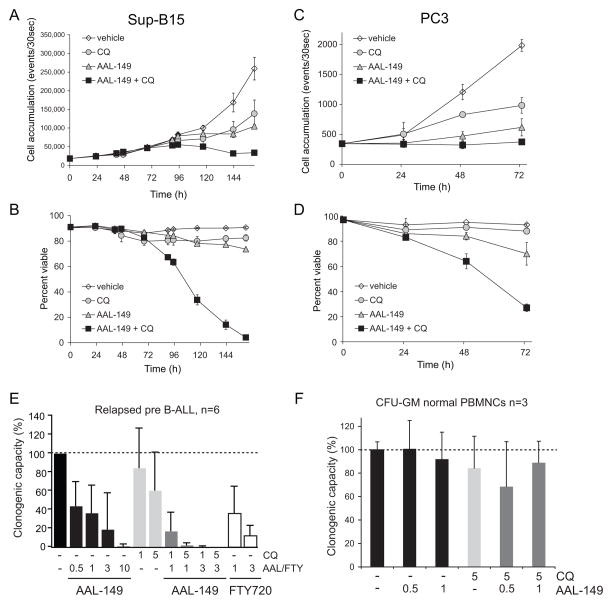
Autophagy is a protective response to nutrient transporter loss (Figure 2F and Supplementary Figure S1B) and tumor cells are often autophagy-deficient [5]. Thus, AAL-149 could be particularly effective when combined with a drug that interferes with autophagy. By inhibiting lysosomal acidification, chloroquine blocks the degradation of autophagosomes preventing nutrient liberation from the recycled material. Chloroquine is not a specific autophagy inhibitor, but it is a relatively safe drug that is already approved for use in humans and is being tested in multiple clinical trials in cancer patients [61]. Combining cytostatic concentrations of AAL-149 and chloroquine killed all Sup-B15 leukemia cells in the culture (Figure 9A–B). Synergy was also observed in PC3 prostate cancer cells (Figure 9C–D). Given these promising results in human cancer cell lines, AAL-149 and chloroquine were evaluated in colony assays with BCR-ABL-positive B-cell acute lymphoblastic leukemia cells isolated from 6 different patients who had relapsed after therapy. AAL-149 blocked colony formation by these primary leukemias as effectively as FTY720 (Figure 9E). As seen in cancer cell lines, combining AAL-149 with chloroquine produced synergy; in fact, adding chloroquine at just 1 μM was sufficient to reverse the resistance to 3 μM AAL-149 observed in one patient-derived sample that led to large error bars under this condition. Because normal cells have a greater autophagic capacity, we hypothesized that AAL-149 might retain FTY720’s selectivity for cancer cells even in combination with chloroquine. In fact, at concentrations that dramatically inhibited colony formation by leukemia cells, AAL-149 had no effect on granulocyte-macrophage colony formation from normal peripheral blood mononuclear cells even when combined with chloroquine (Figure 9E–F). Together, these results strongly suggest that drugs that decrease nutrient transporter levels will be effective and selective anti-cancer agents, particularly in combination with autophagy inhibitors.
Figure 9. AAL-149 and chloroquine synergize to selectively kill human cancer cells.
A,B) Cell accumulation (A) or viability (B) of Sup-B15 leukemia cells treated with 5 μM chloroquine (CQ) and/or 2.5 μM AAL-149 was measured by flow cytometry. C,D) Cell accumulation (C) or viability (D) of PC3 prostate cancer cells treated with 10 μM chloroquine (CQ) and/or 5 μM AAL-149 was measured by flow cytometry. E) Colony formation assays were performed in the presence or absence of AAL-149, FTY720, and/or chloroquine (CQ) (doses in μM) using leukemia cells isolated from 6 different patients with relapsed Philadelphia chromosome positive pre-B cell acute lymphoblastic leukemia (Ph+ B-ALL). The decrease in colonies is statistically significant at all AAL-149 doses (p<0.01 using a two-tailed t test); the decrease when 5 μM chloroquine is added to 1 μM AAL-149 is also significant (p<0.05). F) The formation of granulocyte/macrophage colonies by normal peripheral blood mononuclear cells (PBMNCs) isolated from normal human donors was measured in the presence or absence of AAL-149 and/or CQ (doses in μM). No sample is statistically different from control (p>0.05).
DISCUSSION
Drugs directed at almost every stage of biosynthesis are in or moving towards cancer clinical trials [62, 63], but none target nutrient transporter proteins. The work we present here suggests that sphingolipid-based drugs might be developed to trigger nutrient transporter loss and selectively starve cancer cells to death. Our previous studies indicated that ceramide down-regulates transporters for both amino acids and glucose and selectively kills highly anabolic cells [18]. However, the challenges associated with using ceramide, an extremely hydrophobic molecule, therapeutically are still being overcome. Here we show that FTY720, a water soluble, orally bioavailable sphingolipid with favorable pharmacologic properties limits nutrient transporter expression in vitro and in vivo. Because FTY720 down-regulates transporters for both amino acids and glucose, the development of resistance by switching to alternate fuels might be limited. Moreover, diverse forms of cancer with distinct fuel preferences should be susceptible, particularly when transporter loss is combined with autophagy inhibition. Given their novel mechanism of action, compounds that trigger transporter loss might also be combined with current cancer therapies. These drug combinations might be effective even in aggressive or multidrug resistant tumors such as the relapsed patient leukemias evaluated in Figure 9E.
One of the most striking findings is that drugs that profoundly down-regulate nutrient transporter proteins are highly selective for cancer cells and minimally toxic to normal, primary cells (Figures 5D & 9F and [23]). At the same time, non-transformed cell lines such as FL5.12 and MEFs are as sensitive to FTY720 and AAL-149 as their transformed counterparts. This apparent paradox can be explained by the fact that rapidly proliferating, tissue culture-adapted cell lines have a metabolic profile very similar to cancer cells, exhibiting high rates of glycolysis and protein and membrane synthesis following adaptation to culture conditions where growth factors and nutrients are virtually unlimited [41, 43, 44]. Although these rapidly proliferating cell lines are not locked into their anabolic program by oncogenic mutations, adaptive responses to acute transporter loss would have to be executed very quickly while the cells are under severe bioenergetic stress. When transporters are acutely down-regulated in these non-transformed cells proliferating at full speed, bioenergetic stress likely kills the cells before adaptive changes in metabolism can take place. Primary cells that are not growing as rapidly as FL5.12 cells or MEFs are likely to have more time to make adaptive changes to their metabolic program before a bioenergetic crisis ensues. Consistent with this model, adapting FL5.12 cells to low growth factor or nutrient levels afforded significant protection from FTY720- or AAL-149 induced death (Figures 4A–D and 8D–E).
FTY720 blocks cancer progression and metastasis in a wide variety of animal models, but its mechanism of action at the anti-neoplastic dose had not been determined. FTY720 was suggested by one group to kill cancer cells by activating the serine/threonine phosphatase PP2A which, through an undefined mechanism, was proposed to decrease the level of tyrosine phosphorylation of BCR-ABL and reduce BCR-ABL protein levels and signaling [23]. However, many of the cancer cell lines that are sensitive to FTY720-induced cell death do not express BCR-ABL [19–22, 24]. Moreover, we find that non-transformed, growth factor-dependent cells lines are just as sensitive to the drug as their BCR-ABL transformed counterparts. Our own and published data is instead consistent with a model where FTY720 kills cancer cells by inducing nutrient transporter loss and starvation. As in starving cells, reducing anabolism by pre-treating cells with rapamycin or 2-DG limits the cell death induced by FTY720- and AAL-149 (Figures 4C–D & 8D–E). These treatments do not protect cells from the loss of growth and survival signaling (Supplementary Figure S3A–B). On the other hand, disabling apoptosis efficiently blocks death in cells deprived of growth factor receptor signaling but did not protect cells from FTY720 and AAL-149 (Figures 3E–G & Supplementary Figure S6B). Because cell permeable nutrients rescued these apoptosis-deficient cells (Supplementary Figure S6B), fuel is the limiting factor. Our experiments using autophagy- or AMPK-deficient cells (Figure 2F–G and Supplementary Figure S1B), cell permeable nutrients (Figure 3A, Figure 8C, and Supplementary Figure S6A) and an inhibitor of raft-dependent endocytosis (Figure 3B–C) also strongly support our proposal that bioenergetic stress subsequent to nutrient transporter loss is the principal mechanism behind the selective toxicity of FTY720 and AAL-149 for cancer cells. PP2A could trigger nutrient transporter loss through effects on the actin cytoskeleton, an important player in the clathrin-independent endocytic pathway utilized by these transporters [64]. Alternatively, the Arf6 GTPase that controls the trafficking of these transporters is regulated by PP2A [31, 65, 66]. Now that we have uncovered this novel mechanism of action for FTY720 at the anti-neoplastic dose, future studies will further dissect the contributions of PP2A and Arf6 to sphingolipid- and AAL-149-induced nutrient transporter loss.
While FTY720 is a selective and effective anti-neoplastic agent in animal models, dose-limiting bradycardia prevents its use in human cancer patients. AAL-149 does not activate S1P receptors but down-regulates nutrient transporter proteins and selectively kills cancer cells with similar potency to FTY720 (Figures 8&9). This result clearly demonstrates that the dose-limiting toxicity and anti-cancer effects of FTY720 are separable. Pharmacokinetic and toxicity studies with AAL-149 and other analogs with similar properties will be important next steps. FTY720 is rapidly metabolized to FTY720 phosphate in vivo; phosphorylated FTY720 does not reduce nutrient transporter expression or kill cells (Figure 8A). As AAL-149 is not phosphorylated, lower doses may produce a similar degree of transporter loss. On the other hand, structural modifications may be necessary to improve AAL-149’s solubility or pharmacologic properties. Other sphingolipids, including sphingosine itself, have similar effects on nutrient transporter proteins as FTY720-based drugs ([18] and data not shown). However, the therapeutic potential of these lipids is limited by their hydrophobicity and by their conversion into active metabolites such as S1P. S1P receptor-inactive FTY720 analogs are thus superior therapeutic candidates. In conclusion, our results indicate that nutrient transporter down-regulation could be a feasible, effective, and novel approach to cancer therapy.
Supplementary Material
Acknowledgments
The authors thank Eunkyu Dong, Pinal Patel, and Garret Guenther for their contributions, Diane O’Dowd and Xicui Sun for help with electron microscopy, Marc Sitbon for generously sharing the GFP-HTLV-RBD construct and protein, Volker Brinkmann for providing phosphorylated FTY720 and AAL-149, and Ralph Deberardinis for suggestions and technical advice.
FUNDING: This work was supported by K08 CA100526, R01 GM089919, the Gabrielle’s Angel Foundation, the UC CRCC, and a SIIG from the UCI CORCL to ALE, F31 CA126494 to KRR, and NIH P20 RR17677, VA CDA-2, VA REAP, and ACS IRG 97-219-11 to LJS.
ABBREVIATIONS USED
- 2-DG
2-deoxy-D-glucose
- 4F2hc
4F2 heavy chain
- αKG
dimethyl alpha ketoglutarate
- AMPK
5′-AMP-activated kinase
- Bak
Bcl-2 homologous antagonist/killer
- Bax
Bcl-2-associated X protein
- Bcl-2
B-cell lymphoma 2
- BIM I
bisindolylmaleimide I
- CAT-1
high affinity cationic amino acid transporter 1
- CerS
ceramide synthase
- CQ
chloroquine
- DAPI
4′,6-diamidino-2-phenylindole
- DMS
dimethylsphingosine
- FB1
Fumonisin B1
- FCS
fetal calf serum
- GLUT1
glucose transporter 1
- IL-3
interleukin 3
- MEF
murine embryonic fibroblast
- MS
dimethyl succinate
- mTOR
mammalian target of rapamycin
- OA
okadaic acid
- PMA
phorbol 12-myristate 13-acetate
- PKC
protein kinase C
- PP2A
protein phosphatase 2A
- PTX
pertussis toxin
- S1P
sphingosine-1-phosphate
- TCA cycle
tricarboxylic acid cycle
- TfR
transferrin receptor
Footnotes
AUTHOR CONTRIBUTIONS: Kimberly Rosales, Gurpreet Singh, Kevin Wu, Aimee Edinger, and Eigen Peralta performed in vitro experiments with cell lines. Kimberly Romero and Matt Janes executed the leukemia studies in Figure 5. Michael Lilly performed the PBMNC experiments in Figure 9F and provided the human leukemia samples to Matt Janes who performed the colony assays in Figure 9E. Leah Siskind performed experiments measuring ceramide levels in Bax−/−Bak−/− cells and helped to design the experiments evaluating the contribution of ceramide production to FTY720’s ability to down-regulate transporters. Jie Chen, with input from Michael Bennett, quantified ceramide levels by mass spectrometry. All authors were involved in planning experiments and data analysis. Aimee Edinger conceived the project, prepared the figures, and wrote the paper. David Fruman edited the manuscript and helped to design the in vivo experiments and those using primary human cells.
References
- 1.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 3.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yuneva M, Zamboni N, Oefner P, Sachidanandam R, Lazebnik Y. Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells. J Cell Biol. 2007;178:93–105. doi: 10.1083/jcb.200703099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mathew R, White E. Autophagy in tumorigenesis and energy metabolism: friend by day, foe by night. Curr Opin Genet Dev. 2011;21:113–119. doi: 10.1016/j.gde.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elstrom RL, Bauer DE, Buzzai M, Karnauskas R, Harris MH, Plas DR, Zhuang H, Cinalli RM, Alavi A, Rudin CM, Thompson CB. Akt stimulates aerobic glycolysis in cancer cells. Cancer Res. 2004;64:3892–3899. doi: 10.1158/0008-5472.CAN-03-2904. [DOI] [PubMed] [Google Scholar]
- 7.Yun J, Rago C, Cheong I, Pagliarini R, Angenendt P, Rajagopalan H, Schmidt K, Willson JK, Markowitz S, Zhou S, Diaz LA, Jr, Velculescu VE, Lengauer C, Kinzler KW, Vogelstein B, Papadopoulos N. Glucose deprivation contributes to the development of KRAS pathway mutations in tumor cells. Science. 2009;325:1555–1559. doi: 10.1126/science.1174229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 10.Shaw RJ, Bardeesy N, Manning BD, Lopez L, Kosmatka M, DePinho RA, Cantley LC. The LKB1 tumor suppressor negatively regulates mTOR signaling. Cancer Cell. 2004;6:91–99. doi: 10.1016/j.ccr.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 11.Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 13.Choo AY, Kim SG, Vander Heiden MG, Mahoney SJ, Vu H, Yoon SO, Cantley LC, Blenis J. Glucose addiction of TSC null cells is caused by failed mTORC1-dependent balancing of metabolic demand with supply. Mol Cell. 2010;38:487–499. doi: 10.1016/j.molcel.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paez-Ribes M, Allen E, Hudock J, Takeda T, Okuyama H, Vinals F, Inoue M, Bergers G, Hanahan D, Casanovas O. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narta UK, Kanwar SS, Azmi W. Pharmacological and clinical evaluation of L-asparaginase in the treatment of leukemia. Crit Rev Oncol Hematol. 2007;61:208–221. doi: 10.1016/j.critrevonc.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 17.Kim RH, Coates JM, Bowles TL, McNerney GP, Sutcliffe J, Jung JU, Gandour-Edwards R, Chuang FY, Bold RJ, Kung HJ. Arginine deiminase as a novel therapy for prostate cancer induces autophagy and caspase-independent apoptosis. Cancer Res. 2009;69:700–708. doi: 10.1158/0008-5472.CAN-08-3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guenther GG, Peralta ER, Rosales KR, Wong SY, Siskind LJ, Edinger AL. Ceramide starves cells to death by downregulating nutrient transporter proteins. Proc Natl Acad Sci USA. 2008;105:17402–17407. doi: 10.1073/pnas.0802781105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee TK, Man K, Ho JW, Wang XH, Poon RT, Xu Y, Ng KT, Chu AC, Sun CK, Ng IO, Sun HC, Tang ZY, Xu R, Fan ST. FTY720: a promising agent for treatment of metastatic hepatocellular carcinoma. Clin Cancer Res. 2005;11:8458–8466. doi: 10.1158/1078-0432.CCR-05-0447. [DOI] [PubMed] [Google Scholar]
- 20.Azuma H, Takahara S, Ichimaru N, Wang JD, Itoh Y, Otsuki Y, Morimoto J, Fukui R, Hoshiga M, Ishihara T, Nonomura N, Suzuki S, Okuyama A, Katsuoka Y. Marked prevention of tumor growth and metastasis by a novel immunosuppressive agent, FTY720, in mouse breast cancer models. Cancer Res. 2002;62:1410–1419. [PubMed] [Google Scholar]
- 21.Chua CW, Lee DT, Ling MT, Zhou C, Man K, Ho J, Chan FL, Wang X, Wong YC. FTY720, a fungus metabolite, inhibits in vivo growth of androgen-independent prostate cancer. Int J Cancer. 2005;117:1039–1048. doi: 10.1002/ijc.21243. [DOI] [PubMed] [Google Scholar]
- 22.Azuma H, Takahara S, Horie S, Muto S, Otsuki Y, Katsuoka Y. Induction of apoptosis in human bladder cancer cells in vitro and in vivo caused by FTY720 treatment. J Urol. 2003;169:2372–2377. doi: 10.1097/01.ju.0000064938.32318.91. [DOI] [PubMed] [Google Scholar]
- 23.Neviani P, Santhanam R, Oaks JJ, Eiring AM, Notari M, Blaser BW, Liu S, Trotta R, Muthusamy N, Gambacorti-Passerini C, Druker BJ, Cortes J, Marcucci G, Chen CS, Verrills NM, Roy DC, Caligiuri MA, Bloomfield CD, Byrd JC, Perrotti D. FTY720, a new alternative for treating blast crisis chronic myelogenous leukemia and Philadelphia chromosome-positive acute lymphocytic leukemia. J Clin Invest. 2007;117:2408–2421. doi: 10.1172/JCI31095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yasui H, Hideshima T, Raje N, Roccaro AM, Shiraishi N, Kumar S, Hamasaki M, Ishitsuka K, Tai YT, Podar K, Catley L, Mitsiades CS, Richardson PG, Albert R, Brinkmann V, Chauhan D, Anderson KC. FTY720 induces apoptosis in multiple myeloma cells and overcomes drug resistance. Cancer Res. 2005;65:7478–7484. doi: 10.1158/0008-5472.CAN-05-0850. [DOI] [PubMed] [Google Scholar]
- 25.Janes MR, Limon JJ, So L, Chen J, Lim RJ, Chavez MA, Vu C, Lilly MB, Mallya S, Ong ST, Konopleva M, Martin MB, Ren P, Liu Y, Rommel C, Fruman DA. Effective and selective targeting of leukemia cells using a TORC1/2 kinase inhibitor. Nat Med. 2010;16:205–213. doi: 10.1038/nm.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manel N, Kim FJ, Kinet S, Taylor N, Sitbon M, Battini JL. The ubiquitous glucose transporter GLUT-1 is a receptor for HTLV. Cell. 2003;115:449–459. doi: 10.1016/s0092-8674(03)00881-x. [DOI] [PubMed] [Google Scholar]
- 27.McKearn JP, McCubrey J, Fagg B. Enrichment of hematopoietic precursor cells and cloning of multipotential B-lymphocyte precursors. Proc Natl Acad Sci USA. 1985;82:7414–7418. doi: 10.1073/pnas.82.21.7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Welsch CA, Roth LW, Goetschy JF, Movva NR. Genetic, biochemical, and transcriptional responses of Saccharomyces cerevisiae to the novel immunomodulator FTY720 largely mimic those of the natural sphingolipid phytosphingosine. J Biol Chem. 2004;279:36720–36731. doi: 10.1074/jbc.M406179200. [DOI] [PubMed] [Google Scholar]
- 29.Deves R, Boyd CA. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J Membr Biol. 2000;173:165–177. doi: 10.1007/s002320001017. [DOI] [PubMed] [Google Scholar]
- 30.Verrey F, Closs EI, Wagner CA, Palacin M, Endou H, Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. 2004;447:532–542. doi: 10.1007/s00424-003-1086-z. [DOI] [PubMed] [Google Scholar]
- 31.Eyster CA, Higginson JD, Huebner R, Porat-Shliom N, Weigert R, Wu WW, Shen RF, Donaldson JG. Discovery of new cargo proteins that enter cells through clathrin-independent endocytosis. Traffic. 2009;10:590–599. doi: 10.1111/j.1600-0854.2009.00894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu X, Silver J. Ecotropic murine leukemia virus receptor is physically associated with caveolin and membrane rafts. Virology. 2000;276:251–258. doi: 10.1006/viro.2000.0555. [DOI] [PubMed] [Google Scholar]
- 33.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jager S, Bucci C, Tanida I, Ueno T, Kominami E, Saftig P, Eskelinen EL. Role for Rab7 in maturation of late autophagic vacuoles. J Cell Sci. 2004;117:4837–4848. doi: 10.1242/jcs.01370. [DOI] [PubMed] [Google Scholar]
- 35.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–785. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Proud CG. Nutrient control of TORC1, a cell-cycle regulator. Trends Cell Biol. 2009;19:260–267. doi: 10.1016/j.tcb.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Rubin D, Ismail-Beigi F. Distribution of Glut1 in detergent-resistant membranes (DRMs) and non-DRM domains: effect of treatment with azide. Am J Physiol Cell Physiol. 2003;285:C377–383. doi: 10.1152/ajpcell.00060.2003. [DOI] [PubMed] [Google Scholar]
- 38.Zong WX, Ditsworth D, Bauer DE, Wang ZQ, Thompson CB. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004;18:1272–1282. doi: 10.1101/gad.1199904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pattingre S, Tassa A, Qu X, Garuti R, Liang XH, Mizushima N, Packer M, Schneider MD, Levine B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 40.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–5912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gems D, Partridge L. Stress-response hormesis and aging: “that which does not kill us makes us stronger”. Cell Metab. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Bauer DE, Harris MH, Plas DR, Lum JJ, Hammerman PS, Rathmell JC, Riley JL, Thompson CB. Cytokine stimulation of aerobic glycolysis in hematopoietic cells exceeds proliferative demand. FASEB J. 2004;18:1303–1305. doi: 10.1096/fj.03-1001fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edinger AL, Linardic CM, Chiang GG, Thompson CB, Abraham RT. Differential effects of rapamycin on mammalian target of rapamycin signaling functions in mammalian cells. Cancer Res. 2003;63:8451–8460. [PubMed] [Google Scholar]
- 45.Bandhuvula P, Tam YY, Oskouian B, Saba JD. The immune modulator FTY720 inhibits sphingosine-1-phosphate lyase activity. J Biol Chem. 2005;280:33697–33700. doi: 10.1074/jbc.C500294200. [DOI] [PubMed] [Google Scholar]
- 46.Vessey DA, Kelley M, Zhang J, Li L, Tao R, Karliner JS. Dimethylsphingosine and FTY720 inhibit the SK1 form but activate the SK2 form of sphingosine kinase from rat heart. J Biochem Mol Toxicol. 2007;21:273–279. doi: 10.1002/jbt.20193. [DOI] [PubMed] [Google Scholar]
- 47.Lahiri S, Park H, Laviad EL, Lu X, Bittman R, Futerman AH. Ceramide synthesis is modulated by the sphingosine analog FTY720 via a mixture of uncompetitive and noncompetitive inhibition in an Acyl-CoA chain length-dependent manner. J Biol Chem. 2009;284:16090–16098. doi: 10.1074/jbc.M807438200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berdyshev EV, Gorshkova I, Skobeleva A, Bittman R, Lu X, Dudek SM, Mirzapoiazova T, Garcia JG, Natarajan V. FTY720 inhibits ceramide synthases and up-regulates dihydrosphingosine 1-phosphate formation in human lung endothelial cells. J Biol Chem. 2009;284:5467–5477. doi: 10.1074/jbc.M805186200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spassieva S, Bielawski J, Anelli V, Obeid LM. Combination of C(17) sphingoid base homologues and mass spectrometry analysis as a new approach to study sphingolipid metabolism. Methods Enzymol. 2007;434:233–241. doi: 10.1016/S0076-6879(07)34012-3. [DOI] [PubMed] [Google Scholar]
- 50.Siskind LJ, Mullen TD, Romero Rosales K, Clarke CJ, Hernandez-Corbacho MJ, Edinger AL, Obeid LM. The BCL-2 protein BAK is required for long-chain ceramide generation during apoptosis. J Biol Chem. 2010;285:11818–11826. doi: 10.1074/jbc.M109.078121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rotmann A, Strand D, Martine U, Closs EI. Protein kinase C activation promotes the internalization of the human cationic amino acid transporter hCAT-1. A new regulatory mechanism for hCAT-1 activity. J Biol Chem. 2004;279:54185–54192. doi: 10.1074/jbc.M409556200. [DOI] [PubMed] [Google Scholar]
- 52.Vina-Vilaseca A, Bender-Sigel J, Sorkina T, Closs EI, Sorkin A. Protein kinase C-dependent ubiquitination and clathrin-mediated endocytosis of the cationic amino acid transporter CAT-1. J Biol Chem. 2011;286:8697–8706. doi: 10.1074/jbc.M110.186858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dobrowsky RT, Kamibayashi C, Mumby MC, Hannun YA. Ceramide activates heterotrimeric protein phosphatase 2A. J Biol Chem. 1993;268:15523–15530. [PubMed] [Google Scholar]
- 54.Sanna MG, Liao J, Jo E, Alfonso C, Ahn MY, Peterson MS, Webb B, Lefebvre S, Chun J, Gray N, Rosen H. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J Biol Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- 55.Koyrakh L, Roman MI, Brinkmann V, Wickman K. The heart rate decrease caused by acute FTY720 administration is mediated by the G protein-gated potassium channel I. Am J Transplant. 2005;5:529–536. doi: 10.1111/j.1600-6143.2005.00754.x. [DOI] [PubMed] [Google Scholar]
- 56.Brinkmann V, Lynch KR. FTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunity. Curr Opin Immunol. 2002;14:569–575. doi: 10.1016/s0952-7915(02)00374-6. [DOI] [PubMed] [Google Scholar]
- 57.Sanchez T, Hla T. Structural and functional characteristics of S1P receptors. J Cell Biochem. 2004;92:913–922. doi: 10.1002/jcb.20127. [DOI] [PubMed] [Google Scholar]
- 58.Brinkmann V, Davis MD, Heise CE, Albert R, Cottens S, Hof R, Bruns C, Prieschl E, Baumruker T, Hiestand P, Foster CA, Zollinger M, Lynch KR. The immune modulator FTY720 targets sphingosine 1-phosphate receptors. J Biol Chem. 2002;277:21453–21457. doi: 10.1074/jbc.C200176200. [DOI] [PubMed] [Google Scholar]
- 59.Butler J, Lana D, Round O, LaMontagne K. Functional characterization of sphingosine 1-phosphate receptor agonist in human endothelial cells. Prostaglandins Other Lipid Mediat. 2004;73:29–45. doi: 10.1016/j.prostaglandins.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 60.Sensken SC, Bode C, Graler MH. Accumulation of fingolimod (FTY720) in lymphoid tissues contributes to prolonged efficacy. J Pharmacol Exp Ther. 2009;328:963–969. doi: 10.1124/jpet.108.148163. [DOI] [PubMed] [Google Scholar]
- 61.Solomon VR, Lee H. Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol. 2009;625:220–233. doi: 10.1016/j.ejphar.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 62.Tennant DA, Duran RV, Gottlieb E. Targeting metabolic transformation for cancer therapy. Nat Rev Cancer. 2010;10:267–277. doi: 10.1038/nrc2817. [DOI] [PubMed] [Google Scholar]
- 63.Schnabel J. Targeting tumour metabolism. Nat Rev Drug Discov. 2010;9:503–504. doi: 10.1038/nrd3215. [DOI] [PubMed] [Google Scholar]
- 64.Zeidan YH, Jenkins RW, Hannun YA. Remodeling of cellular cytoskeleton by the acid sphingomyelinase/ceramide pathway. J Cell Biol. 2008;181:335–350. doi: 10.1083/jcb.200705060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Donaldson JG. Multiple roles for Arf6: sorting, structuring, and signaling at the plasma membrane. J Biol Chem. 2003;278:41573–41576. doi: 10.1074/jbc.R300026200. [DOI] [PubMed] [Google Scholar]
- 66.Robertson SE, Setty SR, Sitaram A, Marks MS, Lewis RE, Chou MM. Extracellular signal-regulated kinase regulates clathrin-independent endosomal trafficking. Mol Biol Cell. 2006;17:645–657. doi: 10.1091/mbc.E05-07-0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.