Abstract
UDP-D-apiose/UDP-D-xylose synthase (AXS) catalyzes the conversion of UDP-D-glucuronic acid to UDP-D-apiose and UDP-D-xylose. An acetyl-protected phosphonate analogue of UDP-D-apiose was synthesized and used in an in situ HPLC assay to demonstrate, for the first time, the ability of AXS to interconvert the two reaction products. Density functional theory calculations provided insight into the energetics of this process and the apparent inability of AXS to catalyze the conversion of UDP-D-xylose to UDP-D-apiose. The data suggest that this observation is unlikely to be due to an unfavorable equilibrium, but rather substrate inhibition by the most stable chair conformation of UDP-D-xylose. The detection of xylose cyclic phosphonate as the turnover product uncovers significant new detail about the AXS-catalyzed reaction and supports the proposed retroaldol-aldol mechanism of catalysis.
Keywords: UDP-D-apiose/UDP-D-xylose synthase, phosphonate substrate analogues, catalytic mechanism
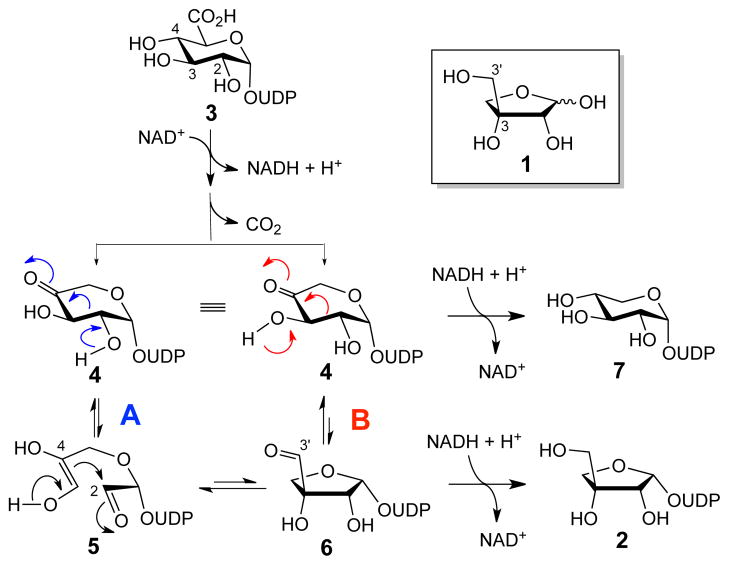
D-Apiose (1) is a rare branched-chain furanose found in the cell wall of plants.1 D-Apiose residues play an important role in plant growth and development by crosslinking cell wall polysaccharides through the formation of a borate tetraester.2 UDP-D-apiose (2), the in vivo D-apiose donor, is formed from UDP-D-glucuronic acid (UDP-GlcA, 3) in an unusual ring-contraction reaction catalyzed by UDP-D-apiose/UDP-D-xylose synthase (AXS). The conversion of 3 to 2 by AXS is NAD+-dependent and generates UDP-D-xylose (7) as a side product.3 The proposed reaction sequence for the formation of 2 (Scheme 1) involves oxidative decarboxylation of 3 to afford a 4-ketoxylose intermediate (UDP-4-KX, 4). Subsequent ring contraction (4 → 6) followed by reduction at C3′ by NADH yields the UDP-D-apiose product.
Scheme 1.
Proposed mechanisms for the AXS-catalyzed biosynthesis of UDP-D-apiose (2) and UDP-D-xylose (7) from UDP-GlcA(3).
Two plausible mechanisms for the ring contraction step have been proposed.4 In the first mechanism (Scheme 1, pathway A), the C2–C3 bond of 4 is cleaved in a retroaldol reaction, yielding an enediol intermediate (5) that cyclizes in an aldol reaction to give 6. In the second mechanism (Scheme 1, pathway B), 6 is formed from 4 directly through a 1,2-shift. In contrast to the stepwise retroaldol-aldol route, the C-C bond migration in this concerted mechanism would bypass the formation of a discrete intermediate such as 5. In both mechanisms, the formation of UDP-D-xylose (7) could result from a premature reduction of the decarboxylated 4-keto intermediate 4 by NADH prior to the rearrangement step.
One curious observation from previous studies is that AXS is unable to convert UDP-D-xylose (7) to UDP-D-apiose (2),5 suggesting that there is no mechanistic link between these two products and that each is formed irreversibly.5c However, testing this hypothesis is challenging due to the instability of UDP-D-apiose in aqueous solution,6 which makes it difficult to directly detect its formation in vitro. One potential strategy to circumvent this issue and gain insight into the mechanism of the AXS-catalyzed ring contraction may be to assay the reaction in the reverse and energetically favorable direction (2 to 7) using stable UDP-D-apiose analogues as the substrate. Accordingly, we designed and synthesized a phosphonate analogue of UDP-D-apiose (8, Figure 1) and examined its interaction with AXS. Compound 8 was chosen because phosphonates are well established as stable analogues of phosphates.7 In addition, density functional theory (DFT) calculations provided insight into the energetics of the AXS-catalyzed reaction. The results are described herein.
Figure 1.
UMP-phosphonate analogues of D-apiose (8) and D-xylose (9).
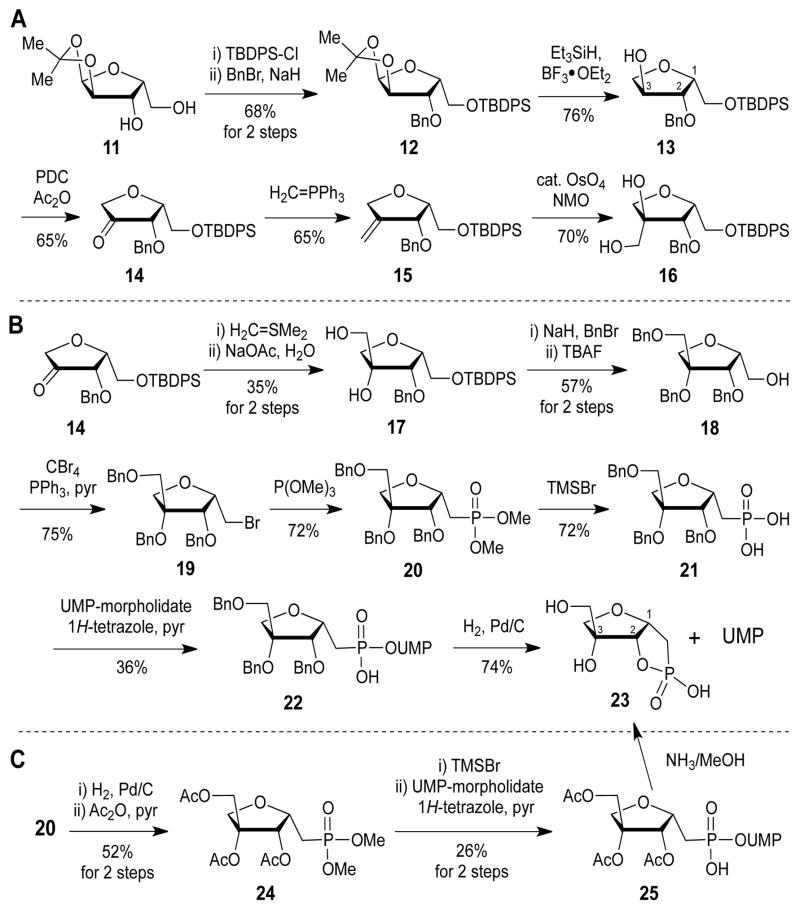
Because of the tertiary carbon centerat C3, the key step in the synthesis of D-apiose analogues is the introduction of the hydroxymethyl moiety. Several synthetic strategies (Scheme 2) were attempted to introduce the C3-methyl branch in the synthesis of the UMP-phosphonate analogue of D-apiose (8, Scheme 2). Starting from isopropylidene-protected xylofuranose (11), sequential protection of the free hydroxyl groups gave protected xylose (12). Deprotection of the isopropylidene group, reduction of the anomeric hydroxyl,8 and oxidation of the 3-hydroxyl moiety afforded a 3-keto-4-deoxy sugar (14). For the introduction of the hydroxymethyl moiety at C3, a Wittig reaction followed by a dihydroxylation protocol was attempted. However, careful NMR assignment (Figure S1A) and X-ray crystallographic analysis (Figure 2A) revealed that the stereochemistry of the product at C3 is opposite to that of the desired product. This result can be rationalized by considering that osmium tetroxide would approach from the less sterically hindered si-face during the hydroxylation step (Figure 2B).
Scheme 2.
Attempts to synthesize the phosphonate analogue of UDP-D-apiose (8).
Figure 2.
X-ray structure of 16 and explanation of the observed stereochemistry for the methylenation of 14 and dihydroxylation of 15.
Introduction of the hydroxymethyl group with the desired configuration was then attempted using ylide chemistry (Scheme 2B). As with OsO4, a sulfur ylide is expected to attack from the si-face. The NOESY NMR spectrum of the hydrolyzed product (17) revealed that it contained the desired configuration (Figure S1B). Consecutive protection of the hydroxyls with benzyl groups, deprotection of the TBDPS group, bromination with CBr4, and phosphonylation afforded a protected apiose phosphonate analogue (20). Hydrolysis of the methyl phosphonate to the corresponding phosphonic acid (21) and coupling with uridine-5′-monophosphate (UMP) morpholidate afforded a benzyl-protected UMP phosphonate analogue of D-apiose (22).9 However, after deprotection of the benzyl groups, a cyclic phosphonate (23) was obtained.
With the hope that this intramolecular cyclization problem could be overcome under mild basic conditions, a tri-O-acetyl protected UMP phosphonate analogue of D-apiose (25) was synthesized (Scheme 2C). However, even under mild conditions (0.2 M NH3 in MeOH, 0 °C), the cyclic phosphonate (23) and UMP were the only products obtained. A possible explanation for these results is that the 2-alkoxide ion formed during the reaction is suitably aligned to attack the vicinal phosphorous center, forming the thermodynamically stable 5-membered cyclic phosphonate with concomitant release of UMP, which is a good leaving group.
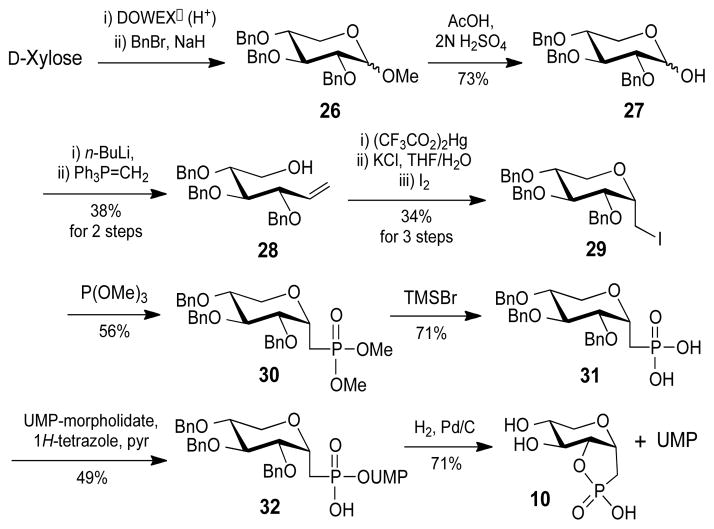
In addition to the phosphonate analogue of D-apiose, synthesis of a UMP phosphonate analogue of D-xylose (9) was also attempted for use as a product standard (Scheme 3). The reducing end of the benzyl-protected D-xylose (27) was converted into a linear alkene (28) by a Wittig reaction and subsequently transformed into iodomethyl compound 29 by a successive oxymercuration-iodination reaction. 10 Only the α-isomer of 29 was isolated, possibly due to an anomeric effect. Using a method similar to that applied for the synthesis of 22, the benzyl-protected UMP phosphonate analogue of D-xylose (32) was obtained. Hydrogenation to remove the benzyl groups also afforded the cyclic phosphonate compound (10) and UMP as the only products.
Scheme 3.
Synthesis of xylose cyclic phosphonate (10).
Although phosphonates are known to be more stable than the corresponding phosphates in terms of their anomeric bond strength, these results suggest that the P-OUMP bond of the phosphonate analogues can still be readily cleaved when the 2-OH group is well aligned for nucleophilic attack. One potential way to overcome the intrinsic instability of 8 is to generate it in situ during the assay of AXS activity. A coupled assay using an acetyl esterase and AXS was therefore designed in which 8 would be generated from the acetyl protected substrate (25). Orange peel acetyl esterase (AE, E.C. 3.1.1.6) was chosen because it is known to deacetylate various peracetylated NDP-sugars in good yield.11 The substrate 25 was incubated with AE for 24 h and shown by MS analysis to be deacetylated to the desired product (8) (see Supporting Information).
Having established that the phosphonate analogue of UDP-D-apiose (8) could be synthesized enzymatically from 25 using AE, an in situ HPLC assay of AXS activity was attempted using dual (UV-visible and Corona charged aerosol) detection.12 The later detection method is able to visualize non-volatile compounds regardless of whether they have a chromophore. As shown in Figure 3 the Corona HPLC traces of the AXS reaction mixtures (panels a and b) contain a broad peak between 11 and 13 min. Comparison with the HPLC traces of the individual reaction components show that NAD+ (panel e) and Tris buffer (panel f) both elute in this region. Xylose cyclic phosphonate (10), the product of the attempted synthesis of the phosphonate analogue of UDP-D-xylose, also elutes within this region (panel d). After a 47 h reaction period, a new peak with a retention time of 10 min is observed, which is consistent with the formation of a small amount of apiose cylic phosphonate (23) (panels b and c). In addition, a shoulder appears to form on the broad peak between 11 and 12 min.
Figure 3.
HPLC traces for the in situ AXS assay with dual detection (black line: Corona detector, red line: UV detector). (a) reaction mixture at 0 h, (b) reaction mixture at 47 h, (c) apiose cyclic phosphonate (23), (d) xylose cyclic phosphonate (10), (e) NAD+, (f) Tris.
To verify that this shoulder indicates the formation of 10, and thus the conversion of 8 to 9 by AXS, the peak was collected between 11 and 12.5 min and analyzed by mass spectrometry (MS). The MS data are indeed consistent with the peak containing both 10 ([M − 1]− = 209) and Tris ([M + 1]+ = 122) (see Supporting Information). To ensure that the species detected by MS corresponds to xylose cyclic phosphonate (10) and not contaminating apiose cyclic phosphonate (23), the isolated peak was fractionated using DEAE ion-exchange chromatography and each fraction was reanalyzed by HPLC. As shown in Figure 4, Tris (11 min) eluted in the H2O wash fractions (f1 and f2), whereas xylose cyclic phosphonate (12 min) eluted at high ammonium bicarbonate concentrations (f14 – f18). There was no evidence for the presence of 23 (10 min) in any of the fractions. These results clearly show that the broad peak indeed contains 10 and indicate that AXS can catalyze the conversion of the phosphonate analogue of UDP-D-apiose (8) to the corresponding UDP-D-xylose analogue (9), which degrades into 10.
Figure 4.
HPLC traces of the isolated 11–12.5 min peak using DEAE ion exchange resin chromatography. For detailed experimental procedures, see Supporting Information.
The observation that AXS can catalyze the reverse reaction, the conversion of the phosphonate analogue of UDP-D-apiose (8) to the corresponding UDP-D-xylose analogue (9), contrasts with the previous result that UDP-D-xylose (7) could not be converted by AXS to UDP-D-apiose (2).5 To gain insight into these different reactivities, the energetics of the reaction were examined by density functional theory (DFT) calculations using the Gaussian 98 software package.13 The calculations (performed at the B3LYP/6-311+G(d,p) level of theory using a scaling factor of 0.9877 and the polarizable continuum model to mimic solvent) show that in aqueous solution, the chair conformation of D-xylose with the hydroxyl groups in the equatorial position (e.g., 7) is only 0.4 kcal/mol more stable than D-apiose (e.g., 2). Such a small difference in free energy is unable to account for the apparent inability of AXS to convert 7 to 2. Surprisingly, however, it was found that the alternative chair conformation of D-xylose, with the hydroxyl groups in the axial position (e.g., 33), is 1.6 kcal/mol more stable than D-apiose.
The less stable conformer of UDP-D-xylose (7) is expected to bind to AXS in a manner analogous to the substrate UDP-GlcA (3), where the 4-H is properly oriented for transfer to NAD+ (Scheme 4). In contrast, the more stable conformer of UDP-D-xylose (33) overlays quite well with the UDP-D-apiose product (2) (Scheme 4, inset). In this binding mode, the catalytic base required for the oxidation of the hydroxymethyl moiety of 2 would interact with the C3 instead of the C4 hydroxyl of 33. Thus, this conformer of UDP-xylose (33) would not be positioned for efficient hydride transfer to NAD+ and its binding to AXS would result in the formation of a dead-end complex. The combined observations give a possible explanation for the apparent inability of AXS to catalyze the conversion of 7 to 2: the former can adopt two conformations in solution where the more stable one inhibits the enzyme. This inhibition may have prevented the detection of 2 under the conditions and time-scale used in the previous studies.5
Scheme 4.
Energy differences between the two conformers of UDP-D-xylose. The less stable conformer(7) can bind productive and be converted to UDP-D-apiose, while the more stable conformation (33) forms a dead-end complex. Inset: Overlay of the most stable conformer of xylose (33, green) and apiose (2, grey).
The detection of xylose cyclic phosphonate (10) instead of 9 as the product of the incubation of 8 with AXS is another significant finding. Since compound 10 must be derived from 9 via nucleophilic attack at the vicinal phosphorous center by the 2-alkoxide anion, the above observation strongly suggests that deprotonation of the 2-OH group occurs during the conversion of UDP-D-apiose (2) to UDP-D-xylose (7). Of the two mechanisms proposed for AXS, only the cleavage of the C2–C3 bond of 4 in a retroaldol process requires the ionization of the 2-OH group (4 → 5, Scheme 1, pathway A). In contrast, the 1,2-shift mechanism (Scheme 1, pathway B) requires deprotonation of the C3 hydroxyl group (4 → 6). Taken together, the current data support the stepwise retroaldol-aldol route as the catalytic mechanism of AXS.
In summary, a protected phosphonate analogue of UDP-D-apiose (8) was synthesized for use in mechanistic studies of AXS. To overcome the intrinsic instability of 8 during chemical synthesis, a coupled assay was developed in which this substrate was generated in situ using acetyl esterase. The data show that AXS can accept phosphonate in addition to the natural phosphate substrates. More importantly, our results reveal that AXS converts 8 into the corresponding UDP-D-xylose analogue (9), which is inconsistent with the assertion that there is no mechanistic link between the biosynthesis of the two products (2 and 7) of the AXS-catalyzed reaction with UDP-GlcA (3).5c Insight into the apparent inability of AXS to convert 7 to 2 was obtained from DFT calculations, which suggest that this observation is unlikely to arise from the endergonicity of the reaction. Instead, it was found that 7 can exist in two stable chair conformations in aqueous solution, the more favorable of which is likely to bind unproductively to AXS and inhibit its activity. This product inhibition of the AXS-catalyzed reaction by UDP-D-xylose may play a physiological role in the regulation of plant cell wall biogenesis. Moreover, the isolation of 10 instead of 9 as the turnover product of 8 with AXS provides strong evidence supporting a retroaldol-aldol mechanism for this intriguing enzyme.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM035906 and GM054346) and the Welch Foundation (F-1511).
Footnotes
Supporting Information. Materials and methods. Spectroscopic and structural data. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.(a) Ishii T, Matsunaga T, Pellerin P, O’Neill MA, Darvill A, Albersheim P. J Biol Chem. 1999;274:13098. doi: 10.1074/jbc.274.19.13098. [DOI] [PubMed] [Google Scholar]; (b) O’Neill MA, Ishii T, Albersheim P, Darvill AG. Annu Rev Plant Biol. 2004;55:109. doi: 10.1146/annurev.arplant.55.031903.141750. [DOI] [PubMed] [Google Scholar]; (c) Beck EZ. Pflanzenphysiol. 1967;57:444. [Google Scholar]; (d) Longland JM, Fry SC, Trewavas AJ. Plant Physiol. 1989;90:972. doi: 10.1104/pp.90.3.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.O’Neill MA, Eberhard S, Albersheim P, Darvill AG. Science. 2001;294:846. doi: 10.1126/science.1062319. [DOI] [PubMed] [Google Scholar]
- 3.(a) Picken JM, Mendicino J. J Biol Chem. 1967;242:1629. [PubMed] [Google Scholar]; (b) Mendicino J. Biochim Biophys Acta. 1974;364:159. doi: 10.1016/0005-2744(74)90143-0. [DOI] [PubMed] [Google Scholar]; (c) Pan YT, Kindel PK. Arch Biochem Biophys. 1977;181:131. doi: 10.1016/0003-9861(77)90427-1. [DOI] [PubMed] [Google Scholar]
- 4.Choi S-h, Ruszczycky MW, Zhang H, Liu H-w. Chem Comm. 2011;47:10130. doi: 10.1039/c1cc13140k. [DOI] [PubMed] [Google Scholar]
- 5.(a) Baron D, Wellmann E, Grisebach F. Biochim Biophys Acta. 1972;258:310. doi: 10.1016/0005-2744(72)90988-6. [DOI] [PubMed] [Google Scholar]; (b) Baron D, Grisebach F. Eur J Biochem. 1973;38:153. [Google Scholar]; (c) Mølhøj M, Verma R, Reiter WD. Plant J. 2003;35:693. doi: 10.1046/j.1365-313x.2003.01841.x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Kindel PK, Watson RR. Biochem J. 1973;133:277. doi: 10.1042/bj1330227. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guyett P, Glushka J, Gu X, Bar-Peled M. Carbohydr Res. 2009;344:1072. doi: 10.1016/j.carres.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Du Y, Linhardt RJ. Tetrahedron. 1998;54:9913. [Google Scholar]; (b) Uchiyama T, Vassilev VP, Kajimoto T, Wong W, Huang H, Lin C-c, Wong C-H. J Am Chem Soc. 1995;117:5395. [Google Scholar]; (c) Compain P, Martin OR. Bioorg Med Chem. 2001;9:3077. doi: 10.1016/s0968-0896(01)00176-6. [DOI] [PubMed] [Google Scholar]; (d) Zhao Z, Hong L, Liu H-w. J Am Chem Soc. 2005;127:7692. doi: 10.1021/ja042702k. [DOI] [PubMed] [Google Scholar]
- 8.Ewing GJ, Robins MJ. Org Lett. 1999;1:635. [Google Scholar]
- 9.Wittmann V, Wong CH. J Org Chem. 1997;62:2144. doi: 10.1021/jo9620066. [DOI] [PubMed] [Google Scholar]
- 10.Garneau S, Qiao L, Chen L, Walker S, Vederas JC. Bioorg Med Chem. 2004;12:6473. doi: 10.1016/j.bmc.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 11.(a) Baisch G, Ohrlein R. Bioorg Med Chem. 1997;5:383. doi: 10.1016/s0968-0896(96)00258-1. [DOI] [PubMed] [Google Scholar]; (b) Waldmann H, Heuser A. Bioorg Med Chem. 1994;2:477. doi: 10.1016/0968-0896(94)80017-0. [DOI] [PubMed] [Google Scholar]
- 12.Tris buffer (11.8 mM) was used in the assay even though it coelutes with 10 under the HPLC conditions used (14 mM NH4OAc, isocratic elution) because AXS showed the best activity in this buffer (e.g. no AXS activity could be detected in phosphate buffer).
- 13.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Jr, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Gonzalez C, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Head-Gordon M, Replogle ES, Pople JA. Gaussian 98. A.9. Gaussian, Inc; Pittsburgh, PA: 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.