SUMMARY
Mammalian A2-adenosine receptor binding subunits (A2AR) can be visualized by covalent labeling with the photoaffinity cross-liking ligand 125I-2-[4-[2-[2-[(4-aminophenyl)methylcarbonyl-amino]ethylaminocarbonyl]ethyl]phenyl]ethylamino-5′-N-ethyl-carboxamidoadenosine or directly with the azide derivative described in this paper. The protein comprising the A2-adenosine receptor binding subunit migrates with a Mr of 45,000 on sodium dodecyl sulfate-polyacrylamide gel electrophoresis. In this study, the glycoproteins representing the radiolabeled A1- and A2-adenosine receptor binding subunit from bovine brain were compared by partial peptide maps and following treatment with exo- and endoglycosidases. Peptide maps using two separate proteases reveal that the A1- and A2-adenosine receptor binding subunits share no common peptide fragments by two-dimensional gel electrophoresis. Endoglycosidase F treatment of labeled A2AR results in a single labeled peptide of Mr 38,000 without intermediate peptides, suggesting a single N-linked carbohydrate chain. The labeled A2AR demonstrates a sensitivity to neuraminidase, as evidenced by an increased mobility on gel electrophoresis, suggesting the receptors contain a glycan component containing terminal sialic acid. Treatment of the labeled A2AR with α-mannosidase reveals two distinct populations of A2ARs, one of which is sensitive and the other resistant to the enzyme. The nonadditivity of sequential treatments with the two exoglycosidases suggests, a heterogeneous population of A2AR containing either complex- or high mannose-type carbohydrate chains. These data suggest the A2AR is a Mr 45,000 glycoprotein with a single carbohydrate chain of either the complex or high mannose type. In addition, the A1- and A2ARs are distinct glycoproteins, as evidenced by their differing molecular weights (before and after deglycosylation) and distinct peptide maps.
Adenosine is a widely distributed nucleoside that mediates a variety of physiological responses such as central nervous system sedation, inhibition of platelet aggregation, and vascular smooth muscle vasodilatation. These effects occur largely through the interaction of adenosine with either the A1-adenosine receptor, which is inhibitory to adenylate cyclase and exhibits the potency order (R)-PIA > NECA > (S)-PIA, or the A2-adenosine receptor, which is stimulatory to adenylate cyclase and displays a distinctly different potency order, where NECA is more potent than (R)-PIA, which is more potent still than (S)-PIA (1).
The A1-adenosine receptor binding subunit, like the β-adrenergic and many other membrane receptors, has been shown to be a glycoprotein (2, 3). Removal of the carbohydrate moiety from the photoaffinity-labeled A1-adenosine receptor binding subunit results in a decrease in the apparent molecular weight of the photolabeled receptor binding subunit from Mr 38,000 to Mr 32,000 on SDS-PAGE (3). This increase in mobility (i.e., decrease in Mr) following endo- and exoglycosidase treatment is thought to be attributable not only to the removal of the carbohydrate moiety (molecular mass effect) but also to changes in the receptor charge density due to alterations in the binding of SDS to the receptor protein (2, 3).
In contrast to the relatively well characterized A1-adenosine receptor, very little is known about the nature of the A2-adenosine receptor, largely due to the dearth of selective high affinity A2-adenosine receptor radioligands and photoaffinity probes. Recently, [3H] CGS 21680 (4) and 125I-PAPA-APEC (5) have been shown to be selective high affinity agonist A2-adenosine receptor radioligands. Photoaffinity cross-linking studies with 125I-PAPA-APEC identified the A2 binding subunit as a Mr 45,000 protein (on SDS-PAGE) that was clearly distinct from the Mr 38,000 A1-adenosine receptor binding subunit.
We have now synthesized the azide derivative of 125I-PAPA-APEC and demonstrated that this direct photoaffinity radioligand labels the same Mr 45,000 band previously identified with the photoaffinity cross-linking probe 125I-PAPA-APEC, but at a nearly 3-fold greater efficiency of photoincorporation. Utilizing this direct A2-adenosine receptor photoaffinity probe, we have demonstrated that the A2 binding subunit is a glycoprotein and is clearly different from the A1-adenosine receptor binding subunit. Furthermore, in bovine striatal membranes, A2-adenosine receptors exist as a heterogeneous population containing either a single complex or a single high mannose-type carbohydrate chain.
Experimental Procedures
Materials
Benzamidine, chloramine T, EDTA, HEPES, leupeptin (L-2884), α-mannosidase (from jack beans; M-7257), MgCl2, neuraminidase (type X from Clostridium perfringens; N-2138), phenylmethylsulfonyl fluoride, sodium azide, sodium nitrite, soybean trypsin inhibitor (T-9003), Staphylococcus aureus V8 protease (P-8400), theophylline, Tris, and Triton X-100 were obtained from Sigma Chemical Co. (St. Louis, MO). ADA and endoglycosidase F (878 740) were purchased from Boehringer Mannheim (Indianapolis, IN). APNEA was a generous gift from Dr. Ray Olsson (University of South Florida) and [125I] AZPNEA was synthesized from APNEA by one of the authors (W.W.B.) as previously reported (6). SANPAH was purchased from Pierce Chemical Co. (Rockford, IL). Na125I (carrier-free) was obtained from Amersham Corp. (Arlington Heights, IL). All other reagents were of the highest available grade and were purchased from standard sources.
Preparation of bovine striatal membranes
The striatal membrane preparation has been recently described (5). Briefly, the striatum was excised from a fresh bovine brain, placed in 20 ml of ice-cold 50 mm Tris (pH 8.26 at 5°), minced, and gently disrupted with a motor-driven Teflon pestle. This suspension was centrifuged at 43,000 × g for 10 min, resuspended in 20 ml of Tris buffer, and recentrifuged at 43,000 × g for an additional 10 min. The final pellet was suspended in sufficient buffer to yield a final concentration of 200 mg of wet striatal tissue/ml. When stored at −70, the frozen membranes were stable for at least 1 month.
Synthesis of 125I-azido-PAPA-APEC
The detailed synthesis of PAPA-APEC will be reported elsewhere.1 Briefly, the parent compound PAPA-APEC was iodinated by the chloramine T method, as previously described (7, 8). The 125I-PAPA-APEC was completely separated from the starting materials by HPLC, using a gradient protocol (curve 8) on a Waters model 680 automated gradient controller with a Waters C18 μ-Bondapak column. The mobile phase was initially composed of 60% methanol and 40% 20 mm ammonium formate (pH 7.8) and attained a final concentration (at 10 min) of 50% methanol and 50% 20 mm ammonium formate.
The 125I-PAPA-APEC emerged as a radioactive peak at ~7 min and was the radioligand previously described in binding and cross-linking experiments (5). This fraction (in the HPLC mobile phase) was then dried completely by lyophilization (typically requiring 4 hr), and the residue was dissolved in 10 µl of 6 N acetic acid with 10 µl of water. This solution was placed on ice and 20 µl of ice-cold sodium nitrite (20 mg/ml of water) were added and allowed to react for 10 min. At the end of this time, the solution was placed in subdued lighting, 10 µl of ice-cold sodium azide (5 mg/ml) were added, and the entire mixture was allowed to react for 5 min on ice. An additional 10 µl of the azide solution were then added, and the solution was removed from ice for a final 5 min of reaction time. Eight microliters of ammonium hydroxide were added to alkalinize the reaction mixture, and this was then injected onto the HPLC column for separation.
The 125I-azido-PAPA-APEC was separated from the other reaction products by an isocratic protocol using a 75% methanol/25% 20 mm ammonium formate (pH 7.8) mobile phase. The azide emerged as a radioactive peak at ~6 min. Thin layer chromatography (85:10:5 chloroform/methanol/glacial acetic acid) confirmed the product purity and the fact that PAPA-APEC (Rf = 0.11) was distinct from 125I-PAPA-APEC (Rf = 0.22) and 125I-azido-PAPA-APEC (Rf = 0.34). Each of the purified radioligands was assumed to have a specific activity of 2200 Ci/mmol.
125I-Azido-PAPA-APEC binding
One-milliliter aliquots of frozen striatal membranes were thawed and suspended in 9 ml of buffer containing 50 mm HEPES and 10 mm MgCl2 adjusted to pH 7.2 (hereafter referred to as 50/10 buffer), with 0.2 units/ml ADA and 0.01% (w/v) CHAPS (added to reduce nonspecific binding).
All binding experiments were performed in subdued lighting, in foil-wrapped polypropylene tubes. The total reaction volume of 250 µl was composed of 150 µl of the membrane suspension (~75 µg of protein), 50 µl of water or competitor (5 mm theophylline), and 50 µl of diluted ligand. Competition curves for both 125I-azido-PAPA-APEC and 125I-PAPA-APEC were performed using 0.5–0.75 nM levels of radioligand and five to eight different concentrations of competitors, ranging from 10−9 to 10−3 M, depending on the competitor. Specific binding represented 50–60% of the total binding at the concentrations of radioligand used. Binding of both radioligands is reversible in the absence of UV light, as expected. Total binding in these experiments was approximately 75,000 cpm in the absence of competitor. The suspension was incubated for 1 hr at 37°, which was sufficient time to ensure the attainment of equilibrium binding.
After incubation, the contents of each tube were filtered over glass filters (Schleicher & Schuell no. 32, treated for 1 hr in 0.3% polyethelenimine) and washed three times with 3-ml aliquots of ice-cold 50/10 buffer containing 0.05% (w/v) CHAPS. The filters were placed in polypropylene tubes and counted in a Packard γ-counter.
Binding studies with 125I-PAPA-APEC were performed in the manner previously outlined (5).
Photoaffinity labeling
One-milliliter aliquots of frozen striatal membranes were thawed, suspended in 15 ml of 50/10 buffer with 0.4 units/ml ADA, and incubated at 37° for 15 min. After centrifugation at 43,000 × g for 5 min, the pellet was resuspended in 15 ml of 50/10 buffer containing 0.01% (w/v) CHAPS and 0.2 units/ml ADA. Labeling was performed in foil-wrapped tubes in a total volume of 1 ml, consisting of 0.8 ml of the membrane suspension (250–300 µg of protein), 0.2 ml of water (control) or competitor, and ~0.8 nM final concentration of 125I-azido-PAPA-APEC. After a 1-hr incubation at 37°, the membranes were placed on ice and washed once with ice-cold 50/10 buffer containing 0.03% (w/v) CHAPS and once with ice-cold 50/10 buffer containing 0.01% (w/v) CHAPS, before being resuspended in 1 ml of 50/10 buffer with 0.01% CHAPS in preparation for photoincorporation. The samples were centrifuged at 43,000 × g for 5 min at the end of each wash.
The 125I-azido-PAPA-APEC/membrane suspension was then poured into an iced Petri dish and exposed to UV light (model UVCG-25 mineral light) for 4 min at a distance of 1 cm. The photolyzed suspension was washed once with 50/10 buffer containing 0.05% CHAPS, centrifuged at 43,000 × g for 5 min, washed again with plain 50/10 buffer, and centrifuged a final time at 43,000 × g for 5 more min before being prepared for deglycosylation or SDS-PAGE.
The striatal membranes for [125I]AZPNEA (A1-adenosine receptor binding subunit) labeling were prepared and labeled exactly as above, except that the 50/10 buffer was replaced with a buffer consisting of 50 mm Tris, 10 mm MgCl2, and 1 mm EDTA, adjusted to pH 8.26 at 5°. Additionally, CHAPS was not added to the incubation buffer but was used in the wash buffer. This labeling procedure has been previously described for bovine brain membranes (3).
Endoglycosidase treatment
The endoglycosidase F treatment follows the procedure originally described for the A1-adenosine receptor (3). Briefly, a 1-ml aliquot of the 125I-aside-PAPA-APEC-labeled membrane suspension was sedimented at 43,000 × g for 5 min, and the resulting pellet was solubilized in 500 µl of pH 6.5 buffer composed of 100 mm NaHPO4, 50 mm EDTA, 0.8% (v/v) Triton X-100, and a proteinase inhibitor cocktail (10−4 m phenylmethylsulfonyl fluoride, 10−4 m benzamidine, 5 µg/ml soybean trypsin inhibitor, and 5 µg/ml leupeptin, all final concentrations) that has been shown to be effective in inhibiting proteolysis in other receptor systems (9).
Five units/ml endoglycosidase F was added to the solubilized 125I-azido-PAPA-APEC-labeled membranes, and the suspension was shake-incubated at 37° for the indicated time (typically 5 hr). At the end of this time, the sample was desalted on a Sephadex G50 column to exchange the sample buffer to one composed of 0.2% SDS and 10 mm Tris at pH 6.8. The eluted sample was frozen in liquid nitrogen and lyophilized overnight. The lyophilized residue was then prepared for SDS-PAGE.
Control samples were prepared exactly as above, except that endoglycosidase F was not added to the solubilized suspension.
Exoglycosidase treatments
Each exoglycosidase treatment utilized a 1-ml aliquot of the 125I-azido-PAPA-APEC-labeled membrane suspension (containing the full complement of proteinase inhibitors), in a manner analogous to those described for previous A1-adenosine receptor deglycosylation experiments (3).
Neuraminidase treatment was begun by washing of 1 ml of the labeled membranes with buffer consisting of 100 mm sodium acetate, 5 mm EDTA, and the above proteinase inhibitor cocktail, adjusted to pH 5 at 25°. After centrifugation at 43,000 × g for 5 min, the pellet was resuspended in 1 ml acetate buffer, 2 units of neuraminidase were added, and the entire sample was shake-incubated at 37° for 6 hr. Ensymatic digestion was halted by dilution of the sample in 40 volumes of 50/10 buffer at pH 7.2. The suspension was sedimented at 43,000 × g for 5 min, and the pellet was finally solubilized in SDS sample buffer.
Control samples were prepared exactly as outlined above, except that neuraminidase was not added to the solubilized suspension.
α-Mannosidase treatment began with a 1-ml aliquot of the photoaffinity-labeled membranes, washed and resuspended in buffer composed o 50 mm sodium citrate, 5 mm EDTA, and the proteinase inhibitor cocktail, adjusted to pH 4.5. Four units/ml α-mannosidase was added to the membrane/ligand suspension and the entire sample was shake-incubated at 25° for 24 hr before being washed with pH 7.2 50/10 buffer, centrifuged at 43,000 × g for 5 min, and solubilized in SDS sample buffer for SDS-PAGE.
The sequential application of these enzymes was performed beginning with the neuraminidase treatment (as outlined above), except, instead of the final pellet being solubilized in SDS buffer, it was washed and suspended in sodium citrate buffer and treated as outlined in the α-mannosidase digestion.
SDS-PAGE
Electrophoresis was performed according to the standard methods outlined by Laemmli (10), in homogeneous slabs of 12%, 15%, or 18% acrylamide.
All samples were solubilized for 45 min in sample buffer containing 10% SDS, 10% glycerol, 25 mm Tris-HCl, and 5% β-mercaptoethanol, adjusted to pH 6.8 at 25°.
After electrophoresis, the gels were dried and exposed to Kodak XAR-5 film with dual intensifying screens for 48 to 72 hr.
Limited proteolysis in SDS-polyacrylamide gels
Limited proteolysis was performed as previously outlined (7, 11, 12). Briefly, the labeled receptor was initially subjected to electrophoresis on a 12% polyacrylamide separating gel. The region of the wet gel containing the labeled receptor was then excised and electrophoresed in the second dimension on a higher percentage (~18% acrylamide) 105-mm separating gel with 35-mm stacking gel. The excised gel section was bonded to the second-dimension stacking gel with 1% agarose, and the upper sample well was fitted with 1 ml of buffer containing the appropriate enzyme, 2% SDS, 10% glycerol, and 50 mm Tris-HCl, adjusted to pH 6.8 at room temperature.
The two-dimensional gels were then dried and subjected to autoradiography as above.
Protein determinations
The protein content of samples was determined by the method of Bradford (13).
Analysis of data
Saturation and competitive binding data were analyzed by a previously described and validated nonlinear least-squares computer algorithm (14). Averages are expressed as means ± standard deviations.
Densitometric analysis of the autoradiographs was performed with a Bio-Rad model 620 video densitometer, using Bio-Rad computer software on an IBM PC/AT computer.
Results
Characterization of 125I-azido-PAPA-APEC
The conversion of amine precursor radioligands to arylazide direct photoaffinity probes has been successfully employed for a variety of receptor ligands (6, 7). Typically, the radioligand retains all essential properties, with agonist ligands remaining agonists after conversion to an arylazide (6). Not surprisingly, the conversion of 125I-PAPA-APEC (the amine precursor) to 125I-azido-PAPA-APEC (the arylazide) has produced a direct photoaffinity probe that exhibits selective, saturable, high affinity A2-adenosine receptor binding, with a dissociation constant (Kd) of 1.2 ± 0.4 nM (three experiments) and a receptor density (Bmax) of 637 ± 85 fmol/mg of protein (three experiments). The presence of 10−8 M Gpp(NH)p decreases both the arylazide binding and labeling by ~10%, confirming the agonist nature of 125I-azido-PAPA-APEC. There was no significant Change in Kd. Competitive binding studies demonstrate the appropriate A2 receptor pharmacology (Table 1), with NECA being more potent than (R)-PIA, which is more potent still than theophylline (two to four experiments). Although the potency order is identical for both the amine and arylazide radioligands, the IC50 values indicate that the competition curves have been shifted to the right with the arylazide derivative. The same pharmacology is reflected in the photoaffinity labeling displayed in Fig. 1.
TABLE 1. A2-Adenosine receptor binding pharmacology.
Binding was performed using the conditions described in Experimental Procedures.
| Competitor | IC50 |
|
|---|---|---|
| 125I-PAPA-APEC | 125I-azido-PAPA-APEC | |
| nm | ||
| PAPA-APEC | 28 ± 6 | 48 ± 9 |
| NECA | 86 ± 41 | 112 ± 36 |
| (R)-PIA | 1,350 ± 423 | 1,500 ± 479 |
| Theophylline | 31,600 ± 4,240 | 35,750 ± 100 |
Fig. 1.
Autoradiograph of 125I-azido-PAPA-APEC-labeled A2-adenosine receptor binding subunit. Bovine striatal membranes were incubated with 0.8 nm 125I-azido-PAPA-APEC, as outlined in Experimental Procedures. Molecular weight markers are shown on the left. Left lane, control labeling (×1000) demonstrating the Mr 45,000 A2-adenosine receptor binding subunit. Labeling is decreased significantly more with 5 µm NECA than 5 µm (R)-PIA or 5 µm (S)-PIA, which is appropriate for A2-adenosine receptor pharmacology. Right lane, labeling in the presence of 5 mm theophylline, representing nonspecific labeling. The Mr 45,000 band (arrow) is the only specifically labeled band and is identical to the band visualized with the cross-linked 125I-PAPA-APEC. This autoradiograph is typical of four experiments.
Fig. 1, control lane shows the Mr 45,000 A2-adenosine receptor subunit. The presence of 5 µm NECA (Fig. 1, second lane) dramatically decreases the labeling of this Mr 45,000 band, whereas 5 µm (R)-PIA has a lesser effect on labeling and 5 µm (S)-PIA has almost no effect at all. Fig. 1, right lane (5 mm theophylline) was used to define nonspecific labeling and clearly shows that the Mr 45,000 band (arrow) is the only specifically labeled band, suggesting that this band is the A2-adenosine receptor binding subunit. This autoradiograph is typical of the results seen in four labeling experiments.
Excision and counting of the radioactivity in the region of the Mr 45,000 band give a calculated 6.5% efficiency of photoincorporation. This finding is in excellent agreement with the efficiency described for other direct photoaffinity probes (6, 7) and is approximately 3-fold higher than the labeling efficiency of cross-linked 125I-PAPA-APEC (5).
Because 125I-azido-PAPA-APEC binds to and labels the A2-adenosine receptor binding subunit with all the appropriate pharmacology and a 3-fold higher efficiency of photoincorporation, we chose to utilize this direct photoaffinity probe in our studies.
Bovine striatal membranes contain both A1- and A2-adenosine receptors, which can be selectively labeled with the agonist direct photoaffinity probes [125I]AZPNEA and 125I-azido-PAPA-APEC, respectively (3). We, therefore, sought to determine whether the photolabeled A1- and A2-adenosine receptor binding subunits shared similar polypeptides.
Partial peptide mapping
Two-dimensional partial peptide mapping with S. aureus V8 protease-digested [125I]AZPNEA-labeled A1-adenosine receptor binding subunit and the 125I-azido-PAPA-APEC-labeled A2-adenosine receptor binding subunit is shown in Fig. 2. The A1 receptor peptide map is seen in Fig. 2, left lane, whereas the A2 receptor peptide map is in Fig. 2, right lane. The uppermost band in each lane represents the undigested receptor and highlights the difference between the intact Mr 38,000 A1-adenosine receptor binding subunit and the Mr 45,000 A2-adenosine receptor binding subunit. The pattern of A1 subunit fragments seen in Fig. 2, left lane, is distinctly different from that seen in Fig. 2, right lane (A2 subunit) and this suggests that these radioligands label different polypeptides. This autoradiograph is typical of the pattern seen in three experiments. A second series of digestions with papain (results not shown) demonstrated a different digestion pattern, but the A1 and A2 subunit partial peptide maps continued to be distinctly different.
Fig. 2.
S. aureus V8 protease partial peptide map. Two-dimensional partial peptide mapping was performed as outlined in Experimental Procedures. One hundred micrograms of S. aureus V8 protease were used in this digestion. Left lane, A1-adenosine receptor binding subunit labeled with [125I]AZPNEA; right lane, A2-adenosine receptor binding subunit labeled with 125I-azido-PAPA-APEC. This peptide map is typical of the results seen in three experiments.
Previous studies have established that the A1-adenosine receptor binding subunit, like many other receptors, is actually a glycoprotein (3). We next investigated the possibility that the differences in the apparent molecular weight of these two receptors and their peptide fragments were all attributable to differences in a glycan component of the receptor binding subunit.
Endoglycosidase F treatment
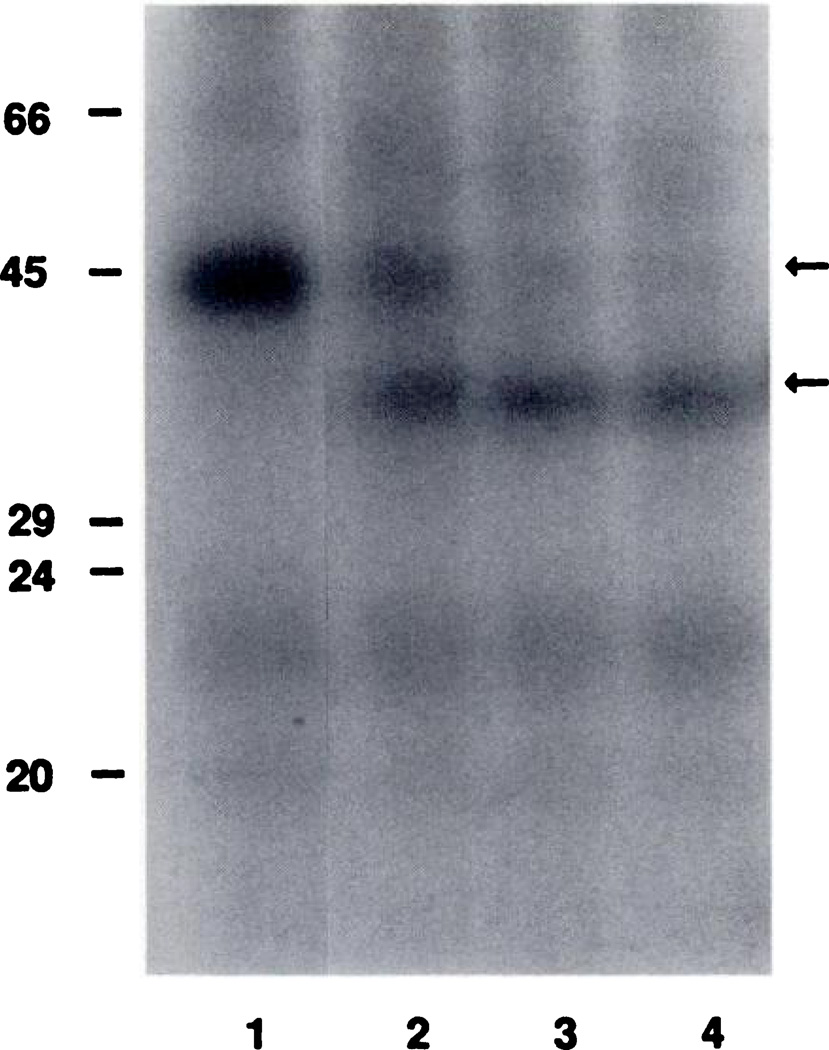
The result of treatment of the 125I-azido-PAPA-APEC-labeled A2-adenosine receptor binding subunit with endoglycosidase F is shown in Fig. 3. Fig. 3, lane 1 is a control lane cut from a longer exposure of the same autoradiograph and serves to highlight the expected Mr 45,000 A2-adenosine receptor binding subunit (upper arrow). Fig. 3, lanes 2 through 4 are the result of treatment of the labeled receptor with endoglycosidase F (5 units/ml) for 30 min, 2.5 hr, and 5 hr, respectively. With increasing digestion times, there is clearly a progressive disappearance of the Mr 45,000 band and an increasing prominence of a Mr 38,000 band (lower arrow). Densitometric analysis of the original autoradiograph reveals that the Mr 45,000 band in Fig. 3, lane 1, has an optical area of 22.8 units (absorbance × millimeters). This is only slightly more (less than 12%) than the combined optical areas of the Mr 45,000 and 38,000 bands seen in Fig. 3, lanes 2 (20.5 units), 3 (21.8 units), or 4 (20.9 units). Prolonged digestions of up to 26-hr duration with 5 units/ml endoglycosidase F and for times as brief as 30 min with only 1 unit/ml endoglycosidase F failed to show new labeled bands, a greater than 12% variation in optical areas, or progressive disappearance of the Mr 38,000 band (data not shown). These findings lead us to believe that the conversion from Mr 45,000 to 38,000 progresses without any intermediary proteins and without any degradation products, being essentially complete at 5 hr (Fig. 3, lane 4).
Fig. 3.
Endoglycosidase F treatment of the 125I-azido-PAPA-APEC-labeled A2-adenosine receptor binding subunit. This autoradiograph is typical of the results seen in three experiments. Lane 1, intact Mr 45,000 A2-adenosine receptor binding subunit (upper arrow) in the absence of enzyme. Lanes 2 through 4, the effect of treatment with 5 units/ml endoglycosidase F for 0.5, 2.5, and 5 hr, respectively. Molecular weight markers (×1000) are shown on the left. The deglycosylated receptor (lower arrow) has an Mr of 38,000 and is the only labeled band generated by the deglycosylation.
Identical aliquots of photoaffinity-labeled membranes (paired controls) were also incubated for up to 26 hr in the absence of endoglycosidase F and showed no degradation of the Mr 45,000 band (data not shown), suggesting that this change in Mr from 45,000 to 38,000 is due to deglycosylation of a glycoprotein A2-adenosine receptor binding subunit.
The only new band noted in Fig. 3, lanes 2 through 4, is the Mr 38,000 band, suggesting that a single endoglycosidase F-sensitive carbohydrate chain is attached to the A2-adenosine receptor subunit polypeptide chain, because additional carbohydrate chains would result in the presence of other labeled bands. The characteristics of this carbohydrate chain were then examined with selective exoglycosidase treatments.
Exoglycosidase treatment
The result of treatment of the photoaffinity-labeled A2-adenosine receptor binding subunit with neuraminidase and α-mannosidase (15) is shown in Fig. 4. Fig. 4, lane 1 is a control lane and serves to demonstrate the undigested Mr 45,000 A2-adenosine receptor binding subunit. When the labeled subunit is treated with 2 units/ml neuraminidase (specifically active in removing terminal sialic acid) for 6 hr, a much broader band extending from Mr 45,000 to 42,000 (Fig. 4, lane 2) is seen. The increased counts in Fig. 4, lane 2, were not typical, as shown in Fig. 4, lane 5 and lane 6, which represent a second experiment where lane 5 is again a control and lane 6, is the A2-adenosine receptor treated with 2 units/ml neuraminidase. The broad band in Fig. 4, lane 2 and 6, does not become more discrete with prolonged treatment, suggesting that the A2 receptor population may be heterogeneous with respect to its sensitivity to neuraminidase.
Fig. 4.
Exoglycosidase treatment of the 125I-azido-PAPA-APEC-labeled A2-adenosine receptor binding subunit. Lane 1, intact Mr 45,000 A2-adenosine receptor binding subunit in the absence of enzyme. Lane 2, treatment of the labeled A2-adenosine receptor binding subunit with 2 units/ml neuraminidase for 6 hr at 37°. Lane 3, result of treatment of the labeled A2-adenosine receptor binding subunit with 4 units/ml α-mannosidase for 24 hr at 25°; lane 4, result of treatment first with 2 units/ml neuraminidase at 37° for 6 hr, followed by 24 hr of treatment at 25° with 4 unitsl/ml α-mannosidase. Lane 5, photoaffinity-labeled receptor in the absence of enzyme from a second experiment. Lane 6, receptor treated with 2 units/ml neuraminidase for 6 hr at 37° from a separate experiment. Lower arrow, mobility of the labeled A2-adenosine receptor following sequential α-mannosidase and neuraminidase treatments and the upper arrow indicates the untreated A2-adenosine receptor. Molecular weight markers (×1000) are shown on the left.
Treatment of the labeled A2-adenosine receptor subunit with 4 units/ml α-mannosidase (specific for cleaving terminal α-linked mannose residues) for 24 hr results in the pattern seen in Fig. 4, lane 3. This lane clearly shows a doublet, with a sharp lower band at Mr 42,000 and a more diffuse upper band that is unaffected by α-mannosidase. The combined optical areas of the Mr 45,000 and 42,000 regions in this lane (20.6 units) are in close agreement with the area found in Fig. 4, lane 1 (21.0 units), and suggests that very little if any of the receptor is lost with α-mannosidase treatment. Again, protracted treatments of up to 60 hr with α-mannosidase failed to eliminate the upper band of the doublet (Fig. 4, lane 3), suggesting that only some of the carbohydrate chains contain terminally α-linked mannose.
If the labeled subunit is sequentially treated, first with neuraminidase (2 units/ml for 6 hr) and then with α-mannosidase (4 units/ml for 24 hr), the result is the single discrete Mr 42,000 band seen in Fig. 4, lane 4 (optical area is 20.6 units). The complete loss of the Mr 45,000 band suggests that the vast majority (if not all) of the carbohydrate chains attached to the A2-adenosine receptor binding subunit polypeptide contain either terminal sialic acid or terminal mannose residues. In addition, the effects of two exoglycosidases are not additive. The observation that neuraminidase does not decrease the apparent molecular weight of the Mr 42,000 peptide created by α-mannosidase treatment suggests that the A2-adenosine receptor does not contain hybrid-type chains with both terminal α-mannose and sialic acid residues.
Discussion
The recently reported radioligands [3H] CGS 21680 (4) and 125I-PAPA-APEC (5) have made it possible to study the A2-adenosine receptor in detail. 125I-PAPA-APEC demonstrates high affinity, highly selective, A2-adenosine receptor binding and, although this ligand can be cross-linked with the heterobifunctional group SANPAH to label the A2 binding subunit, the efficiency of photoincorporation with this technique is only 2 to 2.5% (5). The arylazide derivative, on the other hand, demonstrates an efficiency of 6.5%, along with the same A2-adenosine receptor specificity, selectivity, and pharmacology (Table 1, Fig. 1), making it a superior probe for photoaffinity labeling experiments. Because high quality photoaffinity labeling was essential to these studies, we utilized 125I-azido-PAPA-APEC to label the A2-adenosine receptor binding subunit.
Our original report of the Mr 45,000 A2 receptor binding subunit suggested that this entity was different from the Mr 38,000 A1-adenosine receptor binding subunit (5). Two-dimensional partial peptide mappings with S. aureus V8 protease (Fig. 2) and papain (data not shown) show a distinctive pattern of digestion fragments and confirm that the A1- and A2-adenosine receptor binding subunits are, in fact, different.
Because the A1-adenosine receptor binding subunit is an integral membrane glycoprotein (3), it was conceivable that the differences between the A1- and A2-adenosine receptor binding subunits (both in Mr and peptide mapping) were attributable to differences in the carbohydrate (glycan) chains attached to basically similar polypeptides.
We initially looked for the presence of a carbohydrate chain by treating the labeled A2-adenosine receptor binding subunit with endoglycosidase F, as shown in Fig. 3. The broad specificity of endoglycosidase F activity (cleaving both high mannose- and complex-type carbohydrate chains), along with our protracted incubation times and enzyme-free controls, strongly suggests that the Mr 38,000 band (Fig. 3, lower arrow) seen in Fig. 3 represents the fully deglycosylated (at least in terms of N-linked chains) A2-adenosine receptor binding subunit (15). Clearly then, both the A1- and A2-adenosine receptor binding subunits are glycoproteins.
Because the endoglycosidase F-treated A1- and A2-adenosine receptor binding subunits continue to have different apparent molecular weights (32,000 and 38,000, respectively), it seems likely that this difference reflects a real difference in the A1- and A2-adenosine receptor binding subunit polypeptides.
Parallel experiments in which labeled A2 receptors were incubated under the same conditions as above, in the absence of endoglycosidase F, show no evidence for the generation of the Mr 38,000 or any other labeled protein. Because no intermediate bands are formed, even when the receptor is treated for short times with small amounts of enzyme, and no lower Mr bands are seen after the treatment times are quadrupled, it is likely that only one carbohydrate chain is being cleaved by endoglycosidase F. On this basis, we believe that the A2-adenosine receptor binding subunit has only one site of glycosylation, making it similar to the A1-adenosine receptor but different from the β2-adrenergic receptor, where two glycosylation sites are seen (2).
The nature of the glycan chain was then defined with techniques previously developed for the A1-adenosine receptor (3) and the β-adrenergic receptor (2), using exoglycosidases that cleave specific carbohydrate moieties terminally attached to the glycan chain. Neuraminidase and α-mannosidase are two such specific exoglycosidases (15), and the result of treatment of the A2-adenosine receptor binding subunit with these enzymes is shown in Fig. 4.
Even prolonged neuraminidase treatment of the labeled receptor (Fig. 4, lanes 2 and 6) yielded a broad band extending from Mr 45,000 to 42,000. This broad band may represent a heterogeneity of the receptor population in terms of sensitivity to neuraminidase. The failure of longer digestion times to eliminate the upper portion of this band is reminiscent of the effect neuraminidase had on the β2-adrenergic receptor (2) and provides evidence that some of the labeled A2-adenosine receptor binding subunits do not contain a terminal sialic acid residue.
With α-mannosidase (Fig. 4, lane 3), two photolabeled populations are clearly seen. Here, the Mr 45,000 band is unaffected by α-mannosidase treatment (up to 60 hr in duration) and, by analogy, the distinct ~42,000 band is most likely the A2-adenosine receptor binding subunit devoid of terminal α-linked mannose.
Neuraminidase treatment followed by α-mannosidase treatment (Fig. 4, lane 4) led to the most dramatic effect on mobility, with the generation of a single discrete Mr 42,000 band. The fact that the sequentially treated, photolabeled receptor migrates as a single Mr 42,000 band is likely a coincidence (because sequential treatment with these two exoglycosidases will not fully deglycosylate the receptor) but, because this sequential treatment does eliminate the Mr 45,000 band, virtually all of the glycan chains on the A2-adenosine receptor binding subunit glycoprotein must contain either terminal sialic acid or α-linked mannose.
The endoglycosidase F treatments imply that only one carbohydrate chain is present on each receptor polypeptide. How then can we reconcile the presence of a single glycan chain with two distinctly different terminal sugars?
One possible explanation would be the existence of both sugars on the terminal portions of a single “branched” glycan chain. In this situation, neuraminidase and α-mannosidase treatment would each find that the appropriate substrate was available for deglycosylation and treatment of such a branched chain glycan with either of the enzymes would then lead to a decrease in the Mr of the entire receptor population. This is clearly contradictory to the findings in Fig. 4, lanes 2 and 3, where we always see a portion of the receptors (upper arrow) that are unaffected by each of the selective exoglycosidases.
The only explanation that is fully consistent with our experimental findings requires us to conclude that the A2-adenosine receptor binding subunit (in bovine striatal membranes) contains two receptor populations. Both populations appear to be glycoproteins with the same Mr before (Mr 45,000) and after (Mr 38,000) endoglycosidase F treatment but containing two different types of glycan chains, a high mannose chain (containing terminal α-linked mannose) in one instance and a complex chain (containing terminal sialic acid) in the other. This mixed population of receptors would result in a single labeled band after endoglycosidase F treatment and would be only partially sensitive to individual neuraminidase and α-mannosidase treatment but would be fully sensitive to their sequential application. This is in contrast to the A1-adenosine receptor, which contains a single complex-type carbohydrate chain in a homogeneous population of receptors (3).
Heterogeneous glycoprotein receptor populations have been observed in other receptor systems, such as the β2-adrenergic receptor of hamster lung and rat erythrocyte (2). These β2 receptor glycoproteins contain two carbohydrate chains that can be removed sequentially with endoglycosidase F. In this case, the chains are both of either the high mannose-type or the complex-type, resulting in distinct populations of receptors that are resistant to neuraminidase or α-mannosidase individually but fully sensitive to their sequential application, in much the same way as observed here with the A2-adenosine receptor (2).
We are unable at this time to determine whether the A2-adenosine receptor exists on two separate cell types within the striatum or whether a single cell type expresses this heterogeneous population of receptors. Clearly, further work will be required to determine the reasons for these different patterns of glycosylation in the A2-adenosine receptor binding subunit, and full elucidation of the subunit structure will have to await cloning and sequencing of this physiologically important receptor.
Acknowledgments
We would like to thank Linda Scherich for her excellent assistance in the preparation of this manuscript.
G.L.S. is an Established Investigator of the American Heart Association and this work was supported in part by Grant RO1HL-35134 from the National Heart Lung and Blood Institute, during the tenure of a Grant-in-Aid from the American Heart Association and 3M Riker.
ABBREVIATIONS
- PIA
N6-1-phenyl-2-isopropyladenosine
- ADA
adenosine deaminase
- APNEA
N6-2-(4-aminophenyl)ethyladenosine
- [125I]AZPNEA
[125I]N6-2-(4-azido-3-iodophenyl)ethyladenosine
- CHAPS
3-[(3-chloramidopropyl)dimethylammonio]-1-propanesulfonate
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- HPLC
high performance liquid chromatography
- NECA
N-ethyladenosine-5′-uronic acid
- PAPA-APEC
2-[4-[2-[2-[(4-aminophenyl)methylcarbonyl-amino]ethylaminocarbonyl]ethyl]phenyl]ethylamino-5′-N-ethylcarboxamido adenosine
- 125I-azido-PAPA-APEC
125I-2-[4-[2-[2-[(4-azidophenyl)methylcarbonylamino]ethylaminocarbonyl]ethyl]phenyl]ethylamino-5′-N-ethylcarboxamido adenosine
- SANPAH
N-succinimidyl 6-(4′-azido-2′-nitrophenylamine)hexanoate
- SDS
sodium dodecyl sulfate
- PAGE
polyacrylamide gel electrophoresis
- Gpp(NH)p
guanosine 5′-(β,γ-imido) triphosphate
Footnotes
Manuscript in preparation.
References
- 1.Stiles GL. Adenosine receptors: structure, function and regulation. Trends Pharmacol. Sci. 1986;7:486–490. [Google Scholar]
- 2.Stiles GL, Benovic JL, Caron MG, Lefkowitz RJ. Mammalian β-adrenergic receptors: distinct glycoprotein populations containing high mannose or complex type carbohydrate chains. J. Biol. Chem. 1984;259:8655–8663. [PubMed] [Google Scholar]
- 3.Stiles GL. Photoaffinity crosslinked A1 adenosine receptor binding subunits: homologous glycoprotein expression by different tissues. J. Biol. Chem. 1986;261:10839–10843. [PubMed] [Google Scholar]
- 4.Jarvis MF, Schulz R, Hutchinson AJ, Do UH, Sills MA, Williams M. [3H]CGS21680, a selective A2 adenosine receptor agonist labels A2-receptors in rat brain. J. Pharmacol. Exp. Ther. 1989;251:888–893. [PubMed] [Google Scholar]
- 5.Barrington WW, Jacobson KA, Hutchinson AJ, Williams M, Stiles GL. Identification of the A2 adenosine receptor binding subunit by photoaffinity crosslinking. Proc. Natl. Acad. Sci. USA. 1989;86:6572–6576. doi: 10.1073/pnas.86.17.6572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stiles GL, Daly DT, Olsson RA. Characterization of the A1 adenosine receptor-adenylate cyclase system of cerebral cortex using an agonist photoaffinity ligand. J. Neurochem. 1986;47:1020–1025. doi: 10.1111/j.1471-4159.1986.tb00715.x. [DOI] [PubMed] [Google Scholar]
- 7.Barrington WW, Jacobson KA, Stiles GL. Demonstration of distinct agonist and antagonist conformations of the A2 adenosine receptor. J. Biol. Chem. 1989;264:13157–13164. [PMC free article] [PubMed] [Google Scholar]
- 8.Lavin TN, Nambi P, Heald SL, Jeffs PW, Lefkowitz RJ, Caron MG. 125I-labeled p-azidobenzylcarozolol, a photoaffinity-label for the β-adrenergic receptor characterization of the ligand and photoaffinity labeling of β1- and β2-adrenergic receptors. J. Biol. Chem. 1982;257:12332–12340. [PubMed] [Google Scholar]
- 9.Benovic JL, Stiles GL, Lefkowitz RJ, Caron MG. Photoaffinity labelling of mammalian beta-adrenergic receptors: metal-dependent proteolysis explains apparent heterogeneity. Biochem. Biophys. Res. Commun. 1983;110:504–511. doi: 10.1016/0006-291x(83)91178-6. [DOI] [PubMed] [Google Scholar]
- 10.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature (Lond.) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 11.Bordier C, Crettol-Jarvinen A. Peptide mapping of heterogeneous protein samples. J. Biol. Chem. 1979;254:2565–2567. [PubMed] [Google Scholar]
- 12.Stiles GL, Strasser RH, Caron MG, Lefkowitz RJ. Mammalian beta-adrenergic receptors: structural differences in beta1 and beta2 subtypes revealed by peptide maps. J. Biol. Chem. 1983;258:10689–10694. [PubMed] [Google Scholar]
- 13.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 14.Delean A, Hancock AA, Lefkowitz RJ. Validation and statistical analysis of a computer modeling method for quantitative analysis of radioligand binding data for mixtures of pharmacologic receptor subtypes. Mol. Pharmacol. 1982;21:5–16. [PubMed] [Google Scholar]
- 15.Kornfeld R, Kornfeld S. Structure of glycoproteins and their oligosaccharide units. In: Lennarz WJ, editor. The Biochemistry of Glycoproteins and Proteoglycans. New York: Plenum Press; 1980. pp. 1–34. [Google Scholar]