Abstract
Background
PRX302 is a prostate specific antigen (PSA)–activated pore-forming protein toxin under development as a targeted approach for improving lower urinary tract symptoms (LUTS) caused by benign prostatic hyperplasia (BPH) without affecting sexual function.
Objective
To evaluate the safety and efficacy of PRX302 in men with moderate to severe BPH.
Design, setting, and participants
Eligible subjects were refractory, intolerant, or unwilling to undergo medical therapies for BPH and had International Prostate Symptom Score (IPSS) ≥12, a quality of life (QoL) score ≥3, and prostate volumes between 30 and 80 g. Fifteen patients were enrolled in phase 1 studies, and 18 patients entered phase 2 studies.
Interventions
Subjects received intraprostatic injection of PRX302 into the right and left transition zone via a transperineal approach in an office-based setting. Phase 1 subjects received increasing concentrations of PRX302 at a fixed volume; phase 2 subjects received increasing volumes per deposit at a fixed concentration.
Measurements
IPSS, QoL, prostate volume, maximum flow rate (Qmax), International Index of Erectile Function, serum PSA levels, pharmacokinetics, and adverse events were recorded at 30, 60, 90, 180, 270, and 360 d after treatment with PRX302.
Results and limitations
Sixty percent of men in the phase 1 study and 64% of men in the phase 2 study treated with PRX302 had ≥30% improvement compared to baseline in IPSS out to day 360. Patients also experienced improvement in QoL and reduction in prostate volume out to day 360. Patients receiving ≥1 ml of PRX302 per deposit had the best response overall. PRX302 had no deleterious effect on erectile function. Adverse events were mild to moderate and transient in nature. The major study limitation was the small sample size.
Conclusions
The promising safety profile and evidence of efficacy in the majority of treated subjects in these phase 1 and 2 studies supports further development of PRX302 as a minimally invasive, targeted treatment for BPH.
Keywords: Benign prostatic hyperplasia, Intraprostatic, Lower urinary tract symptoms, Proaerolysin, Prostate-specific antigen, Protease, Protein toxin
1. Introduction
Benign prostatic hyperplasia (BPH) and benign prostatic enlargement (BPE) occur commonly in aging men, resulting in lower urinary tract symptoms (LUTS) [1,2]. BPH/BPE is commonly treated with α-blockers, which reduce urethral resistance caused by smooth muscle overactivity. Transition zone volume (TZV) reduction may also relieve LUTS [3], and such reduction can potentially be achieved with 5α-reductase inhibitors (5-ARIs) [4–6]. For more severe symptoms, reduction can be achieved surgically via transurethral resection of the prostate (TURP) or other minimally invasive surgical techniques (MIST) [7,8]. All of these treatments can have associated effects on sexual function that include erectile dysfunction (ED) and retrograde ejaculation [7,8]. PRX302 is a novel first-in-class targeted therapy for BPH/BPE that has been developed as a less invasive approach than MISTs for achieving a sustained reduction in TZV to improve moderate to severe LUTS without affecting sexual function.
PRX302 is a modified form of proaerolysin, a highly toxic bacterial pore-forming protoxin that requires proteolytic processing by prostate-specific antigen (PSA) for activation (Fig. 1) [9–11]. PSA is a serine protease uniquely produced by the prostate, and benign hyperplastic prostatic tissue is rich in PSA. PRX302 was generated by genetically modifying proaerolysin to remove the native furin protease activation site and replace it with a PSA-recognized sequence (Fig. 1) [11–14]. Nonclinical studies demonstrated that intratumoral injection of PRX302 caused significant regression of PSA-producing human prostate cancers [11]. Although PRX302 was inactive when injected into the non–PSA-producing dog prostate, intraprostatic injection into the PSA-producing monkey prostate proved the ability of PRX302 to effectively and safely ablate PSA-producing prostate tissue [11]. Based on efficacy and safety in nonclinical studies, an open-label phase 1 dose-escalation trial was performed to assess the safety of transperineal injection of PRX302. Subsequently, a phase 2 volume-escalation study was performed to evaluate the effect of PRX302 on alleviating LUTS in men with moderate to severe BPH/BPE. The purpose of this present study was to report the results from these two studies that document the safety and therapeutic activity of a single transperineal, intraprostatic treatment of PRX302 over a follow-up period of 1 yr.
Fig. 1.
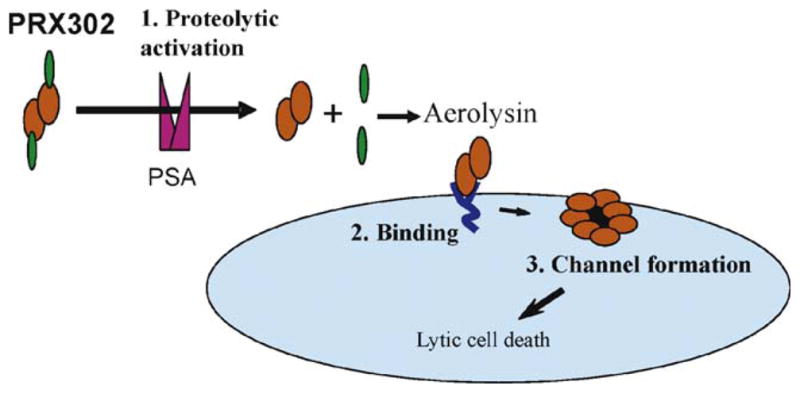
Schematic of PRX302 activation. PRX302 contains a C-terminal inhibitory domain (green) that must be proteolytically removed for activation to occur. Prostate-specific antigen processing can occur in solution as well as after binding of PRX302 to glycosylphosphatidylinositol-anchored proteins (dark blue) on the cell surface. Activated, cell surface–bound aerolysin inserts into membranes and oligomerizes to form a stable heptameric pore (red). Pore formation leads to rapid lytic cell death.
PSA = prostate-specific antigen.
2. Methods
The phase 1 study was conducted at CanMed Clinical Research, Victoria, British Columbia, Canada, and Urological Medical Research, Kitchener, Ontario, Canada, between April 2007 and September 2008. The phase 2 study was conducted at CanMed Clinical Research and Dr. Steinhoff Clinical Research Center, Victoria, British Columbia, Canada, between May and August 2008. Both studies were conducted entirely in office-based settings. Baseline patient characteristics are listed in Table 1. Both studies were approved by local institutional review boards, to which all adverse events were reported. Patients provided written informed consent prior to enrollment. Protox Therapeutics, Vancouver, British Columbia, Canada, was the sponsor of both studies.
Table 1.
Baseline patient characteristics and inclusion and exclusion criteria
| Phase 1 (n = 15) | Phase 2 (n = 18) | |
|---|---|---|
| Age, yr (range) | 64 (52–82) | 66 (49–80) |
| Prior medical BPH therapy, No. (%) | 10 (67) | 4 (22) |
| Prostate volume, g (range) | 45.3 (29.8–80.8) | 51.0 (30.0–74.0) |
| Qmax, ml/s (range) | 11.4 (7.0–14.2) | 10.7 (5.0–15.5) |
| PSA level, ng/ml (range) | 2.2 (0.3–6.1) | 2.9 (0.6–8.9) |
| IPSS (range) | 19.1 (12–26) | 20.1 (15–30) |
| QoL (range) | 4.5 (3–6) | 4.5 (3–6) |
BPH = benign prostatic hyperplasia; Qmax = maximum flow rate; PSA = prostate-specific antigen; IPSS = International Prostate Symptom Score; QoL = quality of life.
Inclusion criteria common to both studies included men 50–80 yr of age with moderate to severe LUTS attributed to BPH/BPE for at least 6 mo prior to study enrollment. Phase 1 subjects had to be refractory, intolerant, or unwilling to use oral medications such as α-blockers or 5-ARIs. Patients on 5-ARIs were excluded from the phase 2 study because of the requirement for a long washout period for this agent (at least 6 mo). Eligible subjects in both studies had to have a baseline International Prostate Symptom Score (IPSS) ≥12, a quality of life (QoL) score ≥3, a maximum flow rate (Qmax) between 5 and 15 ml/s, a postvoid residual (PVR) volume ≤200 ml, and a PSA <10 ng/ml (with negative biopsy if >4 ng/ml). Prostate volumes were between 30 and 80 g, as determined by transrectal ultrasound (TRUS). The ultrasound probe varied from center to center. Subjects were excluded it they had undergone prior minimally invasive treatments for BPH/BPE or had prior TURP.
After administration of local anesthesia, PRX302 was injected transperineally under TRUS guidance using a 22-gauge needle into the transition zone approximately 1 cm away from the right and left side of the urethra. In the phase 1 study, 3–4 deposits of 0.25 ml of PRX302 were made with each injection approximately 1 cm apart as the needle was withdrawn. Cohorts of three were treated with increasing concentrations of PRX302 at a fixed volume. In the phase 2 study, prostate volume was calculated by TRUS, and cohorts of six patients were given six equally divided deposits (ie, three deposits on each side of the transition zone) of PRX302 at a concentration of 3 μg/ml in a total injected volume that was 10%, 20%, or 30% of the total prostate volume. The prostate volume was measured by TRUS. Qmax tracings were reviewed by an independent reviewer, respecting the 2-s rule. IPSS, QoL, Qmax, prostate volume, International Index of Erectile Function (IIEF), serum PSA, and safety assessments were recorded at 30, 90, 180, 270, and 360 d after treatment with PRX302. The severity of adverse events was determined by Common Terminology Criteria for Adverse Events v.3.0. Patients were not permitted to begin other therapies for BPH/BPE over the 360-d observation period; those who chose to do so were considered treatment failures and were withdrawn from the longitudinal follow-up portion of the study.
2.1. Statistical analysis
Summary descriptive statistics (including mean and standard deviation, median, and range or proportion) were determined for subject demographics; subject disposition; subject compliance; and baseline IPSS, QoL, prostate volume, serum PSA levels, and Qmax for each of the five cohorts in phase 1 and three cohorts in phase 2. Changes from screening or predose measurements were calculated and summarized. Clinically significant changes from screening or predose were assessed by paired student t test. Efficacy variables were analyzed as observed (raw data) and as change from baseline scores by analysis of variance.
3. Results
3.1. Phase 1 study
Fifteen subjects were treated in the phase 1 study (Table 1), and 67% of those patients had received prior BPH therapy with α-blockers and/or 5-ARIs. Starting prostate volume and Qmax were comparable among the five cohorts. Cohorts 1–4 were treated as specified in the protocol at a PRX302 concentration of 0.75, 2.25, 7.50, and 10.50 μg/ml, respectively, and a volume/dose of 0.25 ml. Three subjects received a higher total volume/dose of 1.33 ml at a concentration of 0.75 μg/ml. This higher volume was well tolerated. Overall, the total dose (μg/g of prostate) was 0.034 ± 0.004 for cohort 1, 0.102 ± 0.012 for cohort 2, 0.381 ± 0.038 for cohort 3, 0.352 ± 0.062 for cohort 4, and 0.100 ± 0.023 for cohort 1-HV. Subjects received a total intraprostatic injection volume of PRX302 that ranged from 1.5 to 8.0 ml.
3.2. Phase 2 study
Eighteen subjects with moderate to severe BPH/BPE were treated (Table 1). Two patients withdrew following the 6-mo visit. Patients receiving 5-ARIs were ineligible for this study because of the need for a long washout period. Thus, only 22% of patients had received prior BPH/BPE therapy (α-blockers only). Overall, cohort 1 received volumes between 3 and 7.2 ml (0.5–1.2 ml per deposit; total dose: 0.29 ± 0.01 μg/g), cohort 2 received 9.6–15.0 ml (1.6–2.5 ml per deposit; total dose: 0.60 ± 0.01 μg/g), and cohort 3 received 9.0–18.0 ml (1.5–3.0 ml per deposit; total dose: 0.89 ± 0.04 μg/g).
3.3. Safety assessment
No dose-limiting toxicities were reported in the phase 1 study, and the maximum tolerated dose was not reached. Eight of 15 patients (53%) experienced an adverse events (AE), with 6 of 15 patients experiencing an AE that was related to the genitourinary tract (Table 2). Of the 22 reported AEs, all but one were mild to moderate (grade 1 and grade 2) and were transient in nature, resolving with 72 h of treatment with PRX302. A single non–drug-related serious AE was reported in one subject who experience worsening of back pain at 9 mo and underwent herniated disc repair that required hospitalization. No clinically significant changes in hematology or chemistry values were observed. Remarkably, none of the subjects required a urinary catheter at discharge from the outpatient treatment area.
Table 2.
Adverse events from the phase 1 and 2 studies
| Sign or symptoma | Phase 1
|
Phase 2
|
||
|---|---|---|---|---|
| Percent total (n)b | Percent possibly or definitely relatedc | Percent total (n) | Percent possibly or definitely related | |
| Increased urinary frequency | 20 (3) | 7 (1) | 67 (12) | 39 (7) |
| Increased urgency | 20 (3) | 7 (1) | 44 (8) | 33 (6) |
| Dysuria | 20 (3) | 0 | 50 (9) | 33 (6) |
| Hematuria | 0 | 0 | 22 (4) | 0 |
| UTI | 7 (1) | 7 (1) | 11 (2) | 0 |
| Increased PVR | 0 | 0 | 17 (3) | 0 |
| Urinary retention | 0 | 0 | 6 (1) | 0 |
| Fever/chills | 0 | 0 | 22 (4) | 6 (1) |
| Injection site skin infection | 13 (2) | 0 | 0 | 0 |
UTI = urinary tract infection; PVR = postvoid residual.
All adverse events listed were grade 1 or 2.
Percentage of all patients in the study who experienced the indicated sign or symptom.
Physician-reported association of adverse event to PRX302 treatment; choices included “Unrelated,” “Unlikely Related,” “Possibly Related,” and “Definitely Related.”
In the phase 2 study, no dose-limiting toxicities were reported, and the maximum tolerated dose was not reached. Fifteen of 18 patients (80%) experienced an AE, with 11 of 18 patients experiencing an AE related to the genitourinary tract (Table 2). All but two AEs were mild to moderate in severity, and these AEs were also transient in nature. One unrelated grade 3 AE (cutaneous malignancy) and one unrelated grade 4 AE (indolent lymphoma) were reported. Two unrelated severe AEs were also observed: One patient died from a treatment-unrelated cardiac arrest >6 mo after treatment with PRX302 and was therefore withdrawn from the study. A second patient had postoperative sepsis requiring prolonged hospitalization deemed unrelated to PRX302. This patient completed the study to day 360. A second patient voluntarily withdrew from the study (not because of toxicity) after day 180.
3.4. Pharmacokinetics
Samples for plasma and urine pharmacokinetics were obtained from subjects in cohort 4 in phase 1. Samples were analyzed by an enzyme-linked immunosorbent assay (ELISA) with a lower limit of quantification (LLOQ) of 6.00 ng/ml. Plasma PRX302 concentrations were all below the LLOQ for all subjects. Pharmacokinetic urine samples were analyzed by ELISA, with an LLOQ of 0.750 ng/ml. An increase in PRX302 urine concentration was observed 1 min post-treatment (2.43–103.26 ng/ml), and then decreased in all subjects to below LLOQ at 6 h post-treatment.
3.5. Clinical outcomes
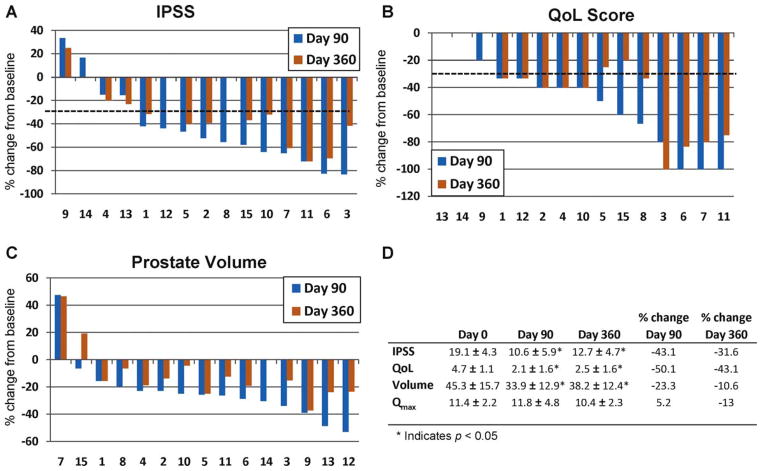
A decrease in total IPSS was observed in all cohorts in the phase 1 study within 30 d following PRX302 treatment administration. The maximum decrease in most of the cohorts was achieved by day 90. However, no relationship between IPSS response and dose was observed. Therefore, because of the small cohort sizes, analysis was performed on IPSS data from all subjects who completed the study to day 90 and day 360. At 90 d, IPSS had declined on average by 8.5 points (Fig. 2). After 1 yr of follow-up, the mean IPSS (12.7 ± 1.2; p < 0.01) was 6.4 points lower than the IPSS level at day 0. In 11 of 15 subjects (73%), the IPSS score decreased by ≥30% at day 90. The majority of subjects in the study (9 of 14 subjects [64%]) maintained this ≥30% reduction out to 1 yr (Fig. 1B).
Fig. 2.
Combined results from all cohorts treated with increasing concentrations of PRX302 in the phase 1 study. The y-axis is the percent change in the parameter compared to the day 0 value; the x-axis denotes individual patient numbers. The percent change in (A) International Prostate Symptom Score (IPSS), (B) quality of life (QoL) score, and (C) prostate volume for each patient at day 90 and day 360 are compared to the day 0 value. The dotted line indicates 30% improvement. (D) The average values plus or minus standard error for IPSS, QoL, prostate volume, and maximum flow rate across all cohorts at 0, 90, and 360 d.
IPSS = International Prostate Symptom Score; QoL = quality of life; Qmax = maximum flow rate.
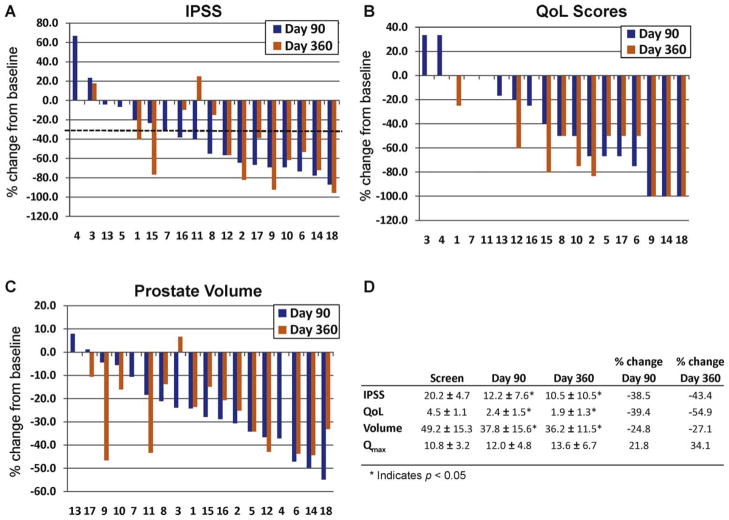
In the phase 2 study, the IPSS values declined in all cohorts (Fig. 3). At 90 d, IPSS values had declined on average by 8.0 points. After 1 yr of follow-up, the mean IPSS (10.5 ± 7.2; p < 0.01) was 9.7 points lower than the IPSS level at day 0. In 12 of 18 subjects (67%), the IPSS value decreased by ≥30% at day 90. The majority of subjects in the study (10 of 16 subjects [63%]) maintained this ≥30% reduction out to 1 yr (Fig. 3).
Fig. 3.
Combined results from all cohorts treated with increasing concentrations of PRX302 in the phase 2 study. The y-axis is the percent change in parameters compared to the day 0 value; the x-axis denotes individual patient numbers. The percent change in (A) International Prostate Symptom Score (IPSS), (B) quality of life (QoL) score; (C) and prostate volume for each patient at day 90 and day 360 compared to the day 0 value. The dotted line indicates 30% improvement. (D) The average values plus or minus standard error for IPSS, QoL, prostate volume, and maximum flow rate across all cohorts at 0, 90, and 360 days.
IPSS = International Prostate Symptom Score; QoL = quality of life; Qmax = maximum flow rate.
3.6. Quality of life scores
QoL scores at screening showed that all subjects were “mostly dissatisfied” or “unhappy” with their current urinary condition for a mean score of 4.5 in both studies. The maximum observed decrease was observed between day 90 and day 180. Overall, no differences among the cohorts were detected, and no significant relationships were observed between QoL changes and prostate size or number of deposits. In the phase 1 study, a 2.2-point improvement in QoL was sustained out to 1 yr, with the majority of subjects showing at least a 33% improvement in QoL scores at day 90 (12 of 15 [80%]) that was maintained out to 1 yr (10 of 14 [71%]; Fig. 2). In the phase 2 study, a 2.6-point improvement at 1 yr was observed, with the majority of subjects showing at least a 33% improvement in QoL scores at day 90 (10 of 18 [56%]) that was maintained out to 1 yr (10 of 16 [69%]; Fig. 3).
3.7. Maximum flow rate
Average screening Qmax was similar in both studies. Although a statistically significant decrease in the mean Qmax was observed at day 90 in the phase 1 study, overall, there was no significant change in the mean Qmax out to 1 yr (Fig. 2D). In contrast, a 2.8 ml/s improvement was noted in the phase 2 patients out to 1 yr, with 11 subjects (61%) showing a ≥3 ml/s improvement (Fig. 3D).
3.8. Prostate volume
TRUS imaging indicated a decrease in prostate mass following PRX302 injection into the transition zone. The majority of subjects in the phase 1 study showed approximately a ≥20% reduction in prostate volume at day 90 (13 of 15 [87%]). The percentage of patients showing ≥20% reduction in volume decreased to 36% (5 of 14) by day 360 after treatment (Fig. 2C). In the phase 2 study, prostate volume was also reduced at all time points and was decreased 27% across the cohorts out to day 360 (p < 0.05 at all points compared to screening volumes; Fig. 3C and 3D). Overall, 12 of 18 patients (67%) showed ≥20% reduction in prostate volume at day 90 and 10 of 16 (63%) by day 360 after treatment (Fig. 3C).
3.9. Effect of volume of administration
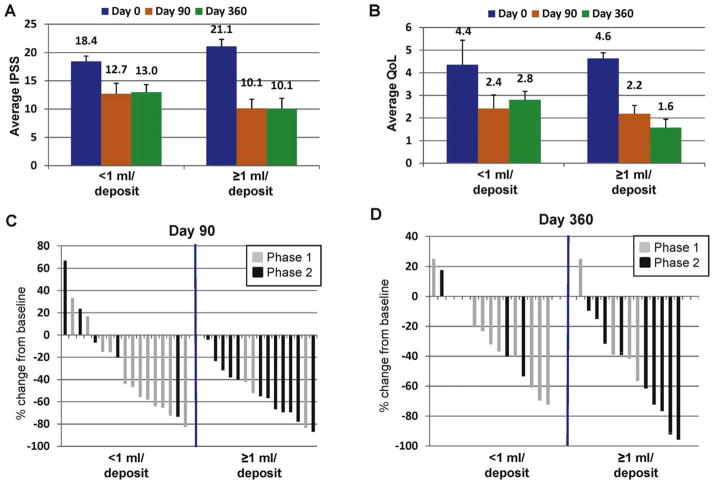
Combined results from phase 1 and phase 2 indicated that 17 of 33 patients received an injection volume per deposit <1 ml, and 16 of 33 patients received a volume ≥1 ml. On this basis, the effect of volume of administration on IPSS, QoL, and prostate volume was determined. In the <1 ml group, IPSS declined 5.4 points from 18.4 to 13.0 at day 360 (Fig. 4A). In this group, 9 of 17 patients (52%) had >30% improvement in IPSS at day 90, with 8 of 15 (53%) at day 360 (Fig. 4C and D). In contrast, in the ≥1 ml group, IPSS declined 10 points at day 360 (Fig. 4A). Thirteen of 16 patients (81%) had >30% IPSS decline at day 90, with 10 of 15 (67%) at day 360 (Fig. 4C and D). QoL scores improved by 36% in the <1 ml group but improved by 67% (from 4.6 at baseline down to 1.6) at day 360 in the ≥1 ml group (Fig. 4B). Prostate volume on average decreased by 18% in the <1 ml group but by 25% in the ≥1 ml group at day 360 (data not shown).
Fig. 4.
Effect of the volume of the PRX302 deposit. Combined average plus or minus standard error for (A) International Prostate Symptom Score (IPSS) and (B) quality of life score in men in the phase 1 and 2 studies receiving either <1 ml or ≥1 ml per deposit of PRX302 at a fixed concentration of 3 μg/ml (p < 0.01 for all data points). (C and D) Percent change in IPSS values at day 90 and day 360 compared to day 0 in men in the phase 1 (gray bars) and phase 2 (black bars) studies receiving <1 ml or ≥1 ml per deposit of PRX302.
IPSS = International Prostate Symptom Score; QoL = quality of life.
3.10. International Index of Erectile Function scores
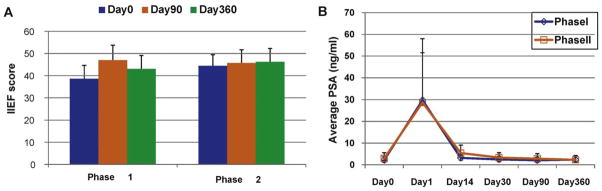
The average erectile function scores at screening were similar in both studies (phase 1: 38.6 ± 6.0; phase 2: 44.4 ± 5.1). At day 90 and day 360, the majority of individual subjects in all cohorts reported no significant change in IIEF scores from their screening scores in both studies (Fig. 5A).
Fig. 5.
(A) Average International Index of Erectile Function score (plus or minus standard error) in combined cohorts from the phase 1 and 2 studies at 0, 90, and 360 days. (B) Average serum prostate-specific antigen levels plus or minus standard error across all cohorts over 360 d.
IIEF = International Index of Erectile Function.
3.11. Serum prostate-specific antigen levels
On day 1 following drug administration, mean PSA levels increased from 2.2 ng/ml to 29.8 ng/ml in the phase 1 study and 2.9 to 28.7 ng/ml in the phase 2 study (Fig. 5B). PSA levels returned to baseline by day 30 and remained at day 0 levels thereafter out to day 360 (Fig. 5B), with half (14 of 28) of the patients evaluable at day 360 having PSA levels 10–70% below baseline.
4. Discussion
These studies have demonstrated that PRX302, a novel PSA-activated pore-forming protein toxin, can be administered safely as an intraprostatic injection in men with moderate to severe BPH/BPE. Although the sample size in both studies is small, the results document the potential for PRX302 to significantly improve LUTS in these patients. Overall, PRX302 treatment resulted in an 8–10-point reduction in IPSS values in the majority of patients, with the duration of treatment effect lasting up to 1 yr. These results, coupled with the excellent safety profile, would potentially position PRX302 in the space between medical therapy and MISTs in the treatment paradigm for BPH/BPE. The reduction in IPSS in these small studies was superior in both magnitude and time to onset to that observed with medical therapies, which typically produce 3–6-point reductions in IPSS values, with clinical improvement often not observed until several weeks to months on therapy [4–6,8]. The IPSS reduction resulting from PRX302 approached that observed for MISTs, which typically reduce IPSS values by 10–12 points [15–17].
Although PRX302 treatment is more invasive than medical therapy in that it involves an injection into the prostate, it is significantly less invasive than currently used MISTs. The administration of PRX302 is a 10–15-min procedure performed in the outpatient setting. Unlike MISTs, patients do not require postprocedure urinary catheterization nor a prolonged recovery period. The most commonly used MISTs are transurethral needle ablation and transurethral microwave thermotherapy, which are associated with ED, urinary tract infection (UTI), strictures, hematuria, and elevated blood pressure postprocedure [16,17]. In contrast, PRX302 produced no significant effect on IIEF scores and no effect on blood pressure in these two studies.
Although the small sample size and multiple dose levels do not allow for definitive conclusions about the potential efficacy of PRX302 in BPH treatment, the preliminary results presented here are encouraging and suggest that further clinical testing is warranted. In this regard, a double-blinded, randomized phase 2 study was recently completed comparing the effect of PRX302 at an injection volume of 20% to placebo. Results from this study will be reported when the follow-up period is complete for all patients. Future studies with PRX302 will define the optimal route of administration. Ongoing follow-up of current study patients will provide further information as to the expected duration of effect on LUTS. PRX302 is a bacterial toxin that can induce an antibody response. Thus, future studies are also necessary to determine the safety and efficacy of repeat dosing.
5. Conclusions
The promising safety profile and evidence of efficacy in the majority of treated subjects in these two studies supports further development of PRX302 as a minimally invasive, targeted treatment for BPH.
Acknowledgments
Funding/Support and role of the sponsor: Protox Therapeutics, Inc., provided support for the design and conduct of the study as well as the management and analysis of the data.
Footnotes
Author contributions: Samuel R. Denmeade had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Denmeade, Merchant, Abi-Habib.
Acquisition of data: Egerdie, Steinhoff, Pommerville.
Analysis and interpretation of data: Denmeade, Merchant, Abi-Habib.
Drafting of the manuscript: Denmeade.
Critical revision of the manuscript for important intellectual content: Merchant.
Statistical analysis: Denmeade, Merchant, Abi-Habib.
Obtaining funding: Denmeade, Merchant.
Administrative, technical, or material support: None.
Supervision: Denmeade.
Other (specify): None.
Financial disclosures: I certify that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Samuel Denmeade is a co-inventor of PRX302 and a consultant for Protox Therapeutics; he has received consulting fees and equity. Rosemina Merchant is an employee of Protox Therapeutics. Ralph Abi-Habib was an employee of Protox Therapeutics at the time of these studies. Peter Pommerville was a paid consultant for Protox Therapeutics at the time of these studies.
References
- 1.Roehrborn CG, Marks L, Harkaway R. Enlarged prostate: a landmark national survey of its prevalence and impact on US men and their partners. Prost Canc Prost Dis. 2006;9:30–4. doi: 10.1038/sj.pcan.4500841. [DOI] [PubMed] [Google Scholar]
- 2.Roehrborn CG. Benign prostatic hyperplasia: an overview. Rev Urol. 2005;7(Suppl 9):S3–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Lepor H. Pathophysiology epidemiology, and natural history of benign prostatic hyperplasia. Rev Urol. 2004;6(Suppl 9):S3–10. [PMC free article] [PubMed] [Google Scholar]
- 4.Debruyne F, Barkin J, van Erps P, et al. Efficacy and safety of long-term treatment with the dual 5 alpha-reductase inhibitor dutasteride in men with symptomatic benign prostatic hyperplasia. Eur Urol. 2004;46:488–95. doi: 10.1016/j.eururo.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Lepor H. The evolution of alpha-blockers for the treatment of benign prostatic hyperplasia. Rev Urol. 2006;8(Suppl 4):S3–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Sandhu JS, Vaughan ED., Jr Combination therapy for the pharmacological management of benign prostatic hyperplasia: rationale and treatment options. Drugs Aging. 2005;22:901–12. doi: 10.2165/00002512-200522110-00002. [DOI] [PubMed] [Google Scholar]
- 7.Deliveliotis C, Liakouras C, Delis A, Skolarikos A, Varkarakis J, Protogerou V. Prostate operations: long-term effects on sexual and urinary function and quality of life. Comparison with an age-matched control population. Urol Res. 2004;32:283–9. doi: 10.1007/s00240-004-0411-0. [DOI] [PubMed] [Google Scholar]
- 8.Harkaway RC, Issa MM. Medical and minimally invasive therapies for the treatment of benign prostatic hyperplasia. Prostate Cancer Prostatic Dis. 2006;9:204–14. doi: 10.1038/sj.pcan.4500869. [DOI] [PubMed] [Google Scholar]
- 9.Howard SP, Buckley JT. Activation of the hole-forming toxin aerolysin by extracellular processing. J Bacteriol. 1985;163:336–40. doi: 10.1128/jb.163.1.336-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckley JT. Crossing three membranes. Channel formation by aerolysin. FEBS Lett. 1992;307:30–3. doi: 10.1016/0014-5793(92)80896-o. [DOI] [PubMed] [Google Scholar]
- 11.Williams SA, Merchant RF, Garrett-Mayer E, Isaacs JT, Buckley JT, Denmeade SR. A prostate-specific antigen-activated channel-forming toxin as therapy for prostatic disease. J Natl Cancer Inst. 2007;99:376–85. doi: 10.1093/jnci/djk065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abrami L, Fivaz M, Decroly E, et al. The pore-forming toxin proaerolysin is activated by furin. J Biol Chem. 1998;273:32656–61. doi: 10.1074/jbc.273.49.32656. [DOI] [PubMed] [Google Scholar]
- 13.Denmeade SR, Lou W, Malm J, Lovgren J, Lilja H, Isaacs JT. Specific and efficient peptide substrates for assaying the proteolytic activity of prostate specific antigen. Cancer Res. 1997;57:4924–30. [PMC free article] [PubMed] [Google Scholar]
- 14.Williams SA, Singh P, Isaacs JT, Denmeade SR. Does PSA play a role as a promoting agent during the initiation and/or progression of prostate cancer? Prostate. 2007;67:312–29. doi: 10.1002/pros.20531. [DOI] [PubMed] [Google Scholar]
- 15.Mattiasson A, Wagrell L, Schelin S, et al. Five-year follow-up of feedback microwave thermotherapy versus TURP for clinical BPH: a prospective randomized multicenter study. Urology. 2007;69:91–6. doi: 10.1016/j.urology.2006.08.1115. [DOI] [PubMed] [Google Scholar]
- 16.Hermann TRW, Gross AJ, Schultheiss D, Kaufmann PM, Jonas U, Burchardt M. Transurethral microwave therapy for the treatment of BPH: still a challenger? World J Urol. 2006;24:389–96. doi: 10.1007/s00345-006-0098-7. [DOI] [PubMed] [Google Scholar]
- 17.Bouza C, Lopez T, Magro A, Navalpotro L, Amate JM. Systemic review and meta-analysis of transurethral needle ablation in symptomatic benign prostatic hyperplasia. BMC Urol. 2006;6:14–30. doi: 10.1186/1471-2490-6-14. [DOI] [PMC free article] [PubMed] [Google Scholar]