Abstract
Objectives
to measure maraviroc total cerebrospinal fluid (CSF) concentrations and compare them with total and unbound plasma concentrations.
Methods
Total maraviroc was measured by reverse phase high performance liquid chromatography with tandem mass spectrometry while ultrafiltration was used for unbound maraviroc.
Results
Maraviroc was detected in all nine CSF/plasma pairs with a median CSF total concentration of 2.4 ng/mL. CSF concentrations exceeded the 50% inhibitory concentration (IC50) of wild-type CCR5-tropic HIV-1 in all specimens.
Conclusions
CSF concentrations are lower than expected based on plasma concentrations and physicochemical characteristics. Unbound maraviroc plasma concentrations may be informative in estimating concentrations in CSF.
Keywords: cerebrospinal fluid, HIV, 50% inhibitory concentration, maraviroc, pharmacokinetics
Introduction
Only antiretrovirals that penetrates the central nervous system (CNS) in therapeutic concentrations will be able to reduce HIV replication in that compartment. Higher antiretroviral concentrations in cerebrospinal fluid (CSF) are associated with reduction of HIV RNA levels in CSF below quantitation limits [1, 2] and perhaps better neurocognitive performance [3, 4]. Maraviroc is the first drug in the class of entry inhibitors blocking CC chemokine receptor 5 (CCR5), a co-receptor critical for cellular entry of R5-tropic viruses and the major co-receptor used for entry into macrophages and microglial cells, both an important virion-producing source in the CNS [5]. The objectives of this study were to measure maraviroc concentrations in CSF and compare them with matched total and unbound plasma concentrations, and to the established in vitro 50% inhibitory concentration (IC50) of wild-type CCR5-tropic HIV-1.
Material and Methods
Subjects with HIV-1 infection enrolled in observational cohort studies conducted at the HIV Neurobehavioral Research Center (HNRC) between 2006 and 2008 were included in the analyses based on self-reported use of maraviroc, availability of stored CSF and matched plasma, and specimens obtained within 16 hours of dosing. All studies were approved by the University of California, San Diego Human Research Protections Program. All subjects were provided with and signed an approved informed consent. Maraviroc was dosed 150 or 300 mg twice daily depending on concurrent use of potent CYP3A inhibitors. Total maraviroc concentrations in plasma and CSF were measured by reverse phase high performance liquid chromatography with tandem mass spectrometry. Unbound maraviroc in plasma was isolated by ultrafiltration. The dynamic range for total plasma maraviroc was 1.9-2000 ng/mL and 0.2-200 ng/mL for CSF and unbound maraviroc. Descriptive and bivariate statistics were generated using standard methods. The in vitro IC50 for wild-type CCR5-tropic HIV-1 used was 0.26 ng/mL (95% CI: 0.23-0.31) [6].
Results
Nine plasma-CSF pairs were obtained from 7 subjects with 5 subjects providing a single pair, and 2 subjects providing 2 pairs. Subjects were predominantly middle-aged (median 47 years; range, 36-64), white (86%) men (100%) with AIDS (86%). Median current CD4+ T lymphocytes were 173/μL (range, 56-475) while nadir was 7/μL (range, 1-129). All CSF specimens and 67% of plasma specimens had HIV RNA at or below the lower limit of detection (1.70 log10 copies/mL).
Median duration of maraviroc use was 2.1 months (Interquartile range [IQR], 1.3-8.4). Five subjects reported use of maraviroc 150 mg twice daily and 2 subjects 300 mg twice daily. All subjects used concurrent antiretrovirals. All subjects reported taking at least 95% of their antiretroviral doses in the 4 days preceding sampling. The last maraviroc dose was taken with food prior to sampling in 67% of the sampling time points.
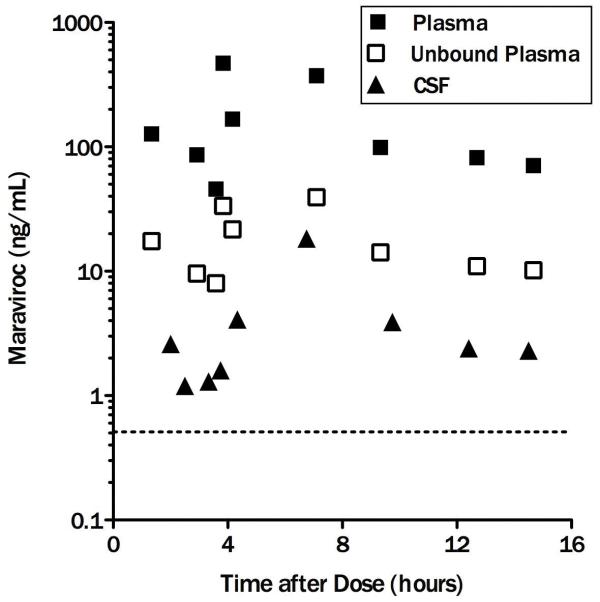
Maraviroc plasma and CSF concentrations are displayed in the figure. Maraviroc was detected in all CSF and plasma specimens with total median concentrations of 2.4 ng/mL (IQR, 1.5-4.0) and 98.9 ng/mL (IQR, 76.3-269.8) respectively. The median maraviroc unbound fraction in plasma was 13% (range, 7-18%). CSF fractional penetrance (i.e. the amount of drug reaching the CSF compartment) was 2.8% of median total and 18.9% of median unbound plasma concentrations. Maraviroc concentrations in CSF exceeded the IC50 of wild-type CCR5-tropic HIV-1 in all specimens by a median of 9.2-fold (IQR: 5.6 – 15.4). Non-statistical correlations between CSF and plasma concentrations (r=0.51 and p=0.17), and CSF concentrations and post dose sampling interval (PDSI) (r=0.33, p=0.38) were observed while correlation between CSF and unbound plasma concentrations was statistically significant (r=0.71, p=0.03). Higher maraviroc concentrations in plasma were not associated with undetectable HIV RNA levels in plasma (p>0.4). The exclusion of individuals on a higher dose of maraviroc (and without ritonavir) did not change the findings.
Figure. Maraviroc concentrations in plasma/total (full squares), plasma/unbound (empty squares) and CSF (full triangles). Horizontal dotted line = IC50.
Discussion
A prior report identified a maraviroc unbound plasma fraction of 24% [7]. However, the median plasma unbound fraction appears lower in this study (13%; 95%CI, 10.6 to 15.1) which may relate to different levels of plasma binding protein. Maraviroc concentrations in CSF exceeded the 50% inhibitory concentration (IC50) required to inhibit wild-type CCR5-tropic HIV-1 in vitro in all CSF specimens. Although only total drug concentrations were measured in CSF, it appears unlikely that protein binding in CSF would result in unbound maraviroc concentrations below the IC50 given the much lower concentration of binding proteins in CSF compared to plasma (100-1000-fold lower) and a pharmacokinetic study measuring bound and unbound indinavir in CSF [8, 9]. The relatively robust correlation between CSF and unbound plasma maraviroc concentrations suggests that the latter may be informative in estimating concentrations in CSF and by extension in brain parenchyma. Unbound maraviroc fraction variability as demonstrated by our and other published data suggests that mere estimation of unbound plasma concentrations from published unbound fraction may not be appropriate and that actual unbound plasma maraviroc concentrations are more accurate. Therefore, interventions focused on increasing maraviroc concentrations in plasma might result in higher CNS concentrations. The median maraviroc concentration in CSF and a CSF-to-plasma ratio of 0.028 are lower than predicted by plasma unbound fraction and physicochemical characteristics of maraviroc, including its relatively low molecular weight and moderate lipophilicity [7], suggesting that other factors such as affinity for the efflux p-glycoprotein transporter may limit distribution to the CSF compartment [10]. Despite these limitations, maraviroc achieved clinically significant CSF concentrations based on wild-type CCR5-tropic HIV-1 in vitro IC50.
CSF maraviroc concentrations in this analysis were comparable to those reported in 2 other small studies with median CSF maraviroc concentrations of 2.58 and 3.63 ng/mL along with a similar CSF-to-plasma ratio 0.022 and 0.03 [11, 12]. The data presented here extends the published findings by reporting actual unbound maraviroc concentrations in plasma. Limitations of this study include the small number of plasma-CSF pairs and subjects and the cross-sectional nature of the analysis.
In summary, observed CSF maraviroc concentrations are lower than expected based on plasma maraviroc concentrations and physicochemical characteristics. Despite this, they exceeded the IC50 in all CSF specimens, suggesting that maraviroc may contribute to control of HIV in the CNS. Additional work will be required to definitively characterize CNS effectiveness and safety of maraviroc.
Acknowledgements
D.C. was involved in data collection and analysis, discussion of the results, and authored the manuscript.
B.M.B. was involved in data analysis, and discussion of the results.
S.L.L was involved in the conception and design of the study, data analysis, discussion of the results, and manuscript preparation.
S.S.R. has contributed to analytic method development and performance for CSF and plasma samples.
R.J.E. was involved in the conception and design of the study, data collection and analysis, and discussion of the results.
D.B.C. was involved in data collection and analysis, and discussion of the results.
A.C.C. was involved in data collection and analysis, and discussion of the results
B.B.G. was involved in data collection and analysis, and discussion of the results..
J.C.M. was involved in data collection and analysis, and discussion of the results.
J.A.M. was involved in data collection and analysis, and discussion of the results.
S.M. was involved in data collection and analysis, and discussion of the results.
I.G. was involved in the conception and design of the study, data collection and analysis, and discussion of the results.
The results of this study were presented as a poster at the International Symposium on NeuroVirology (ISNV), Milan, Italy, October 12-16, 2010
Written and informed consent was obtained for all subjects following review by the University of California, San Diego Human Research Protections Program.
Source of support: This study was supported by a U.S. National Institutes of Health contract (N01 MH22005 – I. Grant) and grant (U01 MH83506 – I. Grant).
References
- 1.Letendre SL, van den Brande G, Hermes A, Woods SP, Durelle J, Marquie Beck J, et al. Lopinavir with ritonavir reduces the HIV RNA level in cerebrospinal fluid. Clin Infect Dis. 2007;54:1511–1517. [Google Scholar]
- 2.Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Letendre SL, McCutchan JA, Childers ME, Woods SP, Lazzaretto D, Heaton RK, et al. Enhancing antiretroviral therapy for human immunodeficiency virus cognitive disorders. Ann. Neurol. 2004;56:416–423. doi: 10.1002/ana.20198. [DOI] [PubMed] [Google Scholar]
- 4.Smurzynski M, Wu K, Letendre S, Robertson K, Bosch RJ, Clifford DB, et al. Effects of central nervous system antiretroviral penetration on cognitive functioning in the ALLRT cohort. AIDS. 2011;25:357–365. doi: 10.1097/QAD.0b013e32834171f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albright AV, Shieh JT, Itoh T, Lee B, Pleasure D, O’Connor MJ, et al. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J Virol. 1999;73:205–213. doi: 10.1128/jvi.73.1.205-213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chem. 2005;49:4721–4732. doi: 10.1128/AAC.49.11.4721-4732.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker DK, Abel S, Comby P, Muirhead GJ, Nedderman AN, Smith DA. Species differences in the disposition of the CCR5 antagonist, UK-427,857, a new potential treatment of HIV. Drug Metab Dispos. 2005;33:587–95. doi: 10.1124/dmd.104.002626. [DOI] [PubMed] [Google Scholar]
- 8.Haas DW, Johnson B, Nicotera J, Bailey VL, Harris VL, Bowles FB, et al. Effects of ritonavir on indinavir pharmacokinetics in cerebrospinal fluid and plasma. Antimicrob Agents Chem. 2003;47:2131–2137. doi: 10.1128/AAC.47.7.2131-2137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adam P, Sobek O, Taborsky L, Hildebrand T, Tutterova O, Zacek P. CSF and serum orosomucoid (alpha-1-acid glycoprotein) in patients with multiple sclerosis: a comparison among particular subgroups of MS patients. Clin Chim Acta. 2003;334:107–110. doi: 10.1016/s0009-8981(03)00229-8. [DOI] [PubMed] [Google Scholar]
- 10.Walker DK, Bowers SJ, Mitchell RJ, Potchoiba MJ, Schroeder CM, Small HF. Preclinical assessment of the distribution of maraviroc to potential human immunodeficiency (HIV) sanctuary sites in the central nervous system (CNS) and gut-associated lymphoid tissue (GALT) Xenobiotica. 2008;38:1330–9. doi: 10.1080/00498250802447409. [DOI] [PubMed] [Google Scholar]
- 11.Tiraboschi JM, Niubo J, Curto J, Podzamczer D. Maraviroc concentrations in cerebrospinal fluid in HIV-infected patients. J Acquir Immune Defic Syndr. 2010;55:606–9. doi: 10.1097/QAI.0b013e3181ef70fe. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz A, Watson V, Else L, Gisslen M. Cerebrospinal fluid maraviroc concentrations in HIV-1 infected patients. AIDS. 2009;23:2537–40. doi: 10.1097/QAD.0b013e328333ae0e. [DOI] [PubMed] [Google Scholar]