Abstract
OBJECTIVE
This study compared birth parameters and the longitudinal course in physical and neurologic development between children with 2 and 3 vessel umbilical cords.
STUDY DESIGN
Our study of the Collaborative Perinatal Project included singletons of at least 24 weeks’ gestation with single umbilical artery at birth and no identifiable congenital anomalies. Demographics that were collected included maternal age, race, smoking status, and socioeconomic index. Delivery data included gestational age, birth-weight, Apgar scores, placental weight, and umbilical cord insertion and length. Growth and neurodevelopmental parameters were collected at various intervals from birth to 7 years.
RESULTS
There were 263 infants with isolated single umbilical artery and 41,415 infants with 3 vessel cords. A random effect model that controlled for potential confounders did not show clinically significant differences in the physical and neurodevelopment measures between these groups.
CONCLUSION
Our study shows no evidence of differential longitudinal physical growth or neurologic outcomes between infants with 2 or 3 vessel cords.
Keywords: growth, longitudinal, neurodevelopment, single umbilical artery
The significance of single umbilical artery (SUA) has been studied for many years with varying outcomes.1-8 The association with various congenital anomalies and increased perinatal morbidity because of intrauterine growth restriction or aneuploidy has been suggested previously.1 The incidence of SUA ranges from 0.3–1% of all pregnancies.2,3 In >80% of cases, SUA is an isolated finding.4 The precise cause of SUA is not fully known. Suggested hypotheses include primary agenesis of 1 umbilical artery during development or atrophy of an umbilical artery at a later time.5 Pregnancy outcomes have not been attributed specifically to either of these possible causes.
Many previous studies have sought to detail the perinatal and neonatal morbidity that is associated with this condition. Perinatal outcomes that have been evaluated previously in the literature include birthweight, gestational age at delivery, fetal anomalies, and neonatal intensive care unit admissions.2,6,7 Although the association between antenatal ultrasound findings and birth outcomes has been evaluated, there have been no data regarding long-term outcomes in affected infants. Our study aims to identify whether there are any long-term implications of SUA on physical and neurologic development. Just as the cause of SUA is unknown, the reason for associated poor outcomes in previous studies has also been unclear. Umbilical cord abnormalities may influence the placental histologic condition if there is reduced blood flow that results from mechanical or vascular causes. Increased placental abnormalities often are associated with poor neonatal outcomes. We have gathered data regarding placental features that were found with SUA in an effort to evaluate the association. The absence of a placental pathologic condition in isolated SUA cases would strengthen the proposition that this finding in isolation is not associated with adverse neonatal outcomes and, therefore, is unlikely to have long-term implications.
Materials and Methods
The Collaborative Perinatal Project (CPP) was used to obtain information regarding infants with SUA. The CPP is a well-known prospective study that enrolled pregnant women from 1959 until 1965 from 12 US medical centers.9,10 The goal of the project was to study the neurologic status of infants and birth outcomes of the pregnancy as they related to various factors. Interviews were conducted with study participants to acquire demographic information, socioeconomic status and behavioral information, and obstetric data were collected prospectively according to a predefined protocol. A socioeconomic index from the US Bureau of the Census was used, which combined education, occupation, and family income into a weighted index.11 Women were observed during pregnancy. At delivery, neonatal anthropometric measurements were taken, and the cord and the placenta were assessed according to specific protocols, which included the number of cord vessels. Children were observed to 7 years of age, at which time a comprehensive battery of physical and neurologic assessments was conducted. Inclusion criteria for this analysis included singleton birth of at least 24 weeks’ gestational age and a known number of umbilical arteries at birth. Study exclusion criteria included any identifiable congenital anomalies, multiple gestation, or gestational age at delivery <24 weeks.
Perinatal outcomes between pregnancies with 2- and 3-vessel cords were compared. The outcomes that were studied included gestational age at delivery, birthweight, and Apgar scores at 1 and 5 minutes. Because the embryologic cause of SUA is unknown, we were interested in the placental findings between the 2 groups. Placental morphologic and histopathologic data were gathered that included placental weight, presence of infarcts, and cord insertion.
Physical growth between the groups was also examined. Various measures of growth, which included head circumference, height, and weight, were collected at birth and again at 4 months, 8 months, 1 year, 3 years, 4 years, and 7 years.
Longitudinal data regarding neurologic development were collected and evaluated between the 2- and 3-vessel cord groups. The neurologic examination was performed by neurologists with specific training in pediatrics or pediatricians with specific training in neurology. All physicians underwent standardized training and were blinded to the child's clinical history. Detailed information regarding the neurologic abnormalities was gathered. At 1 and 7 years, a neurologic assessment was performed that categorized children into 1 of 3 groups: normal, suspect, and abnormal. Neurologists were given detailed instructions regarding instances when an abnormal examination was elicited. If a definite abnormality was found on examination that was associated with a diagnosis, then the child was categorized as abnormal. The suspect category was used in instances in which on examination a child was not completely normal yet did not meet criteria for a definitive syndrome or neurologic diagnosis.
To facilitate interpretation, we combined suspect assessments into the normal group. Combining assessments into the abnormal category was not considered because this would raise the percentage of abnormal neurologic assessments to an unreasonably high level. Additionally, standardized psychologic testing was performed at 3 different ages. The Bayley scales of infant development were used to assess the motor, language, and cognitive abilities at 8 months of age. At the time of CPP, the Bayley scales of infant development were in a preliminary phase, and scores were not normalized as they are today but rather were specific for the CPP. Intelligence testing was performed at 4 years of age, with the Stanford Binet Intelligence scale. At age 7 years, the Weschler Intelligence Scale for Children was used. Both the Stanford Binet and Weschler scales are standardized to a mean of 100, with a standard deviation of 15.
In the unadjusted analysis, we used χ2 tests and the Student t test for categoric and continuous variables, respectively. For all continuous measures, we applied linear regressions with generalized estimating equations and controlled for potential confounders that included maternal age, race, smoking, and the child's sex. For neurologic assessment, we fit a logistic regression with generalized estimating equations.12 A global test was conducted in each regression to see whether the longitudinal trajectories are the same statistically between the study and control groups. A probability value that is < .05 is considered significant. All statistical analyses were performed with SAS software (version 9.1; SAS Institute Inc, Cary, NC).
Results
There were 59,391 pregnancies in the CPP. After excluding subjects with multiple gestations and unknown plurality (n = 3651), fetal deaths (n = 2093), gestational age <24 weeks at delivery (n = 538), infants with unknown number of umbilical arteries (n = 5441), or with anomalies detected at birth (n = 5990), 41,678 infants were included in the final data set. This number included 263 infants (0.7%) with SUA. This group was compared with 41,415 infants with 3-vessel cords.
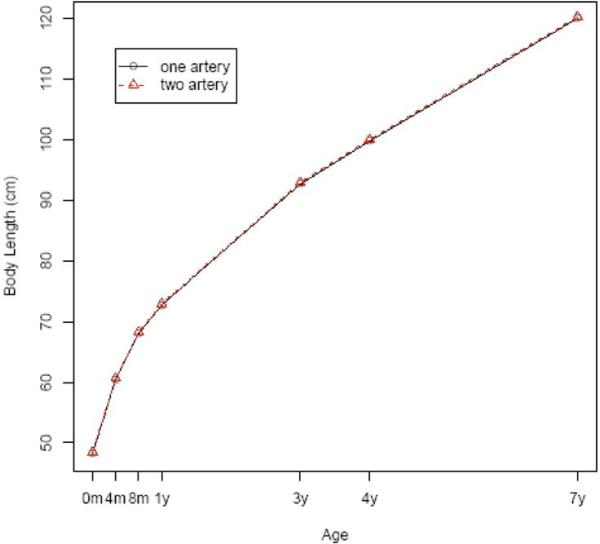
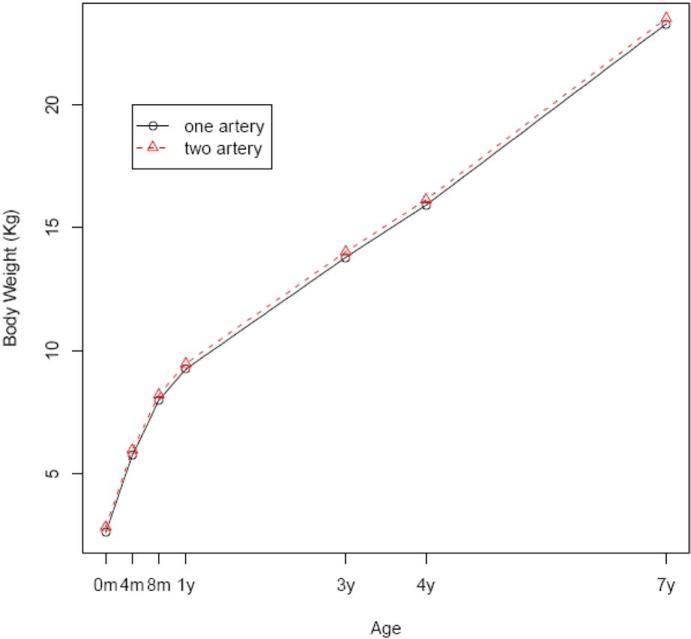
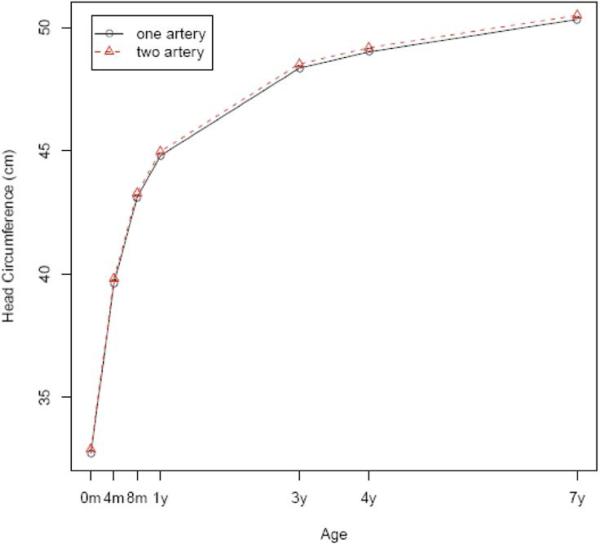
Maternal demographic factors, newborn characteristics, and placental features were compared between the study and control populations (Table 1). There was no difference between these groups with regard to education level, gravidity, or parity. Women with an SUA tended to be older, smoke during pregnancy, and have higher socioeconomic status. The proportion of female infants was higher among the single-artery group than the 2-artery group. However, there was no significant difference in gestational age at delivery or Apgar scores at 1 and 5 minutes. The number of placental infarcts and umbilical cord insertion did not vary significantly between the groups. Infants with an SUA had a slightly smaller placenta but longer umbilical cord. Tables 2 and 3 show cross-sectional comparisons between the 2 groups on child physical and neurologic development. At birth, infants with an SUA appeared smaller, but the difference disappeared thereafter. There were no consistent differences in neurodevelopment at birth, 4 months, 8 months, 1 year, 3 years, 4 years, and 7 years. To see whether the longitudinal physical and neurologic developments were different between the study and control groups, we applied generalized linear models with generalized estimating equations to the data while adjusting for confounders that included maternal demographics, sex of child, and smoking status. All joint tests of equal growth at all the age points are not statistically significant, except in body weight (Figures 1-3). The specific probability values for height, head circumference, weight, intelligence testing, and neurologic measurements are .08, .25, .01, .07, and .06, respectively. Although weight shows statistically significant differences, a closer look reveals that the differences happen only at birth, 4 months, and 7 years of age and are not clinically significant (0.1 kg for birth and 4 months and 0.7 kg for 7 years).
TABLE 1.
Characteristics at baseline by umbilical number
| Arteries |
|||
|---|---|---|---|
| Characteristic | 1 | 2 | P value |
| Age, ya | 25.02 ± 5.6 | 24.08 ± 6.0 | .01 |
| Race, n (%) | |||
| Non-Hispanic white | 173 (65.8) | 19106 (46.1) | .0001 |
| Non-Hispanic black | 77 (29.3) | 19134 (46.2) | |
| Others | 13 (4.9) | 3175 (7.7) | |
| Education, n (%) | |||
| Less than high school | 32 (12.6) | 7265 (17.8) | .06 |
| High school | 185 (72.5) | 28558 (70.0) | |
| Greater than high school | 38 (14.9) | 4956 (12.2) | |
| Smoking status, n (%) | |||
| Nonsmoker | 123 (47.3) | 21840 (53.1) | .01 |
| <1 cigarette/d | 3 (1.1) | 762 (1.9) | |
| 1-5 cigarettes/d | 33 (12.7) | 5374 (13.1) | |
| 6-10 cigarettes/d | 27 (10.4) | 5025 (12.2) | |
| 11-20 cigarettes/d | 54 (20.8) | 6356 (15.4) | |
| ≥21 cigarettes/d | 20 (7.7) | 1769 (4.3) | |
| Socioeconomic index, n (%) | |||
| 0-20 | 14 (5.5) | 4818 (11.9) | .01 |
| 21-40 | 85 (33.6) | 13296 (32.9) | |
| 41-60 | 73 (28.8) | 11914 (29.5) | |
| 61-80 | 52 (20.6) | 6895 (17.0) | |
| 80+ | 29 (11.5) | 3538 (8.7) | |
| Sex, n (%) | |||
| Male | 108 (41.1) | 20642 (49.8) | .004 |
| Female | 155 (58.9) | 20772 (50.2) | |
| Previous pregnancies, n (%) | |||
| 0 | 63 (24.1) | 11652 (28.3) | .33 |
| 1 | 55 (21.0) | 8878 (21.5) | |
| 2 | 41 (15.6) | 6484 (15.7) | |
| ≥3 | 103 (39.3) | 14220 (34.5) | |
| Parity, n (%) | |||
| 0 | 67 (25.5) | 12474 (30.2) | .27 |
| 1 | 58 (22.0) | 9510 (23.0) | |
| 2 | 47 (17.9) | 6795 (16.4) | |
| ≥3 | 91 (34.6) | 12551 (30.4) | |
| Gestational age at delivery, wka | 39.3 ± 3.1 | 39.3 ± 3.2 | .71 |
| Intel1egence quotienta | |||
| 4 y | 100.9 ± 15.7 | 97.6 ± 16 4 | .01 |
| 7 y | 97.2 ± 15.2 | 96.0 ± 14.7 | .26 |
| 1-Minute Apgar score, n (%) | |||
| 0-3 | 11 (4.3) | 2031 (5.1) | .81 |
| 4-6 | 35 (13.7) | 5268 (13.3) | |
| 7-10 | 203 (79.6) | 31590 (79.9) | |
| Incomplete data | 6 (2.4) | 682 (1.7) | |
| 5-Minute Apgar score, n (%) | |||
| 0-3 | 3 (1.2) | 518 (1.3) | .57 |
| 4-6 | 11 (4.4) | 1165 (2.9) | |
| 7-10 | 237 (94.4) | 37903 (95.7) | |
| Incomplete data | 0 | 30 (0.1) | |
| Placental weight, ga | 415.9 ± 92.7 | 436.8 ± 96.2 | .0008 |
| Size of infarcts, n (%) | |||
| None | 188 (76.4) | 30752 (78.4) | .26 |
| All infarcts <3 cm | 53 (21.6) | 7180 (18.3) | |
| At least 1 infarct that measures ≥3 cm | 5 (2.0) | 1281 (3.3) | |
| Cord length, cma | 62.0 ± 15.5 | 58.7 ± 13.3 | .0005 |
| Cord around body, n (%) | |||
| None | 255 (97.7) | 40112 (97.5) | .34 |
| Tight | 2 (0.8) | 131 (0.3) | |
| Loose | 4 (1.5) | 902 (2.2) | |
| Insertion of membranes, n (%) | |||
| Marginal | 184 (75.1) | 29736 (75.7) | .99 |
| Circummarginate | 11 (4.5) | 2126 (5.4) | |
| Marginal and circummarginate | 28 (11.4) | 4237 (10.8) | |
| Circumvallate | 8 (3.3) | 1.39 (2.7) | |
| Marginal and circumvallate | 10 (4.1) | 1575 (4.0) | |
| Circummarginate and circumvallate | 3 (1.2) | 416 (1.1) | |
| Marginal, circummarginate, and circumvallate | 1 (0.4) | 127 (0.3) | |
Continuous variables are presented as mean ± SD.
Chetty-John. Longitudinal outcomes in isolated single umbilical artery. Am J Obstet Gynecol 2010.
TABLE 2.
Cross-sectional analysis for physical and neurologic development
| Arteries |
|||||
|---|---|---|---|---|---|
| 1 |
2 |
||||
| Physical development | n | Mean ± SD | n | Mean ± SD | P valuea |
| Height, cm | |||||
| Birth | 260 | 49.5 ± 2.9 | 40,811 | 49.9 ± 2.8 | .01 |
| 4 mo | 218 | 62.1 ± 3.4 | 35,875 | 62.2 ± 3.2 | .75 |
| 8 mo | 65 | 69.3 ± 2.8 | 15,402 | 69.7 ± 3.1 | .33 |
| 1 y | 214 | 74.4 ± 4.3 | 34,427 | 74.4 ± 3.5 | .72 |
| 3 y | 80 | 93.5 ± 4.5 | 15,661 | 94.4 ± 4.1 | .05 |
| 4 y | 145 | 100.8 ± 5.2 | 27,744 | 101.4 ± 4.6 | .10 |
| 7 y | 190 | 120.7 ± 5.8 | 31,239 | 121.7 ± 5.9 | .02 |
| Head circumference, cm | |||||
| Birth | 260 | 33.5 ± 1.8 | 40,979 | 33.7 ± 1.6 | .01 |
| 4 mo | 219 | 40.4 ± 1.6 | 36,061 | 40.6 ± 1.5 | .03 |
| 8 mo | 66 | 43.7 ± 1.7 | 15,368 | 44.1 ± 1.6 | .04 |
| 1 y | 215 | 45.7 ± 1.7 | 34,796 | 45.8 ± 1.6 | .31 |
| 3 y | 81 | 49.3 ± 1.5 | 15,658 | 49.4 ± 1.6 | .57 |
| 4 y | 142 | 49.8 ± 1.7 | 27,644 | 50.0 ± 1.6 | .25 |
| 7y | 189 | 51.2 ± 1.7 | 31,259 | 51.3 ± 1.6 | .20 |
| Weight, kg | |||||
| Birth | 257 | 3.1 ± 0.5 | 40,930 | 3.2 ± 0.5 | < .0001 |
| 4 mo | 218 | 6.2 ± 0.9 | 35,844 | 6.3 ± 0.9 | .01 |
| 8 mo | 66 | 8.4 ± 1.5 | 15,450 | 8.5 ± 1.1 | .22 |
| 1 y | 213 | 9.7 ± 1.4 | 34,610 | 9.8 ± 1.3 | .09 |
| 3 y | 82 | 14.1 ± 1.8 | 15,780 | 14.3 ± 1.9 | .23 |
| 4 y | 145 | 16.2 ± 2.1 | 27,740 | 16.5 ± 2.3 | .22 |
| 7 y | 189 | 23.2 ± 3.7 | 31,313 | 23.9 ± 4.4 | .03 |
t test.
Chetty-John. Longitudinal outcomes in isolated single umbilical artery. Am J Obstet Gynecol 2010.
TABLE 3.
Cross-sectional analysis for neurologic development
| Arteries |
|||||
|---|---|---|---|---|---|
| 1 |
2 |
||||
| Neurologic development | n | Measure | n | Measure | P value |
| Intel1igence testinga | |||||
| 4 y | 166 | 100.9 ± 15.7 | 29,737 | 97.6 ± 16.4 | .01b |
| 7 y | 188 | 97.2 ± 15.2 | 31,162 | 96.0 ± 14.7 | .26b |
| Mental score at 8 moa | 200 | 79.6 ± 6.2 | 34,276 | 79.8 ± 5.2 | .63b |
| Neurologic condition at birth, n | |||||
| Normal/suspect | 240 | 99.2% | 38,729 | 99.2% | .59c |
| Abnormal | 2 | 0.8% | 302 | 0.8% | |
| Neurologic condition at 4 mo, n | |||||
| Normal/suspect | 189 | 99.5% | 32,986 | 99.6% | .86c |
| Abnormal | 1 | 0.5% | 146 | 0.4% | |
| Neurologic condition at 8 mo, n | |||||
| Normal/suspect | 195 | 97.5% | 33,669 | 98.3% | .36c |
| Abnormal | 5 | 2.5% | 572 | 1.7% | |
| Neurologic condition at 1 y, n | |||||
| Normal/suspect | 208 | 96.7% | 34,510 | 98.9% | .002c |
| Abnormal | 7 | 3.3% | 371 | 1.1% | |
| Neurologic condition at 3 y, n | |||||
| Normal/suspect | 101 | 98.0% | 17,697 | 95.3% | .18c |
| Abnormal | 2 | 2.0% | 879 | 4.7% | |
| Neurologic condition at 7 y, n | |||||
| Normal/suspect | 181 | 95.3% | 30,254 | 96.5% | .36c |
| Abnormal | 9 | 4.7% | 1,102 | 3.5% | |
Data are given as mean ± SD
t test
χ2 test.
Chetty-John. Longitudinal outcomes in isolated single umbilical artery. Am J Obstet Gynecol 2010.
FIGURE 1. Body length.
Body lengths by age of the children with 1 or 2 umbilical arteries, after adjustment for potential confounders
FIGURE 3. Weight.
Body weight by age of the children with 1 or 2 umbilical arteries after adjustment for potential confounders
Comment
The significance of an SUA in pregnancy has been in question in obstetric literature for many years. The finding of SUA on antenatal ultrasound scans has prompted studies that focus on associated perinatal outcomes. There has been evidence to suggest that SUA may be associated with congenital anomalies of the genitourinary and gastrointestinal system and aneuploidy.1,6,13,14 After a comprehensive ultrasound evaluation verifies that no anomalies are present, providers are left to question the significance of this finding in isolation. The consensus of recent literature shows that there is no significant difference in neonatal outcomes when the SUA is found without other anomalies.2,3,8,13 Our study of neonatal diagnosis of SUA also supported that no adverse perinatal outcomes, such as prematurity or increased mortality rate, can be attributed to an SUA alone.
To date, reassuring immediate neonatal measures have been used as a proxy for long-term well-being. This study sought to fill a gap in the current literature by exploring the long-term outcomes of these infants. The data that were evaluated in this study show that there is no evidence of differential longitudinal growth between infants with an SUA or with 3-vessel cords when they are evaluated beyond birth until the age of 8 years. Although weight, length, and head circumference at birth were found to be slightly smaller in the SUA group, this may be attributable to a higher proportion of smokers during pregnancy in that group. After being controlled for potential confounders in the multivariable model, the difference in infant size diminished. Head circumference and neurologic examinations were used as predictors for neurodevelopmental outcome, given that other clinical markers such as Apgar scores are not consistently predictive of cognitive outcomes.15,16 Data collection and analysis did not reveal any consistent or clinically significant difference between groups with regard to neurodevelopment. At 1 year, 3% of infants with an SUA at birth were noted to have abnormal neurologic examination results, compared with 1% of infants with 3-vessel cords. At 4 years, children in the study group had an intelligence test score that was, on average, 4 points higher than the control group. Based on these data, there was no evidence for abnormal long-term neurologic outcomes in infants with isolated SUA.
Placental morphologic and histopathologic findings were compared between groups.Therewerenohistopathologicdifferences between groups. Placental size was approximately 20 g less in the infants with an SUA, compared with those with a 3-vessel cord artery. The umbilical cord length was found to be 3 cm greater in the study group. These morphologic differences may contribute to the overall smaller size of infants with an SUA. In a study by Mu et al,7 there was a more pronounced discrepancy in placental weight between infants with an SUA and a 3-vessel cord artery, which may have been contributory to the increased number of small-for-gestational-age infants in their study group.
Although theoretically, it seems that SUA should contribute to poor perinatal outcomes; physiologically, the fetus has been shown to adopt compensatory mechanisms. Goldkrand et al17 showed that volumetric blood flow in fetuses with an SUA was double that of fetuses with 3-vessel cord arteries. The increased flow and decreased resistance in the SUA are compensatory mechanisms that allow adequate oxygenation, despite this variation in umbilical cord vasculature.
Our study fails to demonstrate any clinically significant difference between infants with an SUA or a 3-vessel cord artery with regard to growth or neurodevelopment. There is variability in the number of the study population at the various time points because of the natural attrition that occurs in longitudinal studies. The time-frame of the study limits some factors that would otherwise be studied because of changes in obstetric practice over the last 50 years. The mode of delivery was also not examined in this study because most of the infants were delivered vaginally during the period of CPP data collection. Prenatal ultrasound findings were not available at the time of this study; therefore, all diagnoses were made at the time of birth. Because our study focused on isolated SUAs at the time of birth, we selected a population of neonates that survived the antepartum and intrapartum periods, which is to be expected in the case of isolated SUA. The time of diagnosis likely would be a confounding factor if our study examined SUA in the context of other congenital anomalies. SUA in fetuses with anomalies that are not compatible with life would not be captured in this study by this mode of diagnosis. Although our study did not use CPP data to analyze births with SUA and anomalies, this was completed by Froehlich and Fujikura.18 They examined singleton births with SUA using CPP data from 1959-1963. The incidence of congenital anomalies in liveborn infants within this study population was 26.2%. The associated congenital malformations were classified into lethal, nonlethal, and borderline malformations. Borderline malformations included findings such as nevi, skin tags, and club feet. Skeletal and gastrointestinal system abnormalities were among the highest incidence in this group. The incidence and types of congenital malformations were comparable with that found in other studies.13,18
In our study criteria, missing data accounted for a large number of our exclusions. Although this is a limitation that we faced, this should not have a signifi-cant impact on our outcome data. If a placenta was not collected during delivery, the information regarding umbilical artery number was not available. These deliveries were not qualitatively different than the others that were included in the study.
The strength of this study lies in the remarkable number of patients and the breadth and comprehensive nature of the data that were collected. The measures of postnatal well-being over a 7-year time period is not available currently in most studies.
In this study population, an SUA was detected at birth. The findings of this study are significantly more valuable when an SUA is detected antenatally. Our data show that overall there is no significant clinical difference in either growth or neurodevelopmental outcomes in infants with an SUA in the newborn period or childhood. With the information from this study and others, providers and patients alike can be reassured that, when found in isolation, SUA is not predictive of poor immediate or long-term outcomes in affected infants.
FIGURE 2. Head circumference.
Head circumference by age of the children with 1 or 2 umbilical arteries after adjustment for potential confounders
Acknowledgments
Supported in part by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health (S.C-J.) and by Intramural Funds from the National Institutes of Health, National Institute of Child Health and Human Development (J.Z.; Z.C.; P.A.; L.S.; M.K.; U.G.).
Footnotes
Presented at the 30th Annual Meeting of the Society for Maternal-Fetal Medicine, Chicago, IL, Feb. 1-6, 2010.
REFERENCES
- 1.Rinehart BK, Terrone DA, Taylor CW, Isler CM, Larmon JE, Roberts WE. Single umbilical artery is associated with an increased incidence of structural and chromosomal anomalies and growth restriction. Am J Perinatol. 2000;17:229–32. doi: 10.1055/s-2000-10002. [DOI] [PubMed] [Google Scholar]
- 2.Bombrys AE, Neiger R, Hawkins S, et al. Pregnancy outcome in isolate single umbilical artery. Am J Perinatol. 2008;25:239–42. doi: 10.1055/s-2008-1061504. [DOI] [PubMed] [Google Scholar]
- 3.Wiegand S, McKenna DS, Croom C, Ventolini G, Sonek JD, Neiger R. Serial sonographic growth assessment in pregnancies complicated by an isolated single umbilical artery. Am J Perinatol. 2008;25:149–52. doi: 10.1055/s-2008-1061502. [DOI] [PubMed] [Google Scholar]
- 4.Martinez-Payo C, Gaitero A, Tamarit I, Garcia-Espantaleon M, Iglesias EI. Perinatal results following the prenatal ultrasound diagnosis of single umbilical artery. Acta Obstet Gynecol Scand. 2005;84:1068–74. doi: 10.1111/j.0001-6349.2005.00884.x. [DOI] [PubMed] [Google Scholar]
- 5.Prucka S, Clemens M, Craven C, McPherson E. Single umbilical artery: what does it mean for the fetus? A case-control analysis of pathologically ascertained cases. Genet Med. 2004;6:54–7. doi: 10.1097/01.gim.0000105743.91723.b0. [DOI] [PubMed] [Google Scholar]
- 6.Gornall AS, Kurinszuk JJ, Konjec JC. Antenatal detection of a single umbilical artery: does it matter? Prenat Diagn. 2003;23:117–23. doi: 10.1002/pd.540. [DOI] [PubMed] [Google Scholar]
- 7.Mu S, Lin C, Chen Y, Sung T, Bai C, Jow G. The perinatal outcomes of asymptomatic isolated single umbilical artery in full term neonates. Pediatr Neonatol. 2008;49:230–3. doi: 10.1016/S1875-9572(09)60016-4. [DOI] [PubMed] [Google Scholar]
- 8.Predanic M, Perni SC, Friedman A, Chervenak F, Chasen S. Fetal growth assessment and neonatal birth weight in fetuses with an isolated single umbilical artery. Obstet Gynecol. 2005;105:1093–7. doi: 10.1097/01.AOG.0000158108.51397.f5. [DOI] [PubMed] [Google Scholar]
- 9.Hardy JB. The Collaborative Perinatal Project: lessons and legacy. Ann Epidemiol. 2003;13:303–11. doi: 10.1016/s1047-2797(02)00479-9. [DOI] [PubMed] [Google Scholar]
- 10.Klebanoff MA. The Collaborative Perinatal Project: a 50-year retrospective. Paediatr Perinat Epidemiol. 2008;23:2–8. doi: 10.1111/j.1365-3016.2008.00984.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Myrianthopoulos NC, French KS. An application of the US Bureau of the Census socioeconomic index to a large, diversified patient population. Soc Sci Med. 1968;2:283–99. doi: 10.1016/0037-7856(68)90004-8. [DOI] [PubMed] [Google Scholar]
- 12.Diggle PJ, Liang KY, Zeger SL. Analysis of longitudinal data. Oxford University Press; Oxford, UK: 1994. [Google Scholar]
- 13.Thummala MR, Raju TN, Langenberg P. Isolated single umbilical artery anomaly and the risk for congenital malformations: a meta-analysis. J Pediatr Surg. 1998;33:580–5. doi: 10.1016/s0022-3468(98)90320-7. [DOI] [PubMed] [Google Scholar]
- 14.Bourke WG, Clarke TA, Mathews TG, et al. Isolated single umbilical artery: the case for routine renal screening. Arch Dis Child. 1993;68:600–1. doi: 10.1136/adc.68.5_spec_no.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badr L, Bookheimer S, Purdy I, Deeb M. Predictors of neurodevelopmental outcome for preterm infants with brain injury: MRI, medical, and environmental factors. Early Hum Dev. 2009;85:279–84. doi: 10.1016/j.earlhumdev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talati AJ, Yang W, Yolton K, Korones SB, Bada HS. Combination of early perinatal factors to identify near-term and term neonates for neuroprotection. J Perinat. 2005;25:245–50. doi: 10.1038/sj.jp.7211259. [DOI] [PubMed] [Google Scholar]
- 17.Goldkrand JW, Pettigrew C, Lentz SU, Clements SP, Bryant JL, Hodges J. Volumetric umbilical artery blood flow: comparison of normal versus the single umbilical artery cord. J Matern Fetal Med. 2001;10:116–21. doi: 10.1080/714052729. [DOI] [PubMed] [Google Scholar]
- 18.Froehlich LA, Fujikura T. Significance of single umbilical artery. Am J Obstet Gynecol. 1966;94:274–9. doi: 10.1016/0002-9378(66)90476-5. [DOI] [PubMed] [Google Scholar]