Abstract
The length of the free-running period (τ) affects how an animal re-entrains after phase shifts of the LD cycle. Those with shorter periods adapt faster to phase advances than those with longer periods, while those with longer periods adapt faster to phase delays than those with shorter periods. The free-running period of humans, measured in temporal isolation units and in forced desychrony protocols in which the day length is set beyond the range of entrainment, ranges from about 23.5 to 26 hours, depending on the individual and the experimental conditions (e.g., temporal isolation vs. forced desychrony). We studied 94 subjects free-running through an ultradian LD cycle, which was a forced desychrony with a day length of 4 h (2.5 h awake in dim light, ~ 35 lux, alternating with 1.5 h for sleep in darkness). Circadian phase assessments were conducted before (baseline) and after (final) three 24-h days of the ultradian LD cycle. During these assessments, saliva samples were collected every 30 min and subsequently analyzed for melatonin. The phase shift of the dim light melatonin onset (DLMO) from the baseline to the final phase assessment gave the free-running period. The mean ± SD period was 24.31 ± .23 h and ranged from 23.7 to 24.9 h. Black subjects had a significantly shorter free-running period than Whites (24.18 ± .23 h, N=20 vs. 24.37 ± .22 h, N=55). We had a greater proportion of women than men in our Black sample, so to check the τ difference we compared the Black women to the White women. Again, Black subjects had a significantly shorter free-running period (24.18 ± .23, N=17 vs. 24.41 ± .23, N=23). We did not find any sex differences in the free-running period. These findings give rise to several testable predictions: on average, Blacks should adapt quicker to eastward flights across time zones than Whites, whereas Whites should adjust quicker to westward flights than Blacks. Also, Blacks should have more difficulty adjusting to night shift work and day sleep, which requires a phase delay. On the other hand, Whites should be more likely to have trouble adapting to the early work and school schedules imposed by society. More research is needed to confirm these results and predictions.
Keywords: circadian rhythms, circadian clock, human, intrinsic period, tau, melatonin, race, forced desynchrony
INTRODUCTION
Wever (1975) published a histogram showing the free-running periods of 137 subjects studied in the famous underground temporal isolation bunker in Andechs, Germany. The average free-running period (τ) was about 25 h, τ was normally distributed, ranging from about 24 to 26 h, and only one subject had a τ < 24 h. For many years, journal papers, text books and encyclopedias stated that humans have a free-running period of 25 h. Since then shorter averages have been reported for τ and it has also been shown that rigorous isolation from time cues is not necessary for humans to free-run.
People free-run in laboratory isolation apartments when given a clock (Middleton et al., 1996, 1997) and in submarines that schedule duty periods and meals according to clock times (Kelly et al., 1999). In fact, Wever (1979) showed that people in the bunker free-ran through an overhead 24 h LD cycle when given access to small auxiliary lights. They believed they were keeping a 24 h routine and that the lights were going on and off irregularly. One of the earliest reports of free-running despite access to time pieces was of a man who lived in a cave for 3 months. He had trouble sleeping at his usual times and eventually gave up trying to adhere to his usual schedule and free-ran with a period of 24.7 h (Mills, 1964).
Depending on the conditions, the average human free-running period can be much shorter than 25 h. Campbell et al. (1993) showed that when subjects were permitted to nap in the German bunkers, those who did nap had a shorter τ than those who did not (24.22 vs. 24.73 h). Middleton et al. (1996) studied men free-running in dim light (< 8 lux) and found an average period of 24.26 h. Middleton et al. noted that the longer τ observed in the Wever studies could be due to the fact that subjects were allowed evening bedside lamps and naps were not allowed. The current reasoning for this is that when subjects are forbidden to nap they will stay awake until they are very sleepy and absolutely sure that it is time for nighttime sleep. They stay up until the sleepiest circadian time, about the time of their temperature minimum (Wever, 1979), which happens to be close to the crossover point in the light phase response curve (PRC) (Revell & Eastman, 2005). Therefore, they will be exposed to light during the phase delay portion of the PRC and sleep through most of the phase advance portion, thus producing a daily phase delay and giving rise to a longer estimate of τ. In support of this idea is the fact that Wever (1979) found that the τ of 5 sighted subjects free-running in complete darkness was 24.48 ± .08h (mean ± SD), which was shorter than the average τ of 147 subjects free-running in constant light (25.00 ± .50 h). Blind free-running subjects, especially those who have been bilaterally enucleated, also cannot be phase delayed by light before their temperature minimum, and the average τ of the free-running blind is, as predicted, shorter than 25 h. Wever (1979) reported a τ of 24.50 ± .42 h in 6 blind people free-running in the bunkers. A study of 11 blind people living in the real world reported a τ of 24.55 ± .31 h (Sack et al., 1992). Lockley et al. (1997) listed the τs of 9 free-running subjects with no eyes, and when we averaged we got 24.50 ± .18 h. A more recent study found an average τ of 24.35 ± .30 h in 16 blind free-runners (Emens et al., 2010).
A popular way to assess τ in sighted people is with a forced desynchrony (FD) protocol in which the LD cycle is beyond the range of entrainment so that the circadian clock free-runs. Hiddinga et al. (1997) measured the temperature rhythm in a FD protocol with 20 h “days” (13.5 h of wake in dim light (< 10 lux) alternating with 6.5 h of dark for sleep) and reported an average τ of 24.30 ± .36 h in 12 men. Then Czeisler et al. (1999) reported τ from a 28 h day FD protocol (18.67 h of wake in dim light (< 15 lux) alternating with 9.33 h dark for sleep) and found that the average τ of 11 young men did not differ from that of 13 older subjects (men and women) and was 24.18 ± .13 h for both (when the τs from melatonin, temperature, and in some subjects, cortisol were averaged). Carskadon et al. (1999) found that the average τ for 10 adolescents (13.7 years old) in a 28 h FD protocol (16.33 h in dim light (15-20 lux), 11.67 h in dark for sleep) was 24.33 ± .21 h when measured by melatonin onsets throughout the FD and 24.30 ± .20 h by the temperature minimum in constant routines before and after the FD. In a retrospective analysis of 25 years of FD studies in dim light (<20 lux), Duffy et al. (2011) reported that the average τ was 24.15 ± .20 h in 157 subjects by body temperature and the same in a subset of 129 subjects whose τs were assess using the melatonin rhythm.
In general, the τs from sighted people in FD studies are shorter compared to blind free-runners, which could be due to the aftereffects of entrainment to a 24 h day. It is well known that when an animal is released from entrainment to short T cycles, e.g., 23 h, τ is shorter than when previously entrained to longer T cycles, e.g. 25 h (Pittendrigh & Daan 1976), and this is also true for humans (Scheer et al., 2007). Furthermore, when rodents are first released from entrainment to a 24 h LD cycle, their τs fall within a narrow range and then gradually disperse in the ensuing months; those with τs longer than 24 h get longer and those with τs shorter than 24 h get shorter (Pittendrigh & Daan, 1976). A study of free-running humans showed that τ gradually lengthened as the days in temporal isolation progressed (Endo et al., 1999). Thus, free-running blind people are expected to have the largest range of τs, because they have been freed from the 24 h LD cycle the longest. Since most people have τs > 24, the average τ of blind people is expected to be longer. Among the other reasons proposed for the longer τ of blind free-runners is that those with τs very close to 24 h are not included in the averages because they are mistakenly considered entrained.
Several factors, besides aftereffects, are known to influence τ in non-human animals. In animals free-running in constant light, light intensity influences τ (Aschoff, 1979). Access to a running wheel changes the τ of free-running rodents (Edgar et al., 1991; Yamada et al., 1988). More recently it was shown that resveratrol (found in many foods such as fruits, tea and wine) shortened the τ of nocturnal primates free-running in DD (Pifferi et al. 2011). Because of all the factors that influence τ, Roenneberg and Merrow (2007) state that “the concept of a stable intrinsic period seems to be an oxymoron.” Therefore, we will refrain from using the term “intrinsic” when referring to the endogenous free-running period (τ).
We have been determining τ since 2002 with an ultradian LD cycle (a 4-h FD protocol) as part of the protocol to generate PRCs (Burgess et al. 2008, 2010; Revell et al., in press; Revell & Eastman, 2005). We first reported a mean ± SD τ of 24.22 ± .22 h with an N of 32 using the melatonin rhythm (Burgess & Eastman 2008). We subsequently increased our sample size to 60 and found a mean tau of 24.24 ± .22 h, but with some unexpected racial differences (Smith et al. 2009a). The τ for self-reported African Americans (hereafter referred to as Blacks) was shorter than for Caucasian Americans (hereafter referred to as Whites), 24.09 ± .17 vs. 24.30 ± .23 h. However, our sample sizes were small (13 Blacks and 35 Whites) and we had many more Black women than Black men. Duffy et al. (2011) recently reported that women had a shorter τ than men (24.09 ± .20 vs. 24.19 ± .19 h by temperature and 24.08 ± .19 vs. 24.18 h ± .19 h by melatonin). We wondered whether our racial difference was due to having a larger proportion of women in the Black group than in the White group. We have now finished the PRC studies and can report our final sample size for τ of 94. Although we still have very few Black men, we have enough Black women to compare to White women. We report a shorter free-running period in Blacks than in Whites, even when only comparing the women.
MATERIALS AND METHODS
Subjects participated in two 5-day lab sessions, from Monday through Saturday, separated by one week. They lived in windowless, light controlled rooms during the lab sessions. For the week before each lab session they were required to keep fixed bed and wake times, tailored to their usual schedule, with fixed time in bed, ranging from 8 to 9 h depending on the individual. The lab sessions started with a baseline phase assessment day in which saliva samples were collected every 30 min in dim light (<5 lux) and later analyzed for melatonin. Then the subjects were put on the 4-h ultradian LD cycle with 2.5 h of wake in dim room light (~35 lux in the angle of gaze) alternating with 1.5 h of dark for sleep for about three 24-h days (18 to 20, 4-h “days”). The dim room light was produced by overhead fluorescent fixtures on a dimmer switch locked to the lowest position. The lab sessions ended with a day for the final phase assessment. During one of the lab sessions, they were given melatonin or exposed to a light box once a day, during the 3 days of ultradian LD cycles; during the other they remained in dim light, counterbalanced. The current report is an analysis of the data which is only from the lab sessions without melatonin or a light box. The free-running period was calculated by taking the change in phase from the baseline to the final phase assessment, dividing this by 4 (because there were 4 days between the phase assessments), and adding 24 h. The protocol diagram can be seen in Burgess et al., 2008, Fig 1; Burgess et al., 2010, Fig 1 or Smith et al., 2009a, Fig S1. The protocol was approved by the Rush University Medical Center Institutional Review Board and conformed to the standard of this journal (Portaluppi et al., 2010). For more methodological details see the papers cited above.
The melatonin profiles for this report were analyzed by first fitting each with a locally weighted least squares (LOWESS) curve (GraphPad Prism; GraphPad Software Inc., La Jolla, CA, USA). Then each curve was normalized by setting the maximum and minimum of the curve to 100% and 0%. The threshold for determining the dim light melatonin onset (DLMO) was 25% of each curve.
Our subjects were young and healthy, mostly in their 20s (ages ranged from 18 to 42, see Table 1), and not taking any prescription medications except for 15 women on oral contraceptives. Subjects completed a questionnaire that asked them to classify themselves as “Hispanic or Latino” or “not Hispanic or Latino.” They were also asked to check at least one racial category which consisted of the 5 required for reporting to the National Institutes of Health (White, Black or African American, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander). Some Hispanics would not endorse a race and all subjects who endorsed Hispanic or Latino were put in the Hispanic category regardless of whether or not they also endorsed a race. There was no difference between the Black and White subjects (or between the Black and White women) in age, morningness-eveningness score (Horne and Ostberg 1976), baseline DLMO, or home bedtime (lights out) or wake time (lights on) (Table 1). Nor were there any differences in these variables between the men and the women (Table 1). Subjects participated in all months of the year. There was no difference in the distribution of Blacks vs. Whites throughout the year.
Table 1.
Subject demographics, baseline dim light melatonin onset (DLMO) and home sleep schedule.
| N | Age | Morningness Eveningness | Baseline DLMO | Home Bedtime | Home Waketime | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| All | 94 | 25.8 | 5.2 | 51.9 | 8.3 | 21:54 | 1.5 | 00:05 | 1.1 | 08:20 | 1.0 |
| All Black | 20 | 24.8 | 4.0 | 51.0 | 7.0 | 22:07 | 1.5 | 23:52 | 1.2 | 08:08 | 1.2 |
| All White | 55 | 26.3 | 4.9 | 51.9 | 9.1 | 21:47 | 1.4 | 00:16 | 1.1 | 08:31 | 1.0 |
| Black Women | 17 | 24.1 | 3.3 | 50.2 | 6.1 | 22:14 | 1.6 | 00:02 | 1.2 | 08:16 | 1.3 |
| White Women | 23 | 25.2 | 4.2 | 53.8 | 9.4 | 21:28 | 1.2 | 23:46 | 1.0 | 08:05 | 0.9 |
| White Men | 32 | 27.0 | 5.2 | 53.6 | 8.8 | 22:02 | 1.5 | 00:38 | 1.0 | 08:49 | 0.9 |
| All Men | 45 | 27.1 | 6.0 | 51.4 | 8.5 | 22:06 | 1.4 | 00:20 | 1.1 | 08:32 | 1.0 |
| All Women | 49 | 24.7 | 4.0 | 52.2 | 8.1 | 21:44 | 1.5 | 23:51 | 1.0 | 08:08 | 1.1 |
NOTE: The categories “Black” and “White” are self-selected and do not include subjects who endorsed “Hispanic” or “Latino.” Home bedtime and wake time were assigned for the week before the lab session based on the subject's habitual sleep schedule and morning and evening commitments, if any.
RESULTS
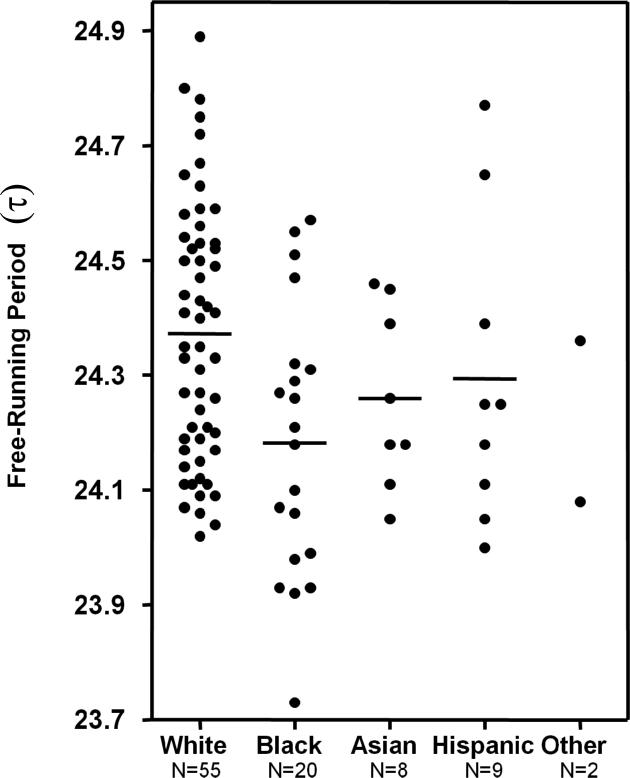
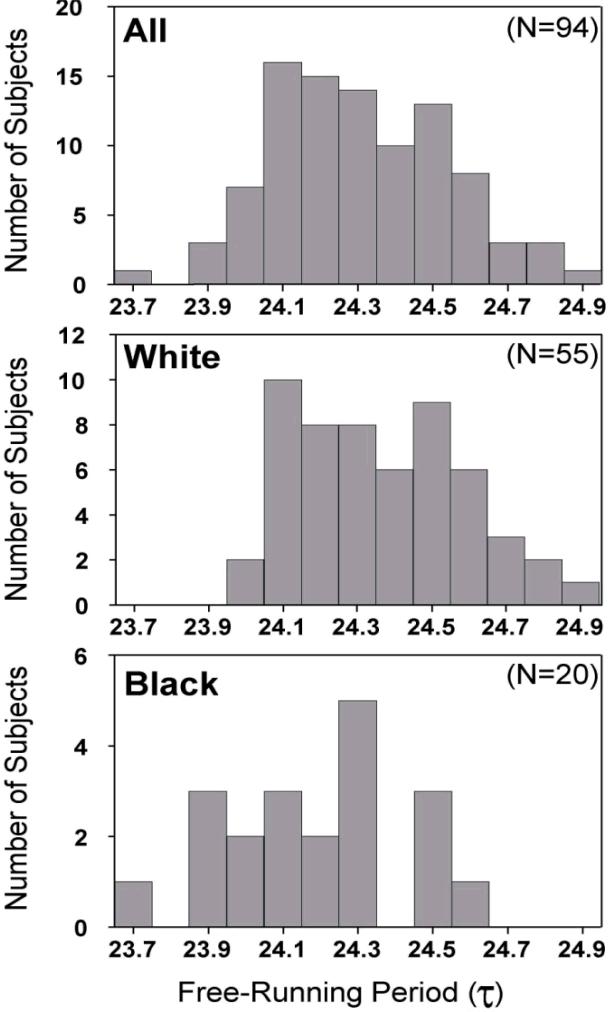
Figure 1 shows the free-running period (τ) for all 94 subjects. Although there is much overlap between Blacks and Whites, the difference in these distributions can be clearly seen. The difference in the distributions can also be seen in the frequency histograms (Fig 2). The difference in τ between all the Blacks and all the Whites was statistically significant, and the difference persisted when only Black women and White women were compared (Table 2). We only had data from 3 Black men, so we cannot do any meaningful comparisons between Black and White men. The differences in the τs between Blacks and Whites are summarized in Table 3. The only subjects who had a τ< 24 h were six Black subjects (five women and one man). The difference between Blacks and Whites in the proportion of subjects with τ < 24 was statistically significant by Fisher's exact tests (all Black subjects, 6/20=30% vs. all White subjects 0/55=0%, p<.0001; Black women 5/17=29.4% vs. White women 0/23=0%, p<.001).
Figure 1.
Free-running period length (τ) for each subject according to self-selected race/ethnicity. Horizontal lines represent means. τ was determined by the phase shift in the dim light melatonin onset (DLMO) from before to after 3 days of free-running through an ultradian LD cycle.
Figure 2.
Frequency histograms of the free-running period length (τ) for the entire sample and for the non-Hispanic Whites and non-Hispanic Blacks, determined by the phase shift in the DLMO from before to after the 3 days of free-running.
Table 2.
Free-running period lengths (τ)
| N | Period (hours) | ||
|---|---|---|---|
| Mean | SD | ||
| All | 94 | 24.31 | .23 |
| All Black | 20 | 24.18* | .23 |
| All White | 55 | 24.37 | .22 |
| Black Women | 17 | 24.18* | .23 |
| White Women | 23 | 24.41 | .23 |
| White Men | 32 | 24.34 | .22 |
| All Men | 45 | 24.32 | .22 |
| All Women | 49 | 24.31 | .25 |
NOTE: τ was determined by the phase shift of the DLMO from before to after 3 days of free-running through an ultradian LD cycle. Here the term Black is equivalent to non-Hispanic Black or non-Hispanic African-American, and White is equivalent to non-Hispanic White or non-Hispanic Caucasian-American.
p < .001, τ was shorter for all Blacks compared to all Whites, and was shorter for Black women compared to White women (t-tests).
Table 3.
Difference between Blacks and Whites in free-running period length (τ)
| Hours | Minutes | |
|---|---|---|
| All Black (N=20) vs. All White (N=55) | .19 | 11.4 |
| Black Women (N=17) vs. White Women (N=23) | .23 | 13.8 |
NOTE: τ was determined by the phase shift of the DLMO from before to after 3 days of free-running through an ultradian LD cycle. Here the term Black is equivalent to non-Hispanic Black or non-Hispanic African-American, and White is equivalent to non-Hispanic White or non-Hispanic Caucasian-American.
We did not find any sex differences in free-running period. The average τs of all the men and all the women are shown in the bottom 2 rows of Table 2. When we compared only the non-Hispanic White women vs. the non-Hispanic White men, again there was no difference (Table 2).
There was a significant correlation between the baseline DLMO and home bedtime (r = .58, p < .0001, N=94). There were weak correlations in the expected direction between τ and phase angle (the interval between the DLMO and the various lights on and lights off signals: sunrise, sunset, wake time, bed time), and a few approached statistical significance. For example, there was a trend for shorter τs to be associated with longer DLMO to sunrise intervals (r = -.19, p = .07, N=94). This correlation was slightly larger, and became statistically significant, when only the non-Hispanic Whites were considered (r = -.31, p = .02, N=55). In other words, subjects with shorter τs had earlier circadian rhythms relative to sunrise.
DISCUSSION
We found a shorter free-running period in Blacks compared to Whites, confirming our previous report (Smith et al., 2009a). This difference held up when we only included women, showing that the race difference was not an artifact due to having a greater proportion of women in the Black group. Duffy et al. (2011) found that τ was 6 min shorter in women than men, so it was conceivable that a group with more women would have a shorter τ. That potential confound does not apply when we compare our Black women to our White women. The Black women had a τ that was about 14 min shorter than the White women, and almost 30% of the Black women had a τ < 24 h, whereas none of the White women had a τ < 24.
We did not find a sex difference in τ when all the men and women were considered regardless of race/ethnicity, or when only the non-Hispanic White men and non-Hispanic White women were compared (the only male and female groups with the same race/ethnicity for which we had sufficient Ns). We also looked at τ calculated by the phase shift in melatonin midpoint, even though many researchers consider the DLMO more accurate (Lewy et al., 1999), because Duffy et al. (2011) used the fitted peak of the melatonin profile as their phase marker, and midpoint is closer to their measure. We still did not find a sex difference (men, N=44, 24.34 ± .18 h; women, N=49, 24.28 ± .22 h; non-Hispanic White men, N=31, 24.38 ± .18 h, non-Hispanic White women, N=23, 24.40 ± .24 h). We do not know the reason for the difference in the results between the two studies, although there were many methodological differences. Wever (1984) did not find a sex difference in the free-running period of the temperature rhythm during spontaneous internal desynchronization, but he did find a shorter τ in women when the rhythms were still internally synchronized.
There was much overlap in the distributions of τs between Blacks and Whites. This makes sense because most African Americans have both African and European ancestry. A recent study of 136 African-Americans (Zakharia et al., 2009) found that their genetic structure was a mixture of European American and indigenous West African. Individuals varied in the amount of European ancestry, with an average ± SD of 21.9% ± 12%.
What real world implications are there for differences in τ between Black and White people? Aschoff et al. (1975) showed that the rate of re-entrainment depends on τ, with a relatively short τ favoring advances and a relatively long τ favoring delays. Therefore, a shorter τ should make it easier to adjust to an eastward flight across time zones, but harder to adjust to a westward flight. A shorter τ should make it more difficult to delay to adjust to night shift work and the delayed (daytime) sleep schedule. On the other hand, a shorter τ should make it easier to advance and adjust to sleeping in the evening before night work, although most shift workers prefer not to sleep during evening family and social hours. We published preliminary data consistent with these predictions, showing that Blacks phase delay less than Whites when put on a delaying sleep schedule with nighttime bright light timed to facilitate the delay, and advance more when put on an advancing sleep schedule with morning light timed to facilitate the advance (Smith et al., 2009a).
In addition to differences in the speed of re-entrainment per se, a racial difference in τ also suggests that there could be racial disparities in disease risk. Night shift work causes circadian misalignment and health problems [reviewed in (Smith et al., 2009b)]. Blacks are more likely than Whites to have jobs that require shift work (McMenamin, 2007; Presser & Ward, 2011; United States Department of Labor and Bureau of Labor Statistics, 2005), and thus their exposure to shift work and its health risks is greater. Shorter τs should make it more difficult to adjust to night shift work, which could compound disease risk in Blacks. Interestingly, Black night and rotating shift workers reported hypertension more often than Black day workers, whereas there was no difference in hypertension between night/rotating shift workers and day workers for Whites (Ceide et al., 2012, Lieu et al., 2012).
Limitations of this study include that we did not have a sufficient sample of Black men, Asians or Hispanics for analyses with these groups. We do not know if the 4 h ultradian (T<24 h) FD used in this study produces a different τ than the infradian (T>24 h) FD cycles more commonly employed, such as 28 h. However, Duffy et al. (2011) found no difference in τ derived from 20 h, 28 h and 42.85 h FD protocols. Finally, there have been no studies to determine whether the light intensity during the FD influences τ. In any case, all our subjects were run in the same light intensity and with the same 4 h FD, so those factors cannot be responsible for the difference in τ between Blacks and Whites.
ACKNOWLEDGEMENTS
This work was supported by grants R01 HL086934 and R01 NR07677 to CIE from the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute, the National Institute of Nursing Research, or the National Institutes of Health. The National Heart Lung and Blood Institute, the National Institute of Nursing Research, and the National Institutes of Health had no involvement in designing the study, data collection, data analysis and interpretation, writing of the manuscript, nor in the decision to submit the manuscript for publication.
We thank the following people for their help with data collection: Daniel Alderson , Elisabeth Beam, Helen Burgess, Jillian Canton, Stephanie Crowley, Erin Cullnan, Rose Diskin, Sarah Garcia, Clifford Gazda, Heather Gunn, Cynthia Hiltz, Heather Holly, Clara Lee, Carlo Legasto, Vanessa Meyer, Jacqueline Munoz, Meredith Rathert, Yelizaveta Sorokin, Jessica Stroup, Christina Suh, Christine Tseng, Nicole Woodrick.
Footnotes
Declaration of Interest: We do not have any conflicts of interest.
REFERENCES
- Aschoff J. Circadian rhythms: Influences of internal and external factors on the period measured in constant conditions. Z Tierpsychol. 1979;49:225–249. doi: 10.1111/j.1439-0310.1979.tb00290.x. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Hoffmann K, Pohl H, Wever R. Re-entrainment of circadian rhythms after phase shifts of the zeitgeber. Chronobiologia. 1975;2:23–78. [PubMed] [Google Scholar]
- Burgess HJ, Eastman CI. Human tau in an ultradian light-dark cycle. J Biol Rhythms. 2008;23:374–376. doi: 10.1177/0748730408318592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol. 2008;586.2:639–647. doi: 10.1113/jphysiol.2007.143180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess HJ, Revell VL, Molina TA, Eastman CI. Human phase response curves to three days of daily melatonin: 0.5 mg versus 3.0 mg. J Clin Endocr Metab. 2010;95:3325–3331. doi: 10.1210/jc.2009-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell SS, Dawson D, Zulley J. When the human circadian system is caught napping: evidence for endogenous rhythms close to 24 hours. Sleep. 1993;16:638–640. [PubMed] [Google Scholar]
- Carskadon MA, Labyak SE, Acebo C, Seifer R. Intrinsic circadian period of adolescent humans measured in conditions of forced desynchrony. Neurosci Lett. 1999;260:129–132. doi: 10.1016/s0304-3940(98)00971-9. [DOI] [PubMed] [Google Scholar]
- Ceide ME, Pandey A, Olafiranye O, Pandy AK, Donat M, Brown CD, Jean-Louis G. Linking sleep duration to night shift-work and hypertension. Sleep. 2012;35:A203. [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Duffy JF, Cain SW, Chang A, Phillips AJK, Munch MY, Gronfier C, Wyatt JK, Dijk D, Wright KP, Czeisler CA. Sex difference in the near-24-hour intrinsic period of the human circadian timing system. PNAS. 2011;108:15602–15608. doi: 10.1073/pnas.1010666108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar DM, Kilduff TS, Martin CE, Dement WC. Influences of running wheel activity on free-running sleep/wake and drinking circadian rhythms in mice. Physiol Behav. 1991;50:373–378. doi: 10.1016/0031-9384(91)90080-8. [DOI] [PubMed] [Google Scholar]
- Emens J, Lewy AJ, Laurie AL, Songer JB. Rest-activity cycle and melatonin rhythm in blind free-runners have similar periods. J Biol Rhythms. 2010;25:381–384. doi: 10.1177/0748730410379080. [DOI] [PubMed] [Google Scholar]
- Endo T, Honma S, Hashimoto S, Honma K. After-effect of entrainment on the period of human circadian system. Jpn J Physiol. 1999;49:425–430. doi: 10.2170/jjphysiol.49.425. [DOI] [PubMed] [Google Scholar]
- Hiddinga AE, Beersma DGM, Van Den Hoofdakker RH. Endogenous and exogenous components in the circadian variation of core body temperature in humans. J Sleep Res. 1997;6:156–163. doi: 10.1046/j.1365-2869.1997.00047.x. [DOI] [PubMed] [Google Scholar]
- Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- Kelly TL, Neri DF, Grill JT, Ryman D, Hunt PD, Dijk DJ, Shanahan TL, Czeisler CA. Nonentrained circadian rhythms of melatonin in submariners scheduled to an 18-hour day. J Biol Rhythms. 1999;14:190–196. doi: 10.1177/074873099129000597. [DOI] [PubMed] [Google Scholar]
- Lewy AJ, Cutler NL, Sack RL. The endogenous melatonin profile as a marker of circadian phase position. J Biol Rhythms. 1999;14:227–236. doi: 10.1177/074873099129000641. [DOI] [PubMed] [Google Scholar]
- Lieu SJ, Curhan GC, Schernhammer ES, Forman JP. Rotating night shift work and disparate hypertension risk in African-Americans. J. Hypertens. 2012;30:61–66. doi: 10.1097/HJH.0b013e32834e1ea3. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Skene DJ, Arendt J, Tabandeh H, Bird AC, Defrance R. Relationship between melatonin rhythms and visual loss in the blind. J Clin Endocr Metab. 1997;82:3763–3770. doi: 10.1210/jcem.82.11.4355. [DOI] [PubMed] [Google Scholar]
- McMenamin TM. A time to work: recent trends in shift work and flexible schedules. Mon Labor Rev. 2007 Dec;130:3–15. [Google Scholar]
- Middleton B, Arendt J, Stone BM. Human circadian rhythms in constant dim light (8 lux) with knowledge of clock time. J Sleep Res. 1996;5:69–76. doi: 10.1046/j.1365-2869.1996.d01-67.x. [DOI] [PubMed] [Google Scholar]
- Middleton B, Arendt J, Stone BM. Complex effects of melatonin on human circadian rhythms in constant dim light. J Biol Rhythms. 1997;12:467–477. doi: 10.1177/074873049701200508. [DOI] [PubMed] [Google Scholar]
- Mills JN. Circadian rhythms during and after three months in solitude underground. J Physiol. 1964;174:217–231. doi: 10.1113/jphysiol.1964.sp007483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pifferi F, Dal-Pan A, Menaker M, Aujard F. Resveratrol dietary supplementation shortens the free-running circadian period and decreases body temperature in a prosimian primate. J Biol Rhythms. 2011;26:271–275. doi: 10.1177/0748730411401788. [DOI] [PubMed] [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. I. The stability and lability of spontaneous frequency. J Comp Physiol. 1976;106:223–252. [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–1929. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Presser HB, Ward BW. Nonstandard work schedules over the life course: a first look. Mon Labor Rev. 2011 Jul;:3–16. [Google Scholar]
- Revell VL, Eastman CI. How to trick Mother Nature into letting you fly around or stay up all night. J Biol Rhythms. 2005;20:353–365. doi: 10.1177/0748730405277233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revell VL, Molina TA, Eastman CI. Human phase response curve to intermittent blue light using a commercially available device. J. Physiol. doi: 10.1113/jphysiol.2012.235416. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roenneberg T, Merrow M. Entrainment of the human circadian clock. Cold Spring Harb Symp Quant Biol. 2007;72:293–299. doi: 10.1101/sqb.2007.72.043. [DOI] [PubMed] [Google Scholar]
- Sack RL, Lewy AJ, Blood ML, Keith LD, Nakagawa H. Circadian rhythm abnormalities in totally blind people: Incidence and clinical significance. J Clin Endocr Metab. 1992;75:127–134. doi: 10.1210/jcem.75.1.1619000. [DOI] [PubMed] [Google Scholar]
- Scheer FA, Wright KP, Jr., Kronauer RE, Czeisler CA. Plasticity of the intrinsic period of the human circadian timing system. PLoS ONE. 2007;2(8):e721. doi: 10.1371/journal.pone.0000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Burgess HJ, Fogg LF, Eastman CI. Racial differences in the human endogenous circadian period. PLoS ONE. 2009a;4(6):e6014. doi: 10.1371/journal.pone.0006014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Fogg LF, Eastman CI. Practical interventions to promote circadian adaptation to permanent night shift work: Study 4. J Biol Rhythms. 2009b;24:161–172. doi: 10.1177/0748730409332068. [DOI] [PubMed] [Google Scholar]
- United States Department of Labor, Bureau of Labor Statistics Workers on flexible and shift schedules in May 2004. 2005 Jul 1;:1–14. [Google Scholar]
- Wever RA. The circadian multi-oscillator system of man. Int J Chronobiol. 1975;3:19–55. [PubMed] [Google Scholar]
- Wever RA. The Circadian System of Man: Results of Experiments Under Temporal Isolation. Springer-Verlag; New York-Heidelberg-Berlin: 1979. p. 276. [Google Scholar]
- Wever RA. Sex differences in human circadian rhythms: Intrinsic periods and sleep fractions. Experientia. 1984;40:1226–1234. doi: 10.1007/BF01946652. [DOI] [PubMed] [Google Scholar]
- Yamada N, Shimoda K, Ohi K, Takahashi S, Takahashi K. Free-access to a running wheel shortens the period of free-running rhythm in blinded rats. Physiol. Behav. 1988;42:87–91. doi: 10.1016/0031-9384(88)90265-x. [DOI] [PubMed] [Google Scholar]
- Zakharia F, Basu A, Absher D, Assimes TL, Go AS, Hlatky MA, Iribarren C, Knowles JW, Li J, Narasimhan B, Sidney S, Southwick A, Myers RM, Quertermous T, Risch N, Tang H. Characterizing the admixed African ancestry of African Americans. Genome Biol. 2009;10:R141. doi: 10.1186/gb-2009-10-12-r141. [DOI] [PMC free article] [PubMed] [Google Scholar]