Abstract
Fibrodysplasia Ossificans Progressiva (FOP) is an autosomal dominant skeletal disorder characterized by widespread and debilitating bone formation in place of soft connective tissue. All mutations associated with FOP map to the BMP type I receptor, ALK2, with the vast majority of patients possessing the ALK2R206H mutation which results in hyperactive signaling. Here, we show that human ALK2R206H exhibits hyperactive signaling both in Drosophila cell culture and in vivo. As true for ALK2R206H–induced signaling in vertebrates, we find that the increase in signaling is also ligand-independent in Drosophila. Using the Drosophila system to identify factors required for this hyperactivity, we identified the type II receptor as a key determinant for mutant ALK2R206H receptor signaling. In addition, we found that the wild-type ALK2 receptor can antagonize, as well as promote, BMP signaling. Due to the heterozygosity typical of FOP, this dual function is of particular interest given that the interplay between the two disparate behaviors of wild-type ALK2 could be shifted by the presence of the hyperactive ALK2R206H mutant receptors. We present our work as a compelling example for the use of Drosophila as a model organism to study the molecular underpinnings of a complex human syndrome such as FOP.
Keywords: BMP signaling, ALK2, ACVR1, FOP, receptor kinase activation, Sax, Gbb, Dpp, Drosophila, type I receptor, type II receptor
INTRODUCTION
Fibrodysplasia Ossificans Progressiva (FOP) is a rare genetic disorder marked by the episodic deposition of heterotopic bone in the place of muscle and connective tissues throughout the life of a patient. All individuals with FOP have been found to carry a point mutation in one copy of their ALK2/ACVR1 genes that encodes a bone morphogenetic protein (BMP) type I receptor (Shore et al., 2006; Kaplan et al., 2009). FOP-associated mutations in ALK2/ACVR1 appear to produce hyperactive receptors, resulting in inappropriate BMP signaling (Billings et al., 2008; Fukuda et al., 2009; Kaplan et al., 2009; van Dinther et al., 2010).
Transforming Growth Factor-β (TGF-β)/BMP type I receptors are highly conserved, transmembrane receptor serine/threonine kinases that are an integral part of the TGF-β/BMP signal transduction pathway acting in a diverse array of cellular and developmental processes. Type I receptors mediate extracellular TGF-β/BMP signals as part of a receptor complex made up of two types of transmembrane serine/threonine kinase receptors, the type I receptors and the related type II receptors. This heteromeric receptor complex has been shown to assemble by two different mechanisms: 1) secreted ligands can induce receptor complex formation by binding the extracellular ligand-binding domains of the type I and type II receptors (Groppe et al., 2008; Nickel et al., 2009), or 2) the type I and type II receptors can interact independently of ligand to generate preformed complexes that then bind ligand to initiate signal transduction (Nohe et al., 2002; Hassel et al., 2003; Ehrlich et al., 2011; Marom et al., 2011). Upon formation of the ligand-bound receptor complex, activation of the type I receptor results from trans-phosphorylation by the constitutive kinase activity of the type II receptor. The cytoplasmic signal transducers, receptor-mediated Smads (R-Smads), are then phosphorylated by the activated type I receptors, enabling nuclear accumulation of co-Smad/R-Smad complexes that interact with other proteins to regulate transcription of specific target genes (Massagué, 1998; Wu and Hill, 2009).
The TGF-β/BMP type I receptors are characterized by a cysteine-rich, extracellular ligand-binding domain, a single-pass transmembrane domain, and a well-conserved, intracellular kinase domain (Massagué, 1998). The intracellular domain contains regulatory regions such as the L45 loop, which confers R-Smad specificity (Feng and Derynck, 1997; Persson et al., 1998), and a glycine-serine rich GS domain required for activation of the type I receptor kinase (Wrana et al., 1994; Franzén et al., 1995). The GS domain, once phosphorylated by the type II receptor, forms a secondary binding site for R-Smads in addition to the L45 loop (Huse et al., 2001).
Based largely on sequence homology and ligand specificity, two main groups emerge from the family of BMP type I receptors represented by the mammalian ALKs and Drosophila Tkv/Sax receptors: ALK3/ALK6/Tkv and ALK1/ALK2 (ACVR1)/Sax (Chen and Massagué, 1999; Newfeld et al., 1999). These two groups exhibit higher affinities for secreted BMP ligands from the two subfamilies, BMP2/4/Dpp and BMP5/6/7/Gbb, respectively. The differential affinity for ligands among type I receptors is thought to reside in the extracellular domain of the receptor, the least conserved domain of the polypeptide. The total number of ligands far outweighs the number of type I and type II receptors and while there is thought to be some functional redundancy, it is clear that the tissue context of various combination of ligands and receptors impacts signaling output. As such, mutations in just the type I receptors are associated with a number of unique diseases including, hereditary hemorrhagic telangiectasia type 2 (HHT2) (ALK1/ACVRL1), juvenile polyposis syndrome (ALK3/BMPR1A), brachydactyly type A2 (ALK6/BMPR1B) and the focus of this study fibrodysplasia ossificans progressiva (FOP) (ALK2/ACVR1) (Abdalla and Letarte, 2006; Bayrak-Toydemir et al., 2006; Wehner et al., 2006; Olivieri et al., 2007; Howe et al., 2001; Zhou et al., 2001; Kim et al., 2003; Lehmann et al., 2003; Lehmann et al., 2006).
Classic, as well as atypical FOP is characterized by progressive, heterotopic ossification that occurs through an endochondral process (Pignolo et al., 2005). Extraskeletal ossification is especially detrimental when it leads to immobilization of joints and restriction of organ function. Mortality associated with FOP often results from respiratory complications due to the fusion of rib bones interfering with the function of muscles, connective tissue and nerves in the intercostal space (Kaplan and Glaser, 2005). Interestingly, for the most part clinical features are not apparent at birth other than great toe malformations, a characteristic that is invariant in classic FOP (Shore et al., 2006). As a general rule the onset of FOP is delayed until early childhood, suggesting that the disease is developmental in nature and may require other triggers. Ossification is episodic and tends to occur in association with trauma or inflammation, thus rendering surgery an ineffective treatment (Kaplan et al., 2008).
Patients with the classic FOP mutation are defined by a 617G>A mutation in one copy of their ALK2 gene resulting in a histidine substitution at arginine 206 (R206H) (Shore et al., 2006), however, a small but growing list of variant mutations in other domains of the receptor are being identified (Billings et al., 2008; Furuya et al., 2008; Bocciardi et al., 2009; Petrie et al., 2009; Ohte et al., 2011). The classic R206H FOP mutation, which lies C-terminal to the GS domain in ALK2, leads to high levels of BMP signaling in a variety of systems (Billings et al., 2008; Shen et al., 2009; Song et al., 2010; van Dinther et al., 2010). Interestingly, the classic FOP mutation alters the identity of the amino acid just N-terminal to the conserved Thr/Gln residue that when mutated to Asp confers constitutive activity to TGF-β and BMP type I receptors (see Fig. 2A) (Wieser et al., 1995; Attisano et al., 1996; Akiyama et al., 1997; Macías-Silva et al., 1998; Chen and Massagué, 1999). Both the classic FOP mutation and the Thr/Gln to Asp mutation emphasize the importance of this region upstream of the type I receptor kinase domain as critical in controlling kinase activity.
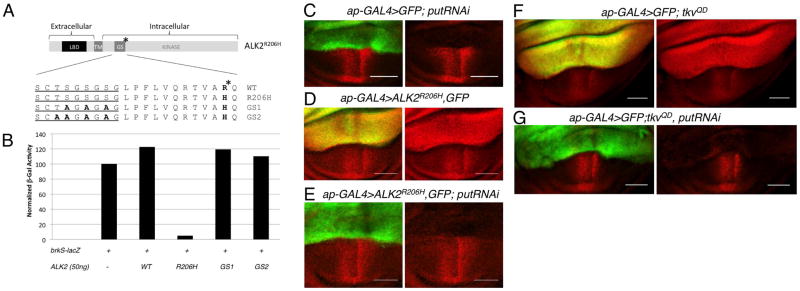
Figure 2.
Hyperactive signaling induced by ALK2R206H requires BMP type II receptor function. A: (Top) Diagram of the full length ALK2R206H receptor drawn to scale. LBD = ligand binding domain. TM= transmembrane domain. GS = glycine-serine rich domain/box. * indicates position of R206H mutation. (Below) Amino acid alignment of GS domains (from ALK2, ALK2R206H, ALK2GS1-R206H (GS1)and ALK2GS2-R206H (GS2). Glycine-serine rich sequence containing serine and threonine targets of type II receptor phosphorylation is underlined. B: brkS-lacZ signaling assay indicates ALK2GS1-R206H (GS1)and ALK2GS2-R206H (GS2) lack BMP signaling activity. Data plotted are mean of two experiments performed in duplicate. C–G: Stimulation of BMP signaling by ALK2R206H and TkvQD in the wing disc requires the type II receptor Punt. Confocal images of pMad distribution (red) in wing pouch of third larval instar wing discs, ap-GAL4 expression domain marked by UAS-GFP (green). C: Expression of putRNAi in dorsal compartment leads to dramatic reduction in pMad. ap-GAL4, UAS-GFP/+; UAS-put RNAi/+ D: Expression of ALK2R206H in dorsal compartment leads to an increase in pMad (red) levels, ap-GAL4, UAS-GFP/UAS-ALK2R206H. E: Coexpression of of putRNAi eliminates pMad increase associated with ALK2R206H as well as endogenous pMad, ap-GAL4,UAS-GFP/UAS-ALK2R206H; UAS-putRNAi/+. F: High levels of pMad are associated with expression of tkvQD in dorsal wing compartment, ap-GAL4, UAS-GFP/+; UAS-tkvQD/+. G: Coexpression of putRNAi eliminates BMP signaling induced by tkvQDas indicated by the loss of pMad, ap-GAL4, UAS-GFP/+; UAS-tkvQD/UAS-putRNAi. Scale bar = 50μm.
While the mutations responsible for FOP have been identified, the molecular details that result in the hyperactive behavior of mutated ALK2/ACVR1 type I receptors are not yet fully understood. Given the multiple roles for BMP signaling in early development, high levels of sustained signaling produced by a constitutively active receptor would certainly affect embryogenesis and result in lethality. Instead, the episodic nature of FOP, as well as the long latency or quiescent period prior to heterotopic bone formation in patients, indicates that the hyperactivity of the mutant receptor must be unleashed. This could occur through a conformational change in the ALK2FOP protein or through a change in its interaction with trans-acting factors that then enable an increase in overall signaling. As such, studies to elucidate the mechanism of ALK2/ACVR1 receptor activation and its function within an organismal context will most certainly advance our understanding of FOP. Finally, identifying the molecular events that are responsible for FOP-induced hyperactive BMP signaling will open up avenues for potential therapeutic approaches.
Drosophila has proven to be an outstanding model organism to study an increasing number of human diseases based on the high degree of molecular and functional conservation observed for genes known to be involved in both the signaling pathways and regulatory mechanisms governing development and homeostasis (Veraksa et al., 2000; Reiter and Bier, 2002; O’Kane, 2003; Bier, 2005; Botas, 2007; Chintapalli et al., 2007; Pandey and Nichols, 2011). Drosophila signaling components are largely non-redundant which circumvents the potential difficulty in interpreting pathway manipulations made in vertebrate systems where two or more closely related proteins may exhibit functional redundancy. As such, the initial identification of the core TGF-β/BMP signaling components benefited from the genetically tractable Drosophila system (Sekelsky et al., 1995; Newfeld et al., 1996; Zhang et al., 1996; Botas, 2007) as have many subsequent studies investigating their developmental roles and mode of action. For example, the role for BMP signaling in wing development has been particularly well-characterized from its importance in wing disc growth and establishing the overall pattern of longitudinal provein and intervein tissue, to its role in the actual differentiation of vein and intervein tissues during pupal development (Rogulja and Irvine, 2005; Bangi and Wharton, 2006a; O’Connor et al., 2006; Affolter and Basler, 2007; Blair, 2007; Rogulja et al., 2008; Oh and Irvine, 2011; Schwank et al., 2011; Wartlick et al., 2011a; Wartlick et al., 2011b). In the larva, a gradient of BMP signaling activity is generated across the wing primordia of the wing imaginal disc, through the action of two Drosophila BMP ligands, Dpp and Gbb, and their interactions with the type I receptors, Sax and Tkv, and the type II receptor, Punt. The resulting phospho-Mad (pMad) gradient reflects the output of BMP signaling, critical for regulating growth, cell survival, and cell fate specification through its transcriptional targets.
The high degree of functional conservation between the Drosophila and vertebrate BMP signaling pathway components is underscored by the interchangeability of their respective signaling components at each level of the pathway. For instance, dpp mutant phenotypes in Drosophila can be rescued by human BMP2 and BMP4, while BMP5, 6 & 7 can apparently rescue gbb lethality (Padgett et al., 1993; Fritsch et al., 2010). In mammalian cell culture, both Dpp and Gbb are capable of inducing bone formation (Sampath et al., 1993). At the receptor level, both of the Drosophila type I receptors Tkv and Sax can bind human BMP2 in combination with exogenous DAF-4, a C. elegans BMP type II receptor (Brummel et al., 1994; Penton et al., 1994). Furthermore, Smads can function in heterologous systems, as Drosophila Mad is able to direct the induction of ventral mesoderm in response to Xenopus BMP4 in Xenopus animal caps (Newfeld et al., 1996).
Previous work from our lab has demonstrated that Sax, the Drosophila ALK2 orthologue, has a dual function in its ability to both promote and to antagonize signaling (Bangi and Wharton, 2006b). Based on the ability of Sax to suppress wing phenotypes associated with gbb or dpp overexpression and the reduction of endogenous sax function to enhance these phenotypes, we hypothesized that Sax antagonizes BMP signaling by directly binding, in particular, its high-affinity ligand Gbb in receptor complexes that are not competent to transduce a signal. Consistent with this proposal, we find that Sax can block Gbb-induced signaling in a quantitative, cell-based signaling assay (Bangi and Wharton, 2006b). The ability of Sax to antagonize BMP signaling alone is borne out by the fact that the other type I receptor Tkv enhances rather than inhibits signaling induced by either Gbb or its high-affinity ligand Dpp. The ability of type I receptors from other organisms to antagonize BMP signaling has not been investigated, although recent studies suggest that ALK2 is able to inhibit activin signaling in MA-10 cells and inhibit BMP6-induced signaling in COS cells (Renlund et al., 2007; van Dinther et al., 2010). These reports coupled with the evolutionary relatedness of ALK2 and Sax raises the possibility that ALK2, like Sax, may have the ability to inhibit BMP signaling.
Here we report on a series of studies that investigated the use of Drosophila as a model to assess the consequences of, as well as the molecular factors required for hyperactive kinase activity associated with FOP mutant receptors. Our findings reveal that the ALK2R206H mutant receptor functions as a ligand-independent hyperactive type I receptor both in vivo and in Drosophila cell culture. Drosophila components are able to mediate the hyperactive signaling of the mutant human receptor and importantly, our findings have contributed to the mechanistic understanding of how defective FOP receptors signal by revealing that the type II receptor is a critical, molecular determinant required for ALK2R206H mutant receptor signaling. Additionally, we investigated the functional similarities between Sax and ALK2 and found that wild-type ALK2 is also able to block BMP signaling but it achieves this inhibition in a manner different from that employed by Sax. These results provide an important advance in our understanding of both the molecular events required for hyperactive signaling by a FOP mutant receptor and the wild-type behavior of the ALK2/ACVR1 receptor. Moreover, these studies provide a new tool for future investigations of the mechanistic attributes and the triggers responsible for activating FOP mutant receptors.
Results
FOP mutant receptor ALK2R206H stimulates increased BMP signaling in Drosophila
In order to test the ability of the human ALK2R206H classical FOP mutant receptor to signal in Drosophila, we generated transgenic lines that allowed us to control the expression of ALK2R206H in a tissue-specific manner (Brand and Perrimon, 1993) and assayed for the ability of ALK2R206H to induce BMP signaling in the developing wing. When UAS-ALK2R206H was expressed under the control of ap-GAL4 or A9-GAL4, lines that primarily express the transcriptional activator Gal4 in the dorsal compartment of the wing imaginal disc, adults were obtained, albeit unable to fully emerge from the pupal case. The wings from these individuals were misshapen and marked by ectopic veins (Fig. 1B; data not shown). In larvae of the same genotype we found a high level of pMad throughout the dorsal compartment of the wing imaginal disc compared to endogenous levels of pMad (Fig. 1C′,D). The ectopic pMad observed in these discs indicate that ALK2R206H is able to stimulate BMP signaling in Drosophila imaginal disc tissues, presumably through the direct phosphorylation of the Drosophila Smad1/5/8 orthologue Mad by its own kinase activity (Fig. 1D). As expected from the known role of BMP signaling in tissue growth (Capdevila and Guerrero, 1994; Haerry et al., 1998; Rogulja and Irvine, 2005; Affolter and Basler, 2007), we observed an increase in the size of the dorsal compartment of ap-GAL4>ALK2R206H wing discs (Fig. 1D) also evident in the adult as downwardly curved wings resulting from the enlargement of the dorsal surface. In a separate, quantitative cell-based BMP signaling assay, we obtained similar results as our in vivo studies indicating that ALK2R206H can induce an increase in BMP signaling (Fig. 1F). This cell-based assay makes use of a lacZ reporter construct under the control of the brinker silencer element (brkS) which is known to quantitatively repress transcription in response to Mad-mediated signaling (Müller et al., 2003; Bangi and Wharton, 2006b; Twombly et al., 2009). S2 cells transfected with a plasmid construct encoding the Drosophila ligand Gbb, exhibited a reduction in β-gal activity reflecting the repression of lacZ transcription as a result of an increase in BMP signaling (Fig. 1F). Cells transfected with a construct encoding the FOP mutant receptor ALK2R206H showed very high levels of BMP signaling (Fig. 1F).
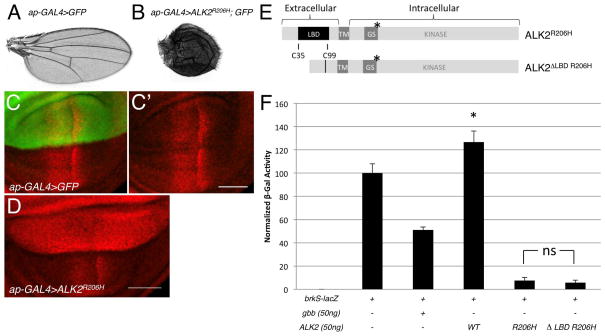
Figure 1.
In the Drosophila system the ALK2R206H FOP mutant receptor stimulates BMP signaling even in the absence of its ligand binding domain. A: Wild-type wing from control ap-GAL4, UAS-GFP/+ adult. B: Wing from ap-GAL4, UAS-GFP/UAS-ALK2R206H adult. C–D: Confocal images of pMad distribution (red) in the wing pouch of third instar larval wing discs. Scale bar= 50μm. C,C′: A representative ap-GAL4, UAS-GFP/+ control wing disc. The dorsal expression domain of ap-GAL4 is marked by GFP expression (green). The ventral compartment lacks expression of GFP. D: A representative ap-GAL4,UAS-GFP/UAS-ALK2R206H wing disc. E: Diagram of full length ALK2R206H and ligand-binding domain deletion mutant ALK2ΔLBD R206H drawn to scale. Amino acids from Cys35 to Cys99 were removed by site-directed mutagenesis. * indicates position of the R206H mutation. F: Quantitative brkS-lacZ assay measuring BMP signaling activity of ALK2R206H and ALK2ΔLBD R206H in S2 cell culture. Data represent mean +/− standard deviation (n=4). ns = not significant, p>0.05 brkS-lacZ +ALK2R206H versus brkS-lacZ+ALK2ΔLBD R206H(p=0.38). * p<0.05 versus brkS-lacZ transfection alone (p=0.005). We interpret this statistical significance to reflect the ability of ALK2 to inhibit endogenous BMP signaling in S2 cells. LBD = ligand binding domain. TM= transmembrane domain. GS = glycine-serine rich domain/box.
Extracellular ligand binding domain is not required for hyperactivity of ALK2R206H
The wild-type ALK2 receptor has been shown to promote Müllerian-inhibiting substance (MIS)-dependent signaling in mammalian systems (Clarke et al., 2001; Visser et al., 2001) and to bind the vertebrate ligands, Activin and BMP7 (Attisano et al., 1993; ten Dijke et al., 1994). However, ALK2R206H has been reported to signal independently of BMP ligands in zebrafish embryos and mammalian cells (Billings et al., 2008; Fukuda et al., 2009; Shen et al., 2009). In Drosophila, orthologues are evident for both BMP ligand subfamilies (BMP2/4 = Dpp and BMP5/6/7 = Gbb) and for the TGF-β/Activin subfamily (Daw, Actβ, Myo, Mav) (Kutty et al., 1998; Lo and Frasch, 1999; Nguyen et al., 2000; Parker et al., 2004; Parker et al., 2006; Moustakas and Heldin, 2009). Given ALK2’s promiscuity in binding ligands from different TGF-β families, we generated an ALK2R206H receptor that lacked the cysteine-rich (C38-C99), ligand-binding domain (LBD) to definitively test for the ability of ALK2R206H to signal independently of a specific ligand in the Drosophila system. By deleting the LBD of ALK2R206H, the mutated receptorwould be precluded from binding any TGF-β/BMP ligands (Fig. 1E). Cells transfected with the ALK2ΔLBD-R206H construct were able to induce BMP signaling at comparable levels to that achieved by the full-length ALK2R206H receptor (Fig. 1F), indicating that the signaling activity of the FOP mutant receptor is not only ligand-independent but that its ability to promote BMP signaling does not even require the presence a large portion of its extracellular domain (ECD) (Fig. 1E).
Type II receptor is required for hyperactivity of ALK2R206H
Given the proximity of the R206H FOP mutation to the GS domain, we questioned whether the hyperactivity of ALK2R206H was in fact dependent on the phosphorylation of its GS domain by the type II receptor as is typical during type I receptor activation, or if the R206H mutant receptor could induce signaling independently of the type II receptor as had been observed for the constitutively activating mutation at the adjacent residue in the TGF-β type I receptor (TβR1T204D) (Wieser et al., 1995; Macías-Silva et al., 1998). The importance of trans-phosphorylation of the ALK2R206H GS domain by the type II receptor kinase was tested by mutating GS domain Ser/Thr residues to Ala and assaying the mutated constructs for signaling activity (Fig. 2A,B). Both ALK2GS1-R206H (three Ser mutated to Ala) and ALK2GS2-R206H (all three Ser and the Thr mutated to Ala) resulted in the abrogation of signaling activity (Fig. 2B) indicating that the identity of these residues as Ser or Thr is critical for signaling, thus suggesting that their phosphorylation is required for the hyperactive signaling of the ALK2R206H receptor.
To test more rigorously the importance of type II receptor function for ALK2R206H hyperactivity, we made use of a UAS-put RNAi construct to knock down the expression of the Drosophila type II receptor, Punt, in vivo. Directed expression of put RNAi to the dorsal compartment of the wing imaginal disc using ap-GAL4 resulted in a dramatic loss of pMad (Fig. 2C) consistent with the requirement for put in BMP signaling (Letsou et al., 1995; Ruberte et al., 1995). The elevated levels of pMad induced by expression of ALK2R206H in the dorsal wing compartment (Fig. 2D) was largely suppressed when put RNAi was coexpressed (Fig. 2E) demonstrating that the activity of ALK2R206H is dependent on the presence of type II receptor function in vivo.
We next tested if the activity of ALK2R206H depends on a specific type II receptor. Given that knocking down endogenous Punt completely suppressed the elevated pMad levels in wing discs induced by ALK2R206H, we tested for the ability of the other Drosophila type II receptor, Wit, to restore hyperactive signaling in this experimental context. Indeed, we found that the expression of wit-HA with ALK2R206H and put RNAi led to elevated pMad indicating that the ability of ALK2R206H to signal is not dependent on a specific type II receptor (Fig. S1). The ability to restore signaling by ALK2R206H with the expression of Wit is consistent with our finding that ALK2R206H signaling requires the presence of a type II receptor.
The QD activating mutation in BMP type I receptors is dependent on type II receptor function
Previous reports have shown that in all members of the TGF-β/BMP family of type I receptors, mutation of the conserved Thr/Gln residue neighboring R206 (in ALK2) to Asp results in constitutive signaling (Wieser et al., 1995; Attisano et al., 1996; Akiyama et al., 1997; Macías-Silva et al., 1998; Chen and Massagué, 1999). The constitutive activity associated with TβR1T204D has been described as TβRII-independent (Wieser et al., 1995). However, as shown above, we found that the presence of a type II receptor is absolutely required for the signaling hyperactivity associated with ALK2R206H. These somewhat conflicting observations led us to question whether activating mutations in other type I receptors have a requirement for type II receptors. Indeed, we found that unlike TβR1T204D, constitutive signaling produced by the Drosophila BMP type I receptor, Tkv, carrying the equivalent mutation (Gln to Asp; TkvQD) is type II receptor-dependent. The high levels of pMad induced by TkvQD are suppressed by knocking down Punt with put RNAi (Fig. 2F,G). These data indicate an inherent difference in how TβR1 responds to an activating mutation compared to how the BMP type I receptor, Tkv, does. It is not yet clear whether this reflects a fundamental difference in the mechanism used by TGF-β and BMP type I receptors as it remains a possibility that TβR1 in the previous studies could have interacted with BMP or Activin type II receptors (Wieser et al., 1995; Chen et al., 1997).
ALK2 can inhibit BMP signaling
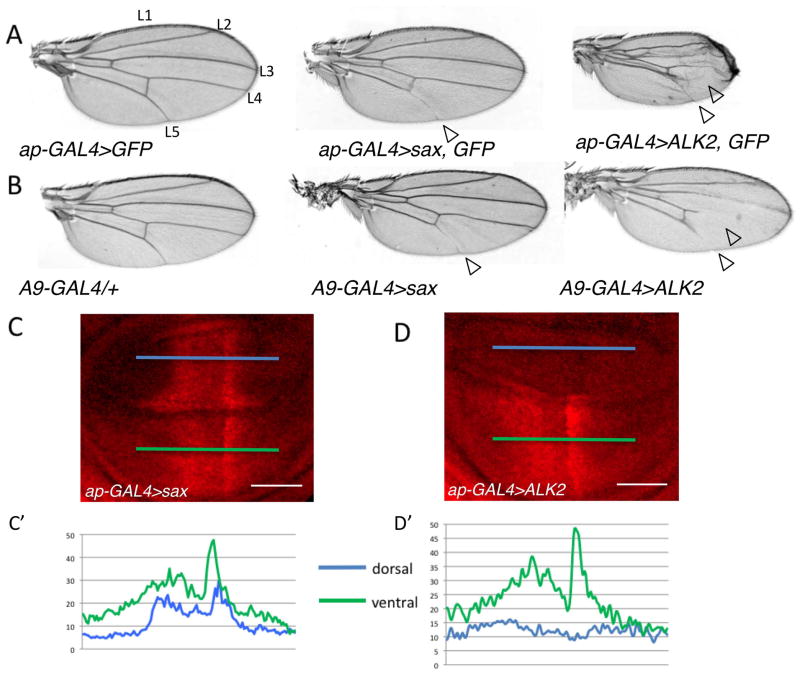
Clearly the FOP mutant receptor, ALK2R206H, exhibits high levels of signaling in Drosophila when expressed in vivo as well as in cell culture (Fig. 1, 2). We have previously shown that the Drosophila ALK2 orthologue, Sax, exhibits a dual function in its transduction of BMP signals (Bangi and Wharton, 2006b). Given this ability of Sax to both antagonize and mediate signaling, we considered the possibility that the hyperactivating ALK2 mutations associated with FOP may actually mask a normal dual function for ALK2. To test for the ability of wild-type ALK2 to inhibit BMP signaling we first compared the in vivo overexpression of wild-type ALK2 to that of Sax, under conditions known to reveal the inhibitory function of Sax (Fig. 3A,B). In both cases, we observed a loss or thinning of longitudinal vein 5 (L5), a phenotype associated with a loss of gbb function, as well as a reduction in the overall size of the wing, an indication of reduced BMP signaling (Wharton et al., 1999). In general, the ectopic expression of ALK2 produced more severe phenotypes than sax including a reduction in wing size and the additional loss of L4 (Fig. 3A, B). Similarly, a more dramatic loss of pMad is observed in the dorsal compartment of the wing pouch in wing imaginal discs when ALK2 is overexpressed compared to the narrowing of pMad distribution in discs from overexpressing Sax (Fig. 3C–D′). Taken together these results indicate that like overexpression of Sax, the overexpression of ALK2 leads to an effective reduction in BMP signaling.
Figure 3.
ALK2 can inhibit endogenous BMP signaling. A–B: Expression of ALK2, like sax, leads to loss of vein tissue (open arrowheads). A: Adult wings from ap-GAL4, UAS-GFP/+ (left), ap-GAL4, UAS-GFP/UAS-sax (middle), and ap-GAL4, UAS-GFP/UAS-ALK2 (right). B: Adult wings from A9-GAL4/+ (left), A9-GAL4/+; UAS-sax/+ (middle) and A9-GAL4/+; UAS-ALK2/+ (right). C–D′: ALK2 reduces pMad levels. C,D: Representative confocal images of pMad distribution (red) in wing pouch of third instar larval wing discs (C) ap-GAL4, UAS-GFP/UAS-sax, (D) ap-GAL4, UAS-GFP/UAS-ALK2. Scale bar = 50μm C′: Average pMad intensity profiles of the dorsal (blue line) and ventral (green line) compartments of ap-GAL4, UAS-GFP/UAS-sax wing discs (n=5). D′: Average pMad intensity profiles of the dorsal (blue line) and ventral (green line) compartments of ap-GAL4, UAS-GFP/UAS-ALK2 wing discs (n=5).
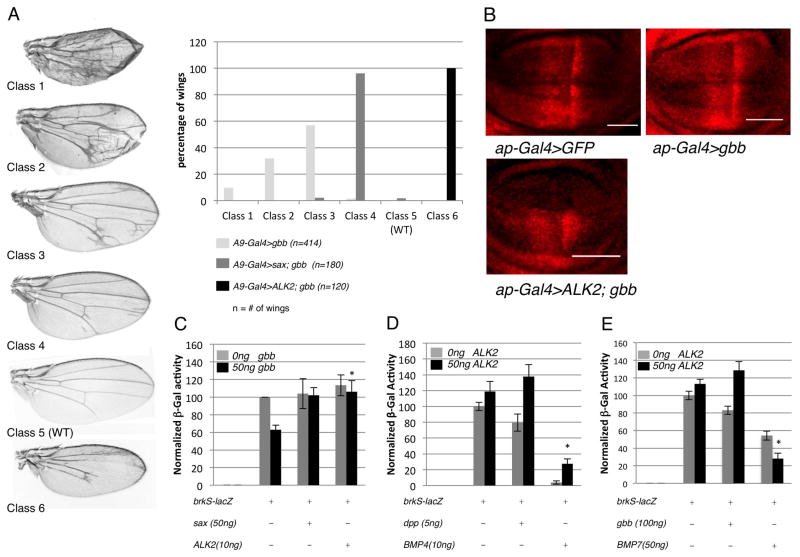
We and others have observed that Sax binds Gbb more effectively than Dpp, and as such we found that Sax can suppress the wing phenotypes produced by overexpression of Gbb better than those produced by the overexpression of Dpp (Haerry et al., 1998; Haerry, 2010). ALK2 has been shown to bind BMP7 but not BMP4 (ten Dijke et al., 1994; Macías-Silva et al., 1998; Greenwald et al., 2003) and given the evolutionary relatedness of Gbb and Dpp to BMP7 and BMP4, respectively (Sampath et al., 1993; Fritsch et al., 2010), we hypothesized that ALK2 would be able to effectively inhibit Gbb-induced BMP signaling. As observed previously, A9-GAL4>UAS-gbb resulted in an array of wing phenotypes marked by ectopic vein material, indicative of an increase in BMP signaling. The distribution of wing phenotypes can be shifted toward the less severe phenotypic classes when sax is coexpressed (Bangi and Wharton, 2006b) (Fig. 4A), consistent with the ability of Sax to antagonize BMP signaling and suppress the ectopic Gbb signaling. In a second set of experiments we made use of this phenotypic assay to test for the ability of ALK2 to antagonize signaling. We found that not only did coexpressing ALK2 with gbb suppress wing defects associated with ectopic Gbb signaling but that all A9-GAL4>UAS-ALK2; UAS-gbb wings exhibited phenotypes consistent with a reduction in endogenous BMP signaling, such as a reduction in wing size and a loss of longitudinal vein material (class 6) (Fig. 4A). An examination of pMad distribution in the wing disc confirmed this conclusion in that not only was the ectopic expression of pMad induced by gbb overexpression eliminated, but pMad associated with endogenous BMP signaling was also been dramatically reduced (Fig. 4B).
Figure 4.
ALK2 can inhibit exogenous, ligand-induced BMP signaling in a ligand-specific manner. A: (Left) Class 1 to Class 4: phenotypic distribution of adult wings from A9-GAL4/+;UAS-gbb9.1/+, Class 5: Wild-type, Class 6: Phenotype of A9-GAL4/+; UAS-ALK2/+; UAS-gbb9.1/+ adult wings. (Right) The shift in the gbb overexpression phenotype associated with coexpression of either sax or Alk2 suggests Gbb-induced signaling is antagonized. B: ALK2 can inhibit the increase in pMad (red) associated with Gbb expression in the dorsal compartment. (top left) ap-GAL4, UAS-GFP/+ (top right) ap-GAL4, UAS-GFP/+;UASgbb9.1/+ (bottom left) ap-GAL4, UAS-GFP/UAS ALK2; UASgbb9.1/+. C: ALK2 can antagonize BMP signaling induced by Gbb as measured by the brkS-lacZ reporter assay in S2 cell culture. Data represent mean +/− standard deviation (n=4). *compared to brkS-lacZ + 50ng gbb p<0.05 (p=0.006). D: ALK2 can antagonize BMP signaling induced by Dpp and human BMP4 (hBMP4) as measured by the brkS-lacZ reporter assay in S2 cell culture. Data represent mean +/− standard deviation (n=6). *compared to brkS-lacZ + 10ng BMP4 p<0.05 (p=0.0006). E: ALK2 enhances BMP signaling induced by mouse BMP7 (mBMP7) in the brkS-lacZ S2 cell culture assay. Data represent mean +/− standard deviation (n=3). *compared to brkS-lacZ + 50ng BMP7 p<0.05 (p=0.005).
The ability of ALK2 to inhibit BMP signaling was also tested in the quantitative, cell-based BMP signaling assay. Cotransfection of either sax or ALK2 with gbb resulted in a suppression of Gbb-induced signaling, indicating that both receptors were capable of inhibiting signaling (Fig. 4C). Interestingly, we found that signaling induced by transfections with either dpp or human BMP4 was also inhibited by ALK2 (Fig. 4D) while signaling induced by transfection of mouse BMP7 (see Fig. S2) was enhanced by ALK2, indicating that the ability of ALK2 to inhibit or promote BMP signaling is ligand-specific (Fig. 4D,E).
Inhibition by ALK2 is ligand-independent
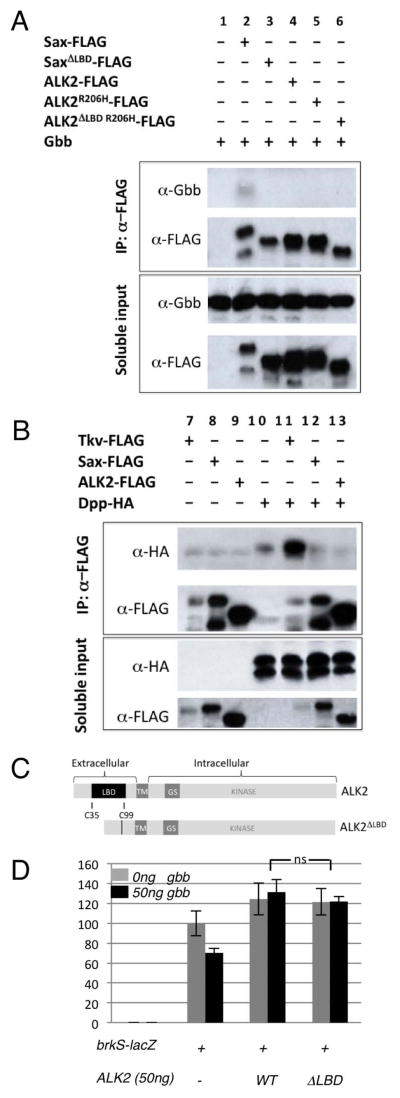
The ability of ALK2 to inhibit signaling induced by BMP4, a ligand it does not bind, coupled with its ability to enhance signaling induced by BMP7, a ligand that it does bind, raises the possibility that ALK2 may only inhibit signaling induced by BMP ligands that ALK2 itself does not bind. We investigated this possibility by testing the ability of ALK2 to bind Gbb and Dpp by co-immunoprecipitation. We were not able to detect an interaction between ALK2 and either Gbb or Dpp (Fig. 5A, lane 4 & Fig. 5B, lane 13, respectively) while the expected association between Gbb and Sax (Fig. 5A, lane 2) was apparent, as was a strong interaction between Dpp and Tkv (Fig. 5B, lane 11) with no to little interaction between Dpp and Sax (lane 12).
Figure 5.
ALK2 does not bind the Drosophila BMPs, Dpp or Gbb. A,B: Gbb co-immunoprecipitates with its high-affinity receptor Sax but not ALK2. Dpp-HA co-immunoprecipitates with its high-affinity receptor Tkv but not ALK2. C: Diagram of the full length ALK2 (LBD = ligand binding domain. TM= transmembrane domain. GS = glycine-serine rich domain/box) and ligand-binding domain deletion mutant ALK2ΔLBD with amino acids from Cys35 to Cys99 removed by site-directed mutagenesis. D: ALK2ΔLBD can inhibit Gbb-induced signaling in S2 cells. Data represent mean +/− standard deviation (n=3). ns (not significant) p>0.05 brkS-lacZ + ALK2WT + 50ng gbb vs. brkS-lacZ + ALK2ΔLBD + 50ng gbb (p=0.3).
It is possible that the affinity of ALK2 for the Drosophila BMP ligands was below the detectable limit of co-immunoprecipitations. Therefore, to more rigorously test for the importance of ALK2-ligand interactions, we deleted the cysteine-rich region of the extracellular (C38-C99) ligand-binding domain of ALK2 (ALK2ΔLBD) (Fig. 5C) and tested for the ability of this mutated receptor to block signaling. Interestingly, we found that ALK2ΔLBD was able to effectively block Gbb induced signaling in S2 cells (Fig. 5D), indicating that the ability of ALK2 to block BMP signaling is independent of a direct interaction with ligand and for that matter independent of a large portion of its extracellular domain. Thus, ALK2 must inhibit BMP signaling by some mechanism other than ligand sequestration.
DISCUSSION
The BMP signaling pathway exhibits a high degree of conservation throughout the metazoans (Newfeld et al., 1999). Consistent with this, we found that when the mutant form of the human ALK2 type I receptor (ALK2R206H), which is associated with the vast majority of fibrodysplasia ossificans progressiva (FOP) cases, (Shore et al., 2006; Billings et al., 2008) is expressed in Drosophila we mimic the misregulation of BMP signaling previously described in vertebrate systems (Billings et al., 2008; Fukuda et al., 2009; Shen et al., 2009; Song et al., 2010; van Dinther et al., 2010). Our results provide clear evidence that the Drosophila BMP signaling components are compatible with the human ALK2 type I receptor, such that the classic FOP mutation ALK2R206H manifests as hyperactive BMP signaling in Drosophila tissues as well. This finding bodes well for the use of the Drosophila system as a future tool to elucidate the molecular details responsible for misregulated BMP signaling associated with FOP despite the obvious differences in the ultimate consequence of this hyperactive signaling in Drosophila versus the formation of heterotopic bone in mammals.
Hyperactive BMP signaling requires type II receptor function
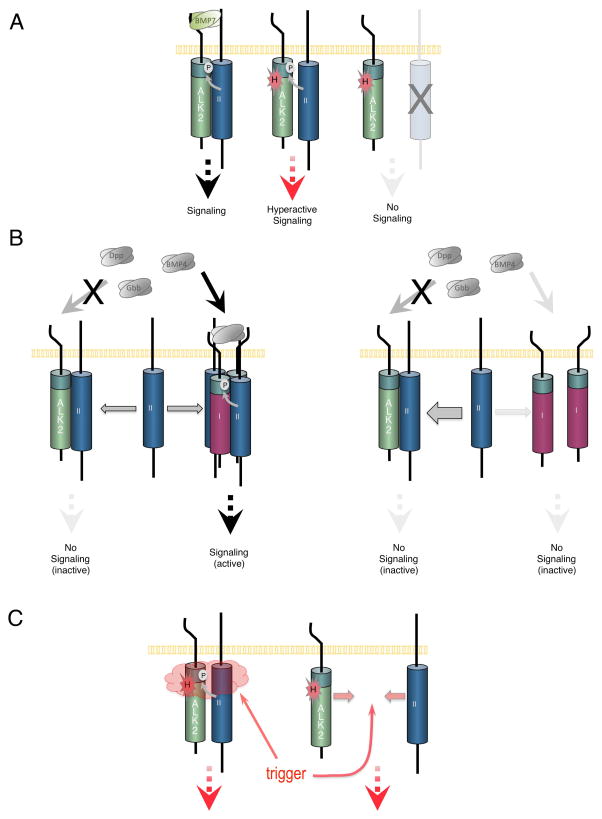
As in mammalian cells, we found that in Drosophila, ALK2R206H is able to induce high levels of phosphorylated Mad, the Drosophila Smad1/5/8 orthologue, in the absence of ligand. The ability of ALK2R206H to induce high levels of pMad is abrogated when the activation domain (GS domain) of the ALK2 receptor is mutated, suggesting that the kinase of ALK2R206H is directly responsible for phosphorylating Mad. While it remains formally possible in the various Drosophila assay systems we have tested that the endogenous Drosophila type I receptors Sax or Tkv are instead responsible for Mad phosphorylation in response to ALK2R206H expression, the level of these receptors should be far lower than ALK2R206H and the dependency of the pMad increase on an intact ALK2 GS domain makes this possibility less likely. Importantly, we found that the hyperactivity of ALK2R206H to signal is completely dependent on the function of a type II receptor kinase, which is responsible for activating type I receptors at their GS domain (Fig. 6A). The dependency of hyperactive signaling by ALK2R206H on the function of a type II receptor had not previously been appreciated in studies of FOP, and the fact that ALK2R206H can signal independently of ligand but requires type II receptor function indicates that ALK2R206H must be able to associate with type II receptors independently of ligand. Taken together, we envision a model in which the classic FOP mutation exposes the serine/threonine residues in the GS domain to phosphorylation by the type II receptor, thus circumventing the requirement for ligand to activate signaling (Fig. 6A).
Figure 6.
Models for ALK2R206H hyperactivity and ALK2 inhibition of BMP signaling. A: (Left) When bound by BMP7, the GS domain (green domain adjacent to membrane) of ALK2 is phosphorylated (P in white circle) by a type II receptor (blue receptor labeled “II”) leading to BMP signal transduction. (Middle) The classic R206H FOP mutation in ALK2 (H in red starburst) circumvents the ligand requirement for signaling by increasing the accessibility of ALK2’s GS domain to the type II receptor resulting in hyperactive signaling. (Right) In the absence of a functional type II receptor, ALK2R206H is not activated and unable to signal. B: (left) While ALK2 is unable to mediate signaling by BMP4, Gbb, or Dpp, these ligands can signal through other type I receptors (purple receptor labeled “I”). (right) In the absence of BMP7 and under conditions when the type I receptor population at the cell surface is enriched for ALK2 (as is the case during experimental overexpression of ALK2), BMP signaling in general is suppressed as a result of the titration of type II receptors away from productive signaling complexes, into inactive complexes with ALK2. C: Various events (trigger) that may act to allow hyperactive signaling of ALK2R206H could further increase GS domain accessibility by disrupting the interaction of a putative inhibitor, or could facilitate the interaction of ALK2R206H with available type II receptors.
Interestingly, our results show that the constitutively active BMP type I receptor TkvQD also shows a dependency for type II receptor function (Fig. 2G). This result is in contrast to that previously shown for the constitutively active TβR1T204D which signals in the absence of the TGF-β type II receptor, TβR-II (Wieser et al., 1995; Chen et al., 1997). While it has not yet been definitively shown that TβR1T204D signals independently of BMP or Activin type II receptors, these apparently conflicting data could reflect a fundamental difference in either the requirement for type II receptors or for the interaction of type I and type II receptors in BMP versus TGF-β signaling. Other key distinctions between TGF-β and BMP receptor signaling have been previously noted. For example, structural studies have shown that the assembly of BMP and TGF-β receptor complexes differ in that the extracellular domains of the BMP type I and II receptors do not contact one another, while an N-terminal extension in the extracellular domain of TGF-β type II receptors has been shown to directly interact with the type I receptor (Kirsch et al., 2000; Allendorph et al., 2006; Groppe et al., 2008). In addition, the minimal receptor complex required for BMP versus TGF-β signaling appears to differ. While a heterotrimeric (type I:type II:type II) BMP receptor complex is minimally required to transduce BMP signals (Isaacs et al., 2010), autonomously functioning TβRI:TβRII (type I:type II) heterodimers have been shown to be sufficient for the transduction of TGF-β signals (Huang et al., 2011).
In addition to divergent type II receptor requirements, mutations that confer hyperactivity or constitutive activity to TGF-β/BMP type I receptors differ in their respective effect on binding of the intracellular inhibitor FKBP12 to the type I receptor. FKBP12 has been proposed to prevent “leaky” ligand-independent signaling by masking the GS domain, until which time ligand binding results in its dissociation and signaling ensues (Chen et al., 1997; Huse et al., 1999; Huse et al., 2001; Wang and Donahoe, 2004). In a number of experiments, it has been shown that the R206H mutation reduces binding of FKBP12 making this an attractive molecular explanation for the hyperactivity displayed by ALK2R206H (Groppe et al., 2007; Shen et al., 2009; Song et al., 2010; Groppe et al., 2011). Curiously, the constitutively active Q207D mutation in ALK2 does not disrupt binding of FKBP12, whereas the equivalent constitutively active mutation in TβR1(T204D) does (Chen et al., 1997). In the case of the Drosophila FKBP12 orthologue FKBP2, our preliminary studies indicate that the loss of FKBP2 function in vivo did not produce phenotypes consistent with a substantial increase in BMP signaling (V. Le, S. Ballard, data not shown). Taken together, there does not appear to be a clear correlation between a loss or disruption of FKBP12 binding and the hyperactivity of mutant type I receptors. While we do not yet understand the mechanisms underlying the differential association of FKBP12 to ALK2R026H versus ALK2Q207D, such differences raise the possibility that in vivo, the constitutively active ALK2Q207D receptor behaves differently from the ALK2R206H FOP mutant receptor.
The finding that ALK2R206H hyperactive signaling depends on type II receptor function provides a new angle in the search for FOP therapeutics. Current strategies for drug development have focused on identifying small molecule inhibitors of type I receptor kinase activity (Yu et al., 2008a; Yu et al., 2008b; Hao et al., 2010). One such inhibitor, dorsomorphin, has been shown to effectively inhibit ALK2R206H kinase activity (Yu et al., 2008a; Fukuda et al., 2009; Shen et al., 2009; van Dinther et al., 2010), but unfortunately dorsomorphin non-specifically inhibits the kinase activity of other BMP type I receptors and appears to exhibit “off-target” effects on VEGF signaling (Yu et al., 2008b; Hao et al., 2010). In addition to future efforts to improve the selectivity of dorsomorphin analogs (Hao et al., 2010), alternative approaches that disrupt FOP-induced signaling are needed. An exciting new prospect for drug development could exploit our recently identified type II receptor requirement for ALK2R206H hyperactivity by focusing on the identification of molecules or factors that specifically block the interaction between ALK2R206H and type II receptors in FOP cells.
The wild-type ALK2 receptor can inhibit BMP signaling
In addition to our studies of ALK2R206H in the Drosophila system, we also analyzed the ability of wild-type ALK2 to mediate signaling. Since FOP is a dominant autosomal disease and all mutations isolated thus far are protein coding point mutations, the FOP mutant receptors must always be expressed in the presence of wild-type ALK2 receptor. Therefore, in order to understand the mechanistic underpinnings of FOP it is critical that we have a full understanding of wild-type ALK2 receptor function in addition to elucidating the consequences of the R206H mutation. Thus, we investigated the possibility that ALK2 can both promote and antagonize signaling, a behavior we discovered is exhibited by the Drosophila ALK2 orthologue, Sax (Bangi and Wharton, 2006b). Our results revealed that wild-type ALK2 receptor is indeed able to inhibit BMP signaling in vivo as well as in Drosophila cell culture. Interestingly, we found that the mechanism by which ALK2 accomplishes signaling inhibition likely differs from that employed by Sax. Whereas Sax likely inhibits signaling via the incorporation of its high-affinity ligand, Gbb, into inactive complexes, ALK2 appears to inhibit signaling induced by ligands that ALK2 itself does not actually bind. We propose that ALK2 accomplishes this inhibition by interacting with a type II receptor, such as Punt, and dominantly prevents Punt’s participation in a signaling complex that is activated by the binding of ligands such as Gbb, Dpp or BMP4 (Fig. 6B). A similar mechanism has been proposed to explain the negative effect of ALK2/ACVR1 on signaling induced by Activin which acts through a different set of core signaling components (Renlund et al., 2007). While specific binding of BMP6 to ALK2 has not been reported, ALK2 has also been observed to inhibit BMP6 induced signaling (van Dinther et al., 2010). However, in the case of BMP7 ligand, which has been shown to bind ALK2 (ten Dijke et al., 1994; Greenwald et al., 2003), Punt would not be excluded from signaling complexes, but rather ALK2 would facilitate BMP7 binding and enhance BMP7-induced signaling (Fig. 6A). In our model, ALK2 would then act as a modifier of receptor complex activity by dictating which BMP ligand the complex can bind. In other words, we propose that ALK2 determines whether the receptor complex that a type II receptor has participated in will be an active or inactive signaling complex depending on which BMP ligand is present (Fig. 6B). Therefore, the ability of ALK2 to regulate signaling based on BMP ligand type may have a profound impact on ligand-specific responses and warrants further investigation to determine if this dual behavior of ALK2 is observed endogenously.
On a separate note, the inability of ALK2 to bind Gbb was unexpected based on the demonstrated ability of ALK2 to bind BMP7 and the evolutionary relatedness of Gbb to the BMP5/6/7 subgroup. While the conserved domains of BMP5, BMP6 and BMP7 can reportedly rescue gbb mutant phenotypes (Fritsch et al., 2010), our results suggest that it is unlikely that Gbb can fully substitute for BMP7 function in vertebrates, specifically for BMP7-induced signaling mediated through ALK2.
Impact of Drosophila models for the study of FOP
Perhaps the least well understood aspect of FOP and most difficult for patients, is the sporadic and progressive nature of the disease. One of the primary difficulties still facing the FOP field is reconciling the molecular events of hyperactive signaling induced by the FOP receptor in animal models with the clinical features that manifest in patients. The sporadic nature of the disease contrasts with the hyperactivity that the mutant receptor displays in experimental assays suggesting that under endogenous conditions the activity of the mutant receptor must be regulated or muted until an event triggers a flare-up.
To date, all FOP patients are heterozygous for mutations in ALK2 regardless of whether they harbor the classic R206H or an atypical mutation. It is possible that one copy of ALK2WT can compete with ALK2FOP receptors for type II availability thereby keeping final output of BMP signaling below a threshold required for bone formation. Therefore, in an endogenous context the relative ratio between FOP type I receptors, wild-type type I receptors, and type II receptors may be a key determinant in whether or not activation of the pathway reaches a threshold necessary for bone formation. Alternatively, at physiological levels, the FOP mutant receptor activity might be inhibited by a different factor in trans and only when the mutant receptor is overexpressed under experimental conditions does it escape this negative regulation. Thus, in the future it will be important to study the behavior of the FOP mutant receptors when expressed at physiologically relevant levels achievable through homologous recombination. Making use of the Drosophila model system to express both mutant and wild-type receptors at endogenous levels will enable in vivo mutagenic screens to identify factors that suppress or enhance the effects of the ALK2R206H activity and in turn provide us with new targets for therapy and treatment. Furthermore, given the correlation between ossification and trauma, it has been suggested that inflammation associated with injury may in some way “trigger” heterotopic ossification. The specific molecule(s) associated with such triggers could act to increase the accessibility of the GS domain to the kinase activity of the type II receptor by disrupting the binding of a putative inhibitor, for example, or they could influence the ability of the ALK2R206H receptor to interact with available type II receptors (Fig. 6C). Although the precise mechanism(s) by which such putative modulators may influence the behavior of ALK2R206H remains unknown, the Drosophila system is a particularly attractive model organism in which to undertake such studies given the high conservation of pathways governing cellular physiology.
In closing, our work has demonstrated the value of using a Drosophila genetic system to study the molecular foundation of altered BMP signaling characteristic of individuals with FOP. Our experiments reveal a requirement for type II receptor function in the hyperactivity displayed by the ALK2R206H mutant receptor, a fact previously unappreciated. In addition, we have also observed the ability of the wild-type ALK2 receptor to inhibit BMP signaling in a ligand-specific manner. How these findings contribute to the sporadic nature of FOP as well as potentially impact our broad understanding of other diseases associated with misregulated type I receptor activity warrants further investigation. We intend to exploit the comprehensive genetic tools in Drosophila system to screen for potential modifiers of FOP mutant receptor activity as a means to bridge this gap.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
Gateway cloning (Invitrogen) was used to clone all cDNAs into the following Drosophila Gateway Vectors: pTWF for GAL4-UAS driven expression in transgenic animals and the Actin5C vector pAWF (C-terminal 3xFLAG) for constitutive expression in cell culture. ALK2 and ALK2R206H cDNAs were a generous gift from Eileen Shore. punt cDNA was a gift from Michael O’Connor. pAW gbb, pAW dppHA and pAW hBMP4 were constructed by Takuya Akiyama.
Ligand-binding domain deletions
Ligand-binding domain deletion mutants were generated by Quikchange Site-directed Mutagenesis (Stratagene). For ALK2ΔLBD, sequences corresponding to Cys35 to Cys 99 were removed using the following primers: fwd 5′-CAA CCC CAA ACT CTA CAT GAA CAG GAA CAT CAC GGC C-3′ and rev 5′-GGC CGT GAT GTT CCT GTT CAT GTA GAG TTT GGG GTT G-3′. For Sax, sequences corresponding to Cys67 to Cys148 were removed using the following primers: fwd 5′-CGC ATC CCA GAT ACA AAA ATG AGG GAG ACT TTC C-3′ and rev 5′-GGA AAG TCT CCC TCA TTT TTG TAT CTG GGA TGC G-3′.
GS domain mutants
Two sets of GS domain mutations were generated in ALK2R206H (Quikchange Site-Directed Mutagenesis). ALK2 GS1-R206H: all three serines were mutated to alanine (TSGSGSG > TAGAGAG) using the following primers: (ALK2 S190,192,194A fwd) 5′-CAG ATT TAT TGG ATC ATT CGT GTA CAG CAG GAG CTG GCG CTG GTC TTC CTT TTC TGG TAC -3′ and (ALK2 S190,192,194A rev) 5′-GTA CCA GAA AAG GAA GAC CAG CGC CAG CTC CTG CTG TAC ACG AAT GAT CCA ATA AAT CTG-3′. ALK2 GS2-R206H: a threonine and all three serines were mutated to alanine (TSGSGSG > AAGAGAG) using the following primers: (ALK2 T189A S190,192,194A fwd) 5′-CAG ATT TAT TGG ATC ATT CGT GTG CAG CAG GAG CTG GCG CTG GTC TTC CTT TTC TGG TAC-3′ and (ALK2 T189A S190,192,194A rev) 5′-GTA CCA GAA AAG GAA GAC CAG CGC CAG CTC CTG CTG CAC ACG AAT GAT CCA ATA AAT CTG-3′.
Drosophila melanogaster strains and crosses
All fly strains were cultured using standard sucrose, yeast extract agar food at 25°C. All fly strains as described in Flybase and obtained from Bloomington Stock Center except where noted: UAS-gbb9.1 (Khalsa et al., 1998), A9-GAL4, UAS- tkvQD (Haerry et al., 1998), UAS-wit-HA31 (Michael O’Connor). punt RNAi (from NIG-FLY, NIG 7904 R-2D). UAS-sax-3xFLAG(1-1M-A), UAS-ALK2-3xFLAG(8-1-9M-1a), and UAS-ALK2R206H-3xFlag(3-4F1-a) were germline transformants derived from constructs described above.
Receptor and gbb overexpression
Receptors and gbb were overexpressed using the UAS-GAL4 system (Brand and Perrimon, 1993). A9-GAL4 and ap-GAL4 drivers express primarily in the dorsal compartment of the wing imaginal disc.
in vivo Gbb signaling assay
A previously described in vivo assay (Bangi and Wharton, 2006b) was used to test for the ability of BMP type I receptors to affect Gbb signaling. Adult wings from the following genotypes were mounted (DPX, EM Sciences) and scored: w A9-GAL4/yw; +/+; UAS-gbb9.1/+ were compared to w A9-GAL4/yw, UAS-sax(1-1M-A)/+; UAS-gbb9.1/+ and w A9-GAL4/yw; UAS-ALK2(8-1-9M-1a)/+; UAS-gbb9.1.
Immunohistochemistry
Everted third instar larvae were dissected and fixed in 4% paraformaldehyde/PBS (v/v) for 20 minutes at room temperature followed by 5 washes in PBST (0.3% Triton X-100). Fixed tissues were then incubated overnight in blocking solution (10% NGS in PBST) at 4°C. After blocking, the cuticles were incubated in primary antibody diluted in blocking solution at the following dilutions: 1:1000 anti-FLAG M2 (Sigma, F3165), 1:1000 anti-HA 3F10 (Roche) and 1:1000 PS3 (Epitomics). Tissues were then washed 5 times with PBST and incubated in secondary antibody in blocking solution at the following dilutions: 1:1000 GAM Alexa Fluor 633, 1:1000 GARt Alexa Fluor405 (in WitHA experiments), 1:1000 GARb Alexa Fluor568. Following 5 washes in PBST, wing discs were removed and mounted in 80% glycerol/0.5% N-propyl gallate. Confocal images were collected using a Zeiss LSM510 Meta confocal laser scanning microscope.
Drosophila Schneider 2 (S2) cell maintenance and Transfections
S2 cells were cultured in Shields and Sang M3 Insect Medium (Sigma S8398) pH 6.5 containing 10% Insect Medium Supplement (Sigma I7267) and 2% Fetal Bovine Serum (F3018). Transient transfections were carried out using Effectene Transfection Reagent (Qiagen 301427).
Quantitative Cell-based BMP Signaling Assay
An adapted protocol based on a previously described assay was used to measure BMP signaling activity (Bangi and Wharton, 2006b; Müller et al., 2003; Twombly et al., 2009). This assay makes use of a reporter construct expressing lacZ under the control of a Su(H) transcriptional activation response element as well as a brk transcriptional silencer element (Su(H)/brkS-lacZ). Cotransfection of the reporter construct with plasmids encoding Su(H) and an activated form of Notch (N*) lead to lacZ transcription while the activation of BMP signaling leads to a repression of lacZ expression by virtue of the BMP-responsive brk silencer element. BMP signaling can thus be measured as a loss of β-galactosidase activity. 2.8×106 cells were cotransfected with Su(H), N*, Su(H)/brkS-lacZ, and luciferase plasmids, all under the control of the actin 5C promoter. Constructs and their concentrations used in this assay are indicated in the figure legends. Cells were harvested and lysed 3 days post-transfection and β-galactosidase activity of cleared lysate was measured using the dual luciferase assay system (Dual-Light, Applied Biosystems) and normalized to luciferase activity which served as a transfection control for each sample. The normalized value obtained from the cleared lysate of cells cotransfected with only Su(H), N*, Su(H)/brkS-lacZ and luciferase was set to 100%. Statistical significance was determined using two-tailed T-Test with significance value of 0.05. Epitope tagged and untagged versions of the type I receptors investigated in this study were compared for signaling activity and showed no significant difference (data not shown).
Co-immunoprecipitation
8×106 S2 cells were transiently transfected with 1ug total DNA at the following ratios:
Receptor-Ligand interaction
300ng pAWF type I receptor constructs and 700ng of either pAW dppHA or pAW gbb; cells were incubated at 25°C for 4 days for protein production. Cells were solubilized in 1% Triton X-100 at 4°C for 1 hour. Cleared lysate was incubated with 1ug anti-FLAG M2 (Sigma F3165) bound to 20uL of Dynabeads Protein G Dynabead (Invitrogen 100-04D) per sample at 4° for 1 hour. An aliquot of cleared lysate was saved as soluble input for western blot analysis. The beads were then washed once with one volume of Wash Buffer 1 (20mM Tris-HCl pH 7.4, 150mM NaCl, 0.2% Triton X-100), twice with one volume of Wash Buffer 2 (20mM Tris-HCl pH 7.4, 150mM NaCl), and boiled for 5 minutes in 50uL 2xSDS buffer. IP and soluble input fractions were run on 12% SDS-PAGE gels and analyzed by western blot using standard protocols. Anti-HA 3F10 (Roche) was used at 0.1ng/uL. Anti-Flag M2 (Sigma) was used at 4ng/uL, mouse anti-Gbb (gift from Guillermo Marquez) was used at a 1:1000 dilution. Secondary antibodies GAM IgG-HRP light-chain specific (Jackson) and GARat HRP (Jackson, preabsorbed) were used at a 1:10,000 dilution.
Image analysis
pMad Intensity Profiles
Intensity profiles of pMad distribution were measured by the Fiji Image Processing Package (http://fiji.sc/wiki/index.php/Fiji). The profiles shown are the average intensity plots measured in the dorsal and ventral compartments of five wing discs and aligned by the posterior and anterior peaks of pMad distribution in the ventral compartment.
Supplementary Material
Acknowledgments
Grant Sponsor: NIH; GM068118
The Center for Research in FOP and Related Disorders; Cali Family Grant
We gratefully acknowledge Eileen Shore for ALK2 and ALK2R206H clones, Petra Seeman for the BMP7 clone and Michael O’Connor for the punt clone. We enjoyed fruitful discussions with Drs. E. Shore, F. Kaplan and J. Groppe. We also thank members of the Wharton lab for their input during this project. We are indebted to the many FOP patients and Dr. Fred Kaplan who inspired us to pursue this work. This work was supported in part by a generous Cali Family Developmental Grant from The Center for Research in FOP and Related Disorders in The Department of Orthopaedic Surgery at The Perelman School of Medicine of The University of Pennsylvania and by a grant from the NIH GM068118 to K.A.W. NIH Training Grant T32 GM007601 to V.Q.L.
References
- Abdalla SA, Letarte M. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet. 2006;43:97–110. doi: 10.1136/jmg.2005.030833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Affolter M, Basler K. The Decapentaplegic morphogen gradient: from pattern formation to growth regulation. Nat Rev Genet. 2007;8:663–674. doi: 10.1038/nrg2166. [DOI] [PubMed] [Google Scholar]
- Akiyama S, Katagiri T, Namiki M, Yamaji N, Yamamoto N, Miyama K, Shibuya H, Ueno N, Wozney JM, Suda T. Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp Cell Res. 1997;235:362–369. doi: 10.1006/excr.1997.3680. [DOI] [PubMed] [Google Scholar]
- Allendorph GP, Vale WW, Choe S. Structure of the ternary signaling complex of a TGF-beta superfamily member. Proc Natl Acad Sci USA. 2006;103:7643–7648. doi: 10.1073/pnas.0602558103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano L, Cárcamo J, Ventura F, Weis FM, Massagué J, Wrana JL. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell. 1993;75:671–680. doi: 10.1016/0092-8674(93)90488-c. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Montalvo E, Massagué J. Activation of signalling by the activin receptor complex. Mol Cell Biol. 1996;16:1066–1073. doi: 10.1128/mcb.16.3.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangi E, Wharton K. Dpp and Gbb exhibit different effective ranges in the establishment of the BMP activity gradient critical for Drosophila wing patterning. Dev Biol. 2006a;295:178–193. doi: 10.1016/j.ydbio.2006.03.021. [DOI] [PubMed] [Google Scholar]
- Bangi E, Wharton K. Dual function of the Drosophila Alk1/Alk2 ortholog Saxophone shapes the Bmp activity gradient in the wing imaginal disc. Development. 2006b;133:3295–3303. doi: 10.1242/dev.02513. [DOI] [PubMed] [Google Scholar]
- Bayrak-Toydemir P, McDonald J, Markewitz B, Lewin S, Miller F, Chou L-S, Gedge F, Tang W, Coon H, Mao R. Genotype-phenotype correlation in hereditary hemorrhagic telangiectasia: mutations and manifestations. Am J Med Genet A. 2006;140:463–470. doi: 10.1002/ajmg.a.31101. [DOI] [PubMed] [Google Scholar]
- Bier E. Drosophila, the golden bug, emerges as a tool for human genetics. Nat Rev Genet. 2005;6:9–23. doi: 10.1038/nrg1503. [DOI] [PubMed] [Google Scholar]
- Billings PC, Fiori JL, Bentwood JL, O’Connell MP, Jiao X, Nussbaum B, Caron RJ, Shore EM, Kaplan FS. Dysregulated BMP signaling and enhanced osteogenic differentiation of connective tissue progenitor cells from patients with fibrodysplasia ossificans progressiva (FOP) J Bone Miner Res. 2008;23:305–313. doi: 10.1359/JBMR.071030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair SS. Wing vein patterning in Drosophila and the analysis of intercellular signaling. Annu Rev Cell Dev Biol. 2007;23:293–319. doi: 10.1146/annurev.cellbio.23.090506.123606. [DOI] [PubMed] [Google Scholar]
- Bocciardi R, Bordo D, Di Duca M, Di Rocco M, Ravazzolo R. Mutational analysis of the ACVR1 gene in Italian patients affected with fibrodysplasia ossificans progressiva: confirmations and advancements. Eur J Hum Genet. 2009;17:311–318. doi: 10.1038/ejhg.2008.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botas J. Drosophila researchers focus on human disease. Nat Genet. 2007;39:589–591. doi: 10.1038/ng0507-589. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brummel TJ, Twombly V, Marqués G, Wrana JL, Newfeld SJ, Attisano L, Massagué J, O’Connor MB, Gelbart WM. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell. 1994;78:251–261. doi: 10.1016/0092-8674(94)90295-x. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J. 1994;13:4459–4468. doi: 10.1002/j.1460-2075.1994.tb06768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Liu F, Massague J. Mechanism of TGFbeta receptor inhibition by FKBP12. EMBO J. 1997;16:3866–3876. doi: 10.1093/emboj/16.13.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Massagué J. Smad1 recognition and activation by the ALK1 group of transforming growth factor-beta family receptors. J Biol Chem. 1999;274:3672–3677. doi: 10.1074/jbc.274.6.3672. [DOI] [PubMed] [Google Scholar]
- Chintapalli VR, Wang J, Dow JAT. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- Clarke TR, Hoshiya Y, Yi SE, Liu X, Lyons KM, Donahoe PK. Müllerian inhibiting substance signaling uses a bone morphogenetic protein (BMP)-like pathway mediated by ALK2 and induces SMAD6 expression. Mol Endocrinol. 2001;15:946–959. doi: 10.1210/mend.15.6.0664. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- van Dinther M, Visser N, de Gorter DJJ, Doorn J, Goumans M-J, de Boer J, ten Dijke P. ALK2 R206H mutation linked to fibrodysplasia ossificans progressiva confers constitutive activity to the BMP type I receptor and sensitizes mesenchymal cells to BMP-induced osteoblast differentiation and bone formation. J Bone Miner Res. 2010;25:1208–1215. doi: 10.1359/jbmr.091110. [DOI] [PubMed] [Google Scholar]
- Ehrlich M, Horbelt D, Marom B, Knaus P, Henis YI. Homomeric and heteromeric complexes among TGF-β and BMP receptors and their roles in signaling. Cell Signal. 2011;23:1424–1432. doi: 10.1016/j.cellsig.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Feng X-H, Derynck R. A kinase subdomain of transforming growth factor-[beta] (TGF-[beta]) type I receptor determines the TGF-[beta] intracellular signaling specificity. EMBO J. 1997;16:3912–3923. doi: 10.1093/emboj/16.13.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzén P, Heldin CH, Miyazono K. The GS domain of the transforming growth factor-beta type I receptor is important in signal transduction. Biochem Biophys Res Commun. 1995;207:682–689. doi: 10.1006/bbrc.1995.1241. [DOI] [PubMed] [Google Scholar]
- Fritsch C, Lanfear R, Ray RP. Rapid evolution of a novel signalling mechanism by concerted duplication and divergence of a BMP ligand and its extracellular modulators. Dev Genes Evol. 2010;220:235–250. doi: 10.1007/s00427-010-0341-5. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Kohda M, Kanomata K, Nojima J, Nakamura A, Kamizono J, Noguchi Y, Iwakiri K, Kondo T, Kurose J, Endo K-ichi, Awakura T, Fukushi J, Nakashima Y, Chiyonobu T, Kawara A, Nishida Y, Wada I, Akita M, Komori T, Nakayama K, Nanba A, Maruki Y, Yoda T, Tomoda H, Yu PB, Shore EM, Kaplan FS, Miyazono K, Matsuoka M, Ikebuchi K, Ohtake A, Oda H, Jimi E, Owan I, Okazaki Y, Katagiri T. Constitutively activated ALK2 and increased SMAD1/5 cooperatively induce bone morphogenetic protein signaling in fibrodysplasia ossificans progressiva. J Biol Chem. 2009;284:7149–7156. doi: 10.1074/jbc.M801681200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya H, Ikezoe K, Wang L, Ohyagi Y, Motomura K, Fujii N, Kira J-I, Fukumaki Y. A unique case of fibrodysplasia ossificans progressiva with an ACVR1 mutation, G356D, other than the common mutation (R206H) Am J Med Genet A. 2008;146A:459–463. doi: 10.1002/ajmg.a.32151. [DOI] [PubMed] [Google Scholar]
- Greenwald J, Groppe J, Gray P, Wiater E, Kwiatkowski W, Vale W, Choe S. The BMP7/ActRII extracellular domain complex provides new insights into the cooperative nature of receptor assembly. Mol Cell. 2003;11:605–617. doi: 10.1016/s1097-2765(03)00094-7. [DOI] [PubMed] [Google Scholar]
- Groppe JC, Shore EM, Kaplan FS. Functional modeling of the ACVR1 (R206H) mutation in FOP. Clin Orthop Relat Res. 2007;462:87–92. doi: 10.1097/BLO.0b013e318126c049. [DOI] [PubMed] [Google Scholar]
- Groppe JC, Wu J, Shore EM, Kaplan FS. In vitro Analyses of the Dysregulated R206H ALK2 Kinase-FKBP12 Interaction Associated with Heterotopic Ossification in FOP. Cells Tissues Organs. 2011 doi: 10.1159/000324230. http://www.ncbi.nlm.nih.gov/pubmed/21525719. [DOI] [PMC free article] [PubMed]
- Groppe J, Hinck CS, Samavarchi-Tehrani P, Zubieta C, Schuermann JP, Taylor AB, Schwarz PM, Wrana JL, Hinck AP. Cooperative assembly of TGF-beta superfamily signaling complexes is mediated by two disparate mechanisms and distinct modes of receptor binding. Mol Cell. 2008;29:157–168. doi: 10.1016/j.molcel.2007.11.039. [DOI] [PubMed] [Google Scholar]
- Haerry TE, Khalsa O, O’Connor MB, Wharton KA. Synergistic signaling by two BMP ligands through the SAX and TKV receptors controls wing growth and patterning in Drosophila. Development. 1998;125:3977–3987. doi: 10.1242/dev.125.20.3977. [DOI] [PubMed] [Google Scholar]
- Haerry TE. The interaction between two TGF-beta type I receptors plays important roles in ligand binding, SMAD activation, and gradient formation. Mech Dev. 2010;127:358–370. doi: 10.1016/j.mod.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Hao J, Ho JN, Lewis JA, Karim KA, Daniels RN, Gentry PR, Hopkins CR, Lindsley CW, Hong CC. In vivo structure-activity relationship study of dorsomorphin analogues identifies selective VEGF and BMP inhibitors. ACS Chem Biol. 2010;5:245–253. doi: 10.1021/cb9002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassel S, Schmitt S, Hartung A, Roth M, Nohe A, Petersen N, Ehrlich M, Henis YI, Sebald W, Knaus P. Initiation of Smad-dependent and Smad-independent signaling via distinct BMP-receptor complexes. J Bone Joint Surg Am. 2003;85-A(Suppl 3):44–51. doi: 10.2106/00004623-200300003-00009. [DOI] [PubMed] [Google Scholar]
- Howe JR, Bair JL, Sayed MG, Anderson ME, Mitros FA, Petersen GM, Velculescu VE, Traverso G, Vogelstein B. Germline mutations of the gene encoding bone morphogenetic protein receptor 1A in juvenile polyposis. Nat Genet. 2001;28:184–187. doi: 10.1038/88919. [DOI] [PubMed] [Google Scholar]
- Huang T, David L, Mendoza V, Yang Y, Villarreal M, De K, Sun L, Fang X, López-Casillas F, Wrana JL, Hinck AP. TGF-β signalling is mediated by two autonomously functioning TβRI:TβRII pairs. EMBO J. 2011;30:1263–1276. doi: 10.1038/emboj.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse M, Chen YG, Massagué J, Kuriyan J. Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell. 1999;96:425–436. doi: 10.1016/s0092-8674(00)80555-3. [DOI] [PubMed] [Google Scholar]
- Huse M, Muir TW, Xu L, Chen YG, Kuriyan J, Massagué J. The TGF beta receptor activation process: an inhibitor- to substrate-binding switch. Mol Cell. 2001;8:671–682. doi: 10.1016/s1097-2765(01)00332-x. [DOI] [PubMed] [Google Scholar]
- Isaacs MJ, Kawakami Y, Allendorph GP, Yoon B-H, Belmonte JCI, Choe S. Bone morphogenetic protein-2 and -6 heterodimer illustrates the nature of ligand-receptor assembly. Mol Endocrinol. 2010;24:1469–1477. doi: 10.1210/me.2009-0496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Shen Q, Lounev V, Seemann P, Groppe J, Katagiri T, Pignolo RJ, Shore EM. Skeletal metamorphosis in fibrodysplasia ossificans progressiva (FOP) J Bone Miner Metab. 2008;26:521–530. doi: 10.1007/s00774-008-0879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Xu M, Seemann P, Connor JM, Glaser DL, Carroll L, Delai P, Fastnacht-Urban E, Forman SJ, Gillessen-Kaesbach G, Hoover-Fong J, Köster B, Pauli RM, Reardon W, Zaidi S-A, Zasloff M, Morhart R, Mundlos S, Groppe J, Shore EM. Classic and atypical fibrodysplasia ossificans progressiva (FOP) phenotypes are caused by mutations in the bone morphogenetic protein (BMP) type I receptor ACVR1. Hum Mutat. 2009;30:379–390. doi: 10.1002/humu.20868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan FS, Glaser DL. Thoracic Insufficiency Syndrome in Patients With Fibrodysplasia Ossificans Progressiva. BMM. 2005;3:213–216. [Google Scholar]
- Khalsa O, Yoon JW, Torres-Schumann S, Wharton KA. TGF-beta/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and establish cell identity in the Drosophila wing. Development. 1998;125:2723–2734. doi: 10.1242/dev.125.14.2723. [DOI] [PubMed] [Google Scholar]
- Kim I-J, Park J-H, Kang HC, Kim K-H, Kim J-H, Ku J-L, Kang S-B, Park SY, Lee J-S, Park J-G. Identification of a novel BMPR1A germline mutation in a Korean juvenile polyposis patient without SMAD4 mutation. Clin Genet. 2003;63:126–130. doi: 10.1034/j.1399-0004.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Sebald W, Dreyer MK. Crystal structure of the BMP-2-BRIA ectodomain complex. Nat Struct Biol. 2000;7:492–496. doi: 10.1038/75903. [DOI] [PubMed] [Google Scholar]
- Kutty G, Kutty RK, Samuel W, Duncan T, Jaworski C, Wiggert B. Identification of a new member of transforming growth factor-beta superfamily in Drosophila: the first invertebrate activin gene. Biochem Biophys Res Commun. 1998;246:644–649. doi: 10.1006/bbrc.1998.8678. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Seemann P, Boergermann J, Morin G, Reif S, Knaus P, Mundlos S. A novel R486Q mutation in BMPR1B resulting in either a brachydactyly type C/symphalangism-like phenotype or brachydactyly type A2. Eur J Hum Genet. 2006;14:1248–1254. doi: 10.1038/sj.ejhg.5201708. [DOI] [PubMed] [Google Scholar]
- Lehmann K, Seemann P, Stricker S, Sammar M, Meyer B, Süring K, Majewski F, Tinschert S, Grzeschik K-H, Müller D, Knaus P, Nürnberg P, Mundlos S. Mutations in bone morphogenetic protein receptor 1B cause brachydactyly type A2. Proceedings of the National Academy of Sciences. 2003;100:12277–12282. doi: 10.1073/pnas.2133476100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letsou A, Arora K, Wrana JL, Simin K, Twombly V, Jamal J, Staehling-Hampton K, Hoffmann FM, Gelbart WM, Massague J, O’Connor MB. Drosophila Dpp signaling is mediated by the punt gene product: a dual ligand-binding type II receptor of the TGF beta receptor family. Cell. 1995;80:899–908. doi: 10.1016/0092-8674(95)90293-7. [DOI] [PubMed] [Google Scholar]
- Lo PC, Frasch M. Sequence and expression of myoglianin, a novel Drosophila gene of the TGF-beta superfamily. Mech Dev. 1999;86:171–175. doi: 10.1016/s0925-4773(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Macías-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- Marom B, Heining E, Knaus P, Henis YI. Formation of Stable Homomeric and Transient Heteromeric Bone Morphogenetic Protein (BMP) Receptor Complexes Regulates Smad Protein Signaling. J Biol Chem. 2011;286:19287–19296. doi: 10.1074/jbc.M110.210377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin C-H. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- Müller B, Hartmann B, Pyrowolakis G, Affolter M, Basler K. Conversion of an extracellular Dpp/BMP morphogen gradient into an inverse transcriptional gradient. Cell. 2003;113:221–233. doi: 10.1016/s0092-8674(03)00241-1. [DOI] [PubMed] [Google Scholar]
- Newfeld SJ, Chartoff EH, Graff JM, Melton DA, Gelbart WM. Mothers against dpp encodes a conserved cytoplasmic protein required in DPP/TGF-beta responsive cells. Development. 1996;122:2099–2108. doi: 10.1242/dev.122.7.2099. [DOI] [PubMed] [Google Scholar]
- Newfeld SJ, Wisotzkey RG, Kumar S. Molecular evolution of a developmental pathway: phylogenetic analyses of transforming growth factor-beta family ligands, receptors and Smad signal transducers. Genetics. 1999;152:783–795. doi: 10.1093/genetics/152.2.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Parker L, Arora K. Identification of maverick, a novel member of the TGF-beta superfamily in Drosophila. Mech Dev. 2000;95:201–206. doi: 10.1016/s0925-4773(00)00338-5. [DOI] [PubMed] [Google Scholar]
- Nickel J, Sebald W, Groppe JC, Mueller TD. Intricacies of BMP receptor assembly. Cytokine Growth Factor Rev. 2009;20:367–377. doi: 10.1016/j.cytogfr.2009.10.022. [DOI] [PubMed] [Google Scholar]
- Nohe A, Hassel S, Ehrlich M, Neubauer F, Sebald W, Henis YI, Knaus P. The mode of bone morphogenetic protein (BMP) receptor oligomerization determines different BMP-2 signaling pathways. J Biol Chem. 2002;277:5330–5338. doi: 10.1074/jbc.M102750200. [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD. Cooperative regulation of growth by Yorkie and Mad through bantam. Dev Cell. 2011;20:109–122. doi: 10.1016/j.devcel.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohte S, Shin M, Sasanuma H, Yoneyama K, Akita M, Ikebuchi K, Jimi E, Maruki Y, Matsuoka M, Namba A, Tomoda H, Okazaki Y, Ohtake A, Oda H, Owan I, Yoda T, Furuya H, Kamizono J, Kitoh H, Nakashima Y, Susami T, Haga N, Komori T, Katagiri T. A novel mutation of ALK2, L196P, found in the most benign case of fibrodysplasia ossificans progressiva activates BMP-specific intracellular signaling equivalent to a typical mutation, R206H. Biochem Biophys Res Commun. 2011;407:213–218. doi: 10.1016/j.bbrc.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Olivieri C, Pagella F, Semino L, Lanzarini L, Valacca C, Pilotto A, Corno S, Scappaticci S, Manfredi G, Buscarini E, Danesino C. Analysis of ENG and ACVRL1 genes in 137 HHT Italian families identifies 76 different mutations (24 novel). Comparison with other European studies. J Hum Genet. 2007;52:820–829. doi: 10.1007/s10038-007-0187-5. [DOI] [PubMed] [Google Scholar]
- O’Connor MB, Umulis D, Othmer HG, Blair SS. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development. 2006;133:183–193. doi: 10.1242/dev.02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Kane CJ. Modelling human diseases in Drosophila and Caenorhabditis. Semin Cell Dev Biol. 2003;14:3–10. doi: 10.1016/s1084-9521(02)00162-3. [DOI] [PubMed] [Google Scholar]
- Padgett RW, Wozney JM, Gelbart WM. Human BMP sequences can confer normal dorsal-ventral patterning in the Drosophila embryo. Proc Natl Acad Sci USA. 1993;90:2905–2909. doi: 10.1073/pnas.90.7.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey UB, Nichols CD. Human disease models in Drosophila melanogaster and the role of the fly in therapeutic drug discovery. Pharmacol Rev. 2011;63:411–436. doi: 10.1124/pr.110.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Stathakis DG, Arora K. Regulation of BMP and activin signaling in Drosophila. Prog Mol Subcell Biol. 2004;34:73–101. doi: 10.1007/978-3-642-18670-7_4. [DOI] [PubMed] [Google Scholar]
- Parker L, Ellis JE, Nguyen MQ, Arora K. The divergent TGF-beta ligand Dawdle utilizes an activin pathway to influence axon guidance in Drosophila. Development. 2006;133:4981–4991. doi: 10.1242/dev.02673. [DOI] [PubMed] [Google Scholar]
- Penton A, Chen Y, Staehling-Hampton K, Wrana JL, Attisano L, Szidonya J, Cassill JA, Massagué J, Hoffmann FM. Identification of two bone morphogenetic protein type I receptors in Drosophila and evidence that Brk25D is a decapentaplegic receptor. Cell. 1994;78:239–250. doi: 10.1016/0092-8674(94)90294-1. [DOI] [PubMed] [Google Scholar]
- Persson U, Izumi H, Souchelnytskyi S, Itoh S, Grimsby S, Engström U, Heldin CH, Funa K, ten Dijke P. The L45 loop in type I receptors for TGF-beta family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 1998;434:83–87. doi: 10.1016/s0014-5793(98)00954-5. [DOI] [PubMed] [Google Scholar]
- Petrie KA, Lee WH, Bullock AN, Pointon JJ, Smith R, Russell RGG, Brown MA, Wordsworth BP, Triffitt JT. Novel mutations in ACVR1 result in atypical features in two fibrodysplasia ossificans progressiva patients. PLoS ONE. 2009;4:e5005. doi: 10.1371/journal.pone.0005005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignolo RJ, Suda RK, Kaplan FS. The Fibrodysplasia Ossificans Progressiva Lesion. BMM. 2005;3:195–200. [Google Scholar]
- Reiter LT, Bier E. Using Drosophila melanogaster to uncover human disease gene function and potential drug target proteins. Expert Opin Ther Targets. 2002;6:387–399. doi: 10.1517/14728222.6.3.387. [DOI] [PubMed] [Google Scholar]
- Renlund N, O’Neill FH, Zhang L, Sidis Y, Teixeira J. Activin receptor-like kinase-2 inhibits activin signaling by blocking the binding of activin to its type II receptor. J Endocrinol. 2007;195:95–103. doi: 10.1677/JOE-07-0281. [DOI] [PubMed] [Google Scholar]
- Rogulja D, Irvine KD. Regulation of cell proliferation by a morphogen gradient. Cell. 2005;123:449–461. doi: 10.1016/j.cell.2005.08.030. [DOI] [PubMed] [Google Scholar]
- Rogulja D, Rauskolb C, Irvine KD. Morphogen control of wing growth through the Fat signaling pathway. Dev Cell. 2008;15:309–321. doi: 10.1016/j.devcel.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruberte E, Marty T, Nellen D, Affolter M, Basler K. An absolute requirement for both the type II and type I receptors, punt and thick veins, for dpp signaling in vivo. Cell. 1995;80:889–897. doi: 10.1016/0092-8674(95)90292-9. [DOI] [PubMed] [Google Scholar]
- Sampath TK, Rashka KE, Doctor JS, Tucker RF, Hoffmann FM. Drosophila transforming growth factor beta superfamily proteins induce endochondral bone formation in mammals. Proc Natl Acad Sci USA. 1993;90:6004–6008. doi: 10.1073/pnas.90.13.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G, Dalessi S, Yang S-F, Yagi R, de Lachapelle AM, Affolter M, Bergmann S, Basler K. Formation of the long range Dpp morphogen gradient. PLoS Biol. 2011;9:e1001111. doi: 10.1371/journal.pbio.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky JJ, Newfeld SJ, Raftery LA, Chartoff EH, Gelbart WM. Genetic characterization and cloning of mothers against dpp, a gene required for decapentaplegic function in Drosophila melanogaster. Genetics. 1995;139:1347–1358. doi: 10.1093/genetics/139.3.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Q, Little SC, Xu M, Haupt J, Ast C, Katagiri T, Mundlos S, Seemann P, Kaplan FS, Mullins MC, Shore EM. The fibrodysplasia ossificans progressiva R206H ACVR1 mutation activates BMP-independent chondrogenesis and zebrafish embryo ventralization. J Clin Invest. 2009;119:3462–3472. doi: 10.1172/JCI37412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Xu M, Feldman GJ, Fenstermacher DA, Cho T-J, Choi IH, Connor JM, Delai P, Glaser DL, LeMerrer M, Morhart R, Rogers JG, Smith R, Triffitt JT, Urtizberea JA, Zasloff M, Brown MA, Kaplan FS. A recurrent mutation in the BMP type I receptor ACVR1 causes inherited and sporadic fibrodysplasia ossificans progressiva. Nat Genet. 2006;38:525–527. doi: 10.1038/ng1783. [DOI] [PubMed] [Google Scholar]
- Song G-A, Kim H-J, Woo K-M, Baek J-H, Kim G-S, Choi J-Y, Ryoo H-M. Molecular consequences of the ACVR1(R206H) mutation of fibrodysplasia ossificans progressiva. J Biol Chem. 2010;285:22542–22553. doi: 10.1074/jbc.M109.094557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twombly V, Bangi E, Le V, Malnic B, Singer MA, Wharton KA. Functional Analysis of saxophone, the Drosophila Gene Encoding the BMP Type I Receptor Ortholog of Human ALK1/ACVRL1 and ACVR1/ALK2. Genetics. 2009;183:563–579. doi: 10.1534/genetics.109.105585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veraksa A, Del Campo M, McGinnis W. Developmental patterning genes and their conserved functions: from model organisms to humans. Mol Genet Metab. 2000;69:85–100. doi: 10.1006/mgme.2000.2963. [DOI] [PubMed] [Google Scholar]
- Visser JA, Olaso R, Verhoef-Post M, Kramer P, Themmen AP, Ingraham HA. The serine/threonine transmembrane receptor ALK2 mediates Müllerian inhibiting substance signaling. Mol Endocrinol. 2001;15:936–945. doi: 10.1210/mend.15.6.0645. [DOI] [PubMed] [Google Scholar]
- Wang T, Donahoe PK. The immunophilin FKBP12: a molecular guardian of the TGF-beta family type I receptors. Front Biosci. 2004;9:619–631. doi: 10.2741/1095. [DOI] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Kicheva A, Bittig T, Seum C, Jülicher F, González-Gaitán M. Dynamics of Dpp signaling and proliferation control. Science. 2011a;331:1154–1159. doi: 10.1126/science.1200037. [DOI] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Jülicher F, Gonzalez-Gaitan M. Understanding morphogenetic growth control - lessons from flies. Nat Rev Mol Cell Biol. 2011b;12:594–604. doi: 10.1038/nrm3169. [DOI] [PubMed] [Google Scholar]
- Wehner L-E, Folz BJ, Argyriou L, Twelkemeyer S, Teske U, Geisthoff UW, Werner JA, Engel W, Nayernia K. Mutation analysis in hereditary haemorrhagic telangiectasia in Germany reveals 11 novel ENG and 12 novel ACVRL1/ALK1 mutations. Clin Genet. 2006;69:239–245. doi: 10.1111/j.1399-0004.2006.00574.x. [DOI] [PubMed] [Google Scholar]
- Wharton KA, Cook JM, Torres-Schumann S, de Castro K, Borod E, Phillips DA. Genetic analysis of the bone morphogenetic protein-related gene, gbb, identifies multiple requirements during Drosophila development. Genetics. 1999;152:629–640. doi: 10.1093/genetics/152.2.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate T beta R-I, the downstream signaling component in the TGF-beta receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrana JL, Attisano L, Wieser R, Ventura F, Massagué J. Mechanism of activation of the TGF-beta receptor. Nature. 1994;370:341–347. doi: 10.1038/370341a0. [DOI] [PubMed] [Google Scholar]
- Wu MY, Hill CS. Tgf-beta superfamily signaling in embryonic development and homeostasis. Dev Cell. 2009;16:329–343. doi: 10.1016/j.devcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- Yu PB, Deng DY, Lai CS, Hong CC, Cuny GD, Bouxsein ML, Hong DW, McManus PM, Katagiri T, Sachidanandan C, Kamiya N, Fukuda T, Mishina Y, Peterson RT, Bloch KD. BMP type I receptor inhibition reduces heterotopic [corrected] ossification. Nat Med. 2008a;14:1363–1369. doi: 10.1038/nm.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008b;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Feng X, We R, Derynck R. Receptor-associated Mad homologues synergize as effectors of the TGF-beta response. Nature. 1996;383:168–172. doi: 10.1038/383168a0. [DOI] [PubMed] [Google Scholar]
- Zhou XP, Woodford-Richens K, Lehtonen R, Kurose K, Aldred M, Hampel H, Launonen V, Virta S, Pilarski R, Salovaara R, Bodmer WF, Conrad BA, Dunlop M, Hodgson SV, Iwama T, Järvinen H, Kellokumpu I, Kim JC, Leggett B, Markie D, Mecklin JP, Neale K, Phillips R, Piris J, Rozen P, Houlston RS, Aaltonen LA, Tomlinson IP, Eng C. Germline mutations in BMPR1A/ALK3 cause a subset of cases of juvenile polyposis syndrome and of Cowden and Bannayan-Riley-Ruvalcaba syndromes. Am J Hum Genet. 2001;69:704–711. doi: 10.1086/323703. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.