Abstract
Background & Aims
Klotho deficiency in hypomorphic KL mice leads to premature senescence and phenotype consistent with impaired mineral homeostasis. Klotho has anti-inflammatory properties protecting from NO-induced endothelial dysfunction, reduces the expression of endothelial adhesion molecules, and may contribute to T-cell dysfunction. Since defective Ca2+/Pi homeostasis leading to osteopenia/osteoporosis is frequently associated with human IBD, we investigated the changes in Klotho gene expression as a consequence of experimental colitis.
Methods
We utilized three murine IBD models: TNBS colitis, microflora-induced colitis in gnotobiotic IL-10−/− mice, and adoptive CD4+CD45RBhigh T-cell transfer colitis. These studies were followed by in vitro approaches using renal epithelial cells (mpkDCT4 and mIMCD3), and the cloned murine KL gene promoter.
Results
Renal expression of Klotho mRNA and protein was significantly inhibited in all three models of human IBD. This degree of inhibition was correlated with the severity of colitis, and was reversed by neutralizing anti-TNF antibodies. In vitro, TNF resulted in a significant inhibition of KL expression and was further potentiated by IFN-γ. TNF/IFN-γ combination resulted in increased iNOS expression and significantly elevated the concentration of NO in medium. The effect of IFN-γ could be reproduced by cell exposure to SNAP (NO donor), and reversed by iNOS inhibitor, L-NIL. The cytokine effects were transcriptionally mediated since Klotho mRNA stability remained unaffected, while reporter constructs with the mKL gene promoter displayed significant downregulation in transiently transfected renal epithelial cells.
Conclusions
These novel findings could help explain several extraintestinal complications including abnormalities in bone homeostasis in patients with chronic colitis.
Keywords: kidney, mineral homeostasis, distal convoluted tubules, bone metabolism
Introduction
α-Klotho was initially identified in mice after an unrelated transgene was fortuitously inserted into the 5′ flanking region of the α-Kl gene, thus creating a hypomorphic allele with dramatically reduced Kl expression in homozygous mice. Klotho hypomorphs present many accelerated age-related disorders that mimic human aging including a short lifespan, infertility, arteriosclerosis, ectopic calcification, skin atrophy, osteoporosis and emphysema1. The Kl gene encodes a 130 kDa type I membrane protein, predominantly expressed in kidney distal convoluted tubules, with its large extracellular domain composed of two repeats that share sequence homology with members of the β-glycosidase family, with relatively weak β-glucuronidase activity2. The extracellular repeats can be enzymatically cleaved from the membrane and released in detectable quantities into the cerebrospinal fluid or circulating blood3. A second klotho variant of approximately 70 kDa exists as the result of alternative splicing which removes half of the C-terminal end together with the membrane binding domain. Among the several functions attributed to this gene are: regulation of Ca2+/Pi homeostasis, regulation of insulin-like growth factor 1 (IGF1) signaling, reduction of oxidative stress through de-repression of forkhead box O (FOXO) transcription factors, and protection of the endothelium through induction of eNOS, and inhibition of TNF-α induced NF-κB activity and expression of endothelial adhesion molecules4–5.
The contribution of Kl to mineral homeostasis is complex. Due to the described β-glucuronidase activity, Klotho positively affects active renal Ca2+ reabsorption by stabilizing and increasing the activity of TRPV5 (transient receptor potential vanilloid 5) calcium channel. Klotho-mediated removal of the terminal sialic acids from TRPV5 N-glycan chains leads to galectin-1 binding to TRPV5, which decreases TRPV5 endocytosis, and enhances its retention at the cell surface6–7, thus leading to increased renal Ca2+ reabsorption. Klotho may also indirectly affect transepithelial Ca2+ through α-Klotho-dependent surface recruitment of Na+/K+-ATPase in response to low extracellular ionized Ca2+8. This mechanism has been implicated in enhancing the release of PTH in response to decreased extracellular calcium ([Ca2+]o) 9, an event that leads to a rapid increase in renal Ca2+ reabsorption. Impaired Klotho expression and activity would likely affect the regulation of electrochemical gradients in the plasma membrane, since the fluctuations of Na+/K+-ATPase activity determine the activities of other ATPases, ion channels, and ion exchangers from the Ca2+/cation antiporter superfamily, such as NCX and NCKX.
α-Klotho participates in the signaling mechanism for FGF23, a potent circulating, osteoblast-derived phosphaturic hormone. Klotho binding converts the FGF receptors (FGFR1c, FGFR3c, and FGFR4) from its inactive to a high affinity state10–11, thus permitting FGF23 to exert its physiological functions. One of these is inhibition of 1,25(OH)2D3 synthesis (the active metabolite of vitamin D)12–13. It is now believed that overproduction of 1,25(OH)2D3 may be the primary effector of the aging-like phenotype in KL-deficient mice14.
By extension, Klotho may also be an indispensable co-factor in FGF23-mediated induction of phosphaturia through inhibition of the apical expression and activity of the NaPi-IIa, a Na+/Pi cotransporter in the proximal tubule epithelium15 and inhibition of the intestinal Pi absorption via decreased activity and brush border membrane expression of NaPi-IIb in the intestine16, although these mechanisms have not been directly investigated.
Several other physiological functions have been attributed to Klotho, such as protection against endothelial dysfunction by regulating NO production, or participation in intracellular signaling pathways including p53/p21, cAMP, protein kinase C (PKC) and Wnt signaling. These have been recently and comprehensively reviewed elsewhere 4.
The wide spectrum of Klotho biological activities suggests that its dysregulated expression, particularly under inflammatory conditions, could have profound consequences. Renal expression of Klotho has been shown to be downregulated in the rat model of acute LPS-mediated inflammatory stress 17. The spleen and other immune organs from Klotho knockout mice are underdeveloped and B-cell development and differentiation are impaired1, 18. Expression of Klotho protein was described on the surface of human CD4+ lymphocytes19, where it was significantly decreased proportionally to the advancing age, but it was also heavily suppressed in the CD4+ cells of rheumatoid arthritis (RA) patients. A similar association of Klotho in the immune cells of patients with idiopathic inflammatory bowel disease (IBD), a disease sharing many commonalities with RA, has not been investigated. The latter group of inflammatory disorders is of particular interest due to high occurrence of osteopenia and osteoporosis as two of the main extraintestinal complications of chronic IBD20. The pathogenesis of the metabolic bone disease in IBD is likely multifactorial with contributing factors including altered Pi, Ca2+ and vitamin D3 homeostasis, inflammatory cytokines, glucocorticoid treatment, decreased gonadal function and overall poor nutrition and low physical activity. Our group has recently demonstrated a detrimental effect of experimental colitis and TNF on intestinal Pi absorption21 and on osteoblast expression of the Phex gene22, effects likely contributing to defective bone mineralization in IBD-related metabolic bone disease.
Here, we demonstrate that renal Kl expression is repressed in three distinct animal models of acute and chronic colitis, and implicate the role of TNF and IFN-γ in the mechanism of this inhibition. A decrease in Klotho expression correlated with the disease severity and could be ameliorated by anti-TNF antibody. In the in vitro studies with immortalized distal convoluted tubule epithelial cells, TNF and IFN-γ inhibited Kl gene transcription, with IFN-γ potentiating the effects of TNF by induction of iNOS and NO production. These results provide the first evidence of the IBD-associated inflammatory process adversely affecting renal expression of Klotho, an event with potentially profound systemic consequences, including mineral homeostasis, vascular health and aging.
Methods
Reagents
The sources of major reagents used in the study are listed in detail in the Supplement.
Murine colitis models
TNBS colitis was induced in BALB/c mice as described earlier23. A subgroup of TNBS treated mice was administered a neutralizing hamster anti-mouse monoclonal anti-TNF antibody (clone TN3–19.12; azide-free, endotoxin level <.001; eBioscience, San Diego, CA). 250 μg of the antibody were injected intraperitoneally 4 hours before induction of colitis and 3 days following induction. Mice that died before day 7 were not included in the experiment. On day 7 post-induction, mice were sacrificed by CO2 narcosis followed by cervical dislocation.
Specific pathogen-free wild type (WT) 129/SvEv mice and germ-free IL-10−/− mice on the same genetic background were obtained from the National Gnotobiotic Rodent Resource Center at the University of North Carolina, Chapel Hill. Germ-free IL-10−/− mice were transferred to the SPF facility and kept in sterile cages two days prior to colonizing them with SPF fecal bacteria. Mice were sacrificed 8 weeks post-colonization to allow development of moderate to severe colitis.
Adoptive T-cell transfer colitis was induced by intraperitoneal injection of 0.5×106 naïve, flow-sorted (FACSAria, Beckton-Dickinson, Franklin Lake, NJ) CD4+CD45RBhigh lymphocytes (98% purity) into Rag-2−/− host (both C57BL/6)24. Control (PBS-injected) and colitic mice were sacrificed 8 weeks after transfer.
All methods in this study were approved by the Institutional Animal Care and Use Committee of the University of Arizona or the University of North Carolina at Chapel Hill.
Evaluation of colitis and sample collection
Mice were monitored for weight loss as well as signs of rectal bleeding and diarrhea. Paraffin-embedded sections were taken from the proximal and distal colon and histological damage was evaluated by a veterinary pathologist in an unbiased fashion in hematoxylin-eosin (H&E)-stained sections as described previously 25–26. Direct visualization of the colon in vivo was performed using a “Coloview system” (Karl Storz Veterinary Endoscopy) as described 27. At the end of the experimental period, kidneys were extracted, flash frozen in liquid nitrogen, and stored at −70°C for RNA and protein isolation. Sections of the proximal and distal colon were used for tissue explant cultures and cytokine ELISA as described earlier28 and briefly explained in the supplement. Mesenteric lymph node cells were prepared and stimulated ex vivo with CD3/CD28 antibodies as described in the supplement.
Cell Culture
Immortalized mouse distal convoluted tubule cells (mpkDCT) were generated in A. Vandewalle’s laboratory by microdissection from a SV-PK/Tag transgenic mouse and cultured as described earlier29. Mouse inner medullary collecting duct (mIMCD-3) cell line derived from a mouse transgenic for the early region of SV40 [Tg(SV40E)bri/7]30 were obtained from American Type Culture Collection (ATCC, Manassas, VA) and cultured in DMEM:F12 medium with 10% fetal bovine serum. Cells were treated with TNF (20 ng/mL) and/or IFN-γ (100 U/mL) for 2–24 hours. RNA stability studies required a 30 minute pretreatment with actinomycin D (ActD; 1 ng/mL) prior to addition of cytokines. For nitric oxide donor experiments, SNAP (a nitric oxide donor) was added to the medium, and medium containing SNAP was replaced every 5 hours for a combined 20 hour exposure. At completion, medium was collected for a nitrate/nitrite assay using the Nitric Oxide Quantitation kit according to manufacturer’s protocol (Active Motif, Carlsbad, CA), while cells were washed with PBS and used for RNA isolation.
RNA Extraction and Real-time RT-PCR
Total renal RNA was extracted and Klotho, iNOS, TBP, or β-Actin mRNA expression was analyzed by real-time RT-PCR as described in more detail in ref. 31 and in the Supplement.
Klotho immunoblotting and ELISA
Western blot and ELISA analysis of renal Klotho protein is described in more detail in the Supplement. The developed ELISA protocol was reliable and reproducible with kidney lysates, but failed to detect circulating Klotho in mouse serum, likely due to sensitivity issues, or epitope targeting.
mKlotho reporter gene construct and transfections
1099 nt fragment of the murine Klotho gene regulatory sequence spanning -1085 nt to +14 nt relative to the described transcription start site32 was amplified from mouse genomic DNA using the following primers: forward 5′-CTTTGAGCCTCGAGATGTTTCCCAATGAAT-3′ and the reverse 5′-GCCCTCCCGGCTCCCGCAGCAAGCTTGCCCG-3′, with XhoI and HindIII adapters, respectively. The amplicon was ultimately subcloned into pGL4.72-basic plasmid (Promega) using pGEM-T Easy cloning plasmid (Promega, Madison, WI) as a shuttle vector. pGL4.72 plasmid contains a Rapid Response Renilla luciferase (hrLucCP), engineered to include the protein degradation sequences hCL1 and hPEST. Shorter 5′-deletion constructs were made in the same fashion and were fully sequenced to confirm fidelity. mIMCD3 cells were then transfected with 0.2 ug the promoter constructs using TransIT-LT reagent (Mirus) in 24-well plates according to manufacturer’s protocol. 16 hours post- transfection, cells were incubated with vehicle or TNF and/or IFN-γ for 24 hours, luciferase activity was measured using the Renilla Luciferase activity assay (Promega), and normalized to protein concentrations.
Statistical Analysis
Statistical significance was determined by the Student’s t-test or analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (PLSD) test, using the Statview software package version 4.53 (SAS Institute; Cary, NC). Data are expressed as means ± S.E.
Results
Downregulation of Klotho in TNBS colitis
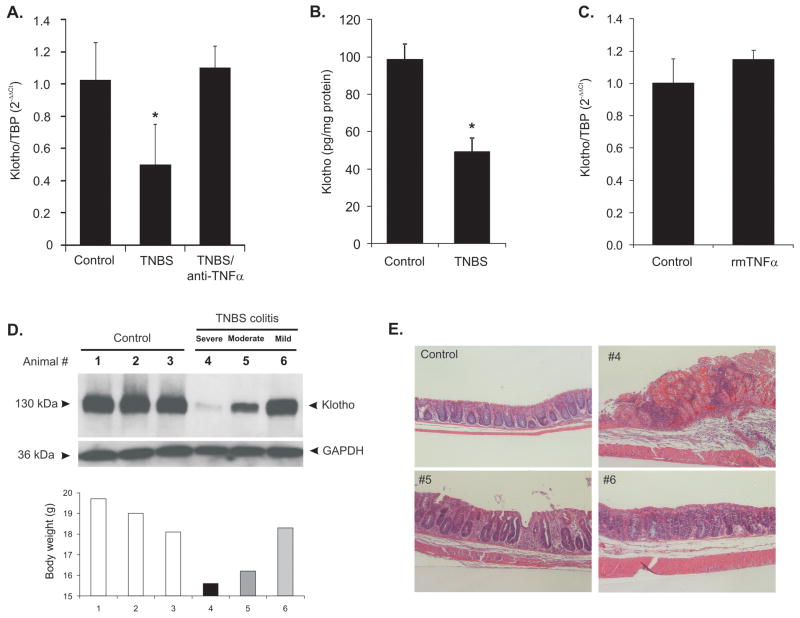
TNBS colitis is an acute remitting model of hapten-induced colonic inflammation characterized by weight loss, rectal bleeding, mucosal neutrophilic and lymphocytic infiltration, mucosal erosions and in severe cases, death. The progression of the disease is quite variable in mice, ranging from extreme moribundity to nearly complete recovery on day 7 post-administration. Within the limitations of this model, it allowed us to not only assess the average expression of Klotho in all experimental mice, but to correlate the extent of inflammation/recovery with the degree of inhibition of renal Klotho expression. In all studied mice (n=9), TNBS colitis resulted in significant inhibition of Klotho mRNA (Fig. 1A) and protein (Fig. 1B) expression in the kidney, an effect which was completely abrogated by treatment with a neutralizing anti-TNF antibody (Fig. 1A). However, exogenous TNF injected at a dose sufficient to inhibit expression of the Phex gene in the bone [15μg/100g body weight22) was not sufficient to reproduce the effects of colitis on Klotho expression (Fig. 1C). Analysis of individual colitic animals demonstrated significant variation in the extent of this inhibition, which highly correlated with the body weight loss and the histological mucosal damage, as exemplified by data from animals #4–6 in Fig. 1D and 1E. ELISA analysis failed to detect the circulating soluble form of Klotho in mouse serum (data not shown).
Figure 1. The effects of TNBS colitis on renal expression of Klotho.
(A) Klotho mRNA expression in control mice, TNBS-treated mice, and mice treated with TNBS and anti-TNF antibody. Anti TNF antibody was given to mice (250 μg/mouse) 4 hours prior to TNBS enema and again on day 3 after TNBS treatment. (n=6–8). (B) ELISA analysis of renal Klotho protein in tissue lysates from control and TNBS-treated mice (n-=5) (C) Exogenous recombinant murine TNF (15μg/100 g body weight) is not sufficient to downregulate Klotho mRNA in vivo (n=3). Decreased expression of Klotho protein correlates with the severity of colitis in individual TNBS-treated mice as exemplified in mice selected (animal #4–6) by varying weight loss (D) and distal colonic histology (E).
Downregulation of Klotho in IL-10 deficiency colitis
Germ-free IL-10−/− mice and genetically matched wild-type control were transferred to SPF conditions and colonized with SPF microflora two days later. After 8 weeks, WT mice showed no evidence of macroscopic inflammation and displayed a semi-translucent mucosa with well-defined vascularization associated with a healthy colon (Fig. 2A, upper panel) as assayed by miniature endoscopy. In contrast, IL-10−/− mice exhibited mucosal thickening and loss of apparent vasculature associated with macroscopic intestinal inflammation (Fig. 2A, lower panel). Consistent with previously published data [28, 33], histological analysis of the proximal and distal colon of IL-10−/− mice 8 weeks post-colonization showed a characteristic pattern of inflammation associated with crypt hyperplasia, lymphocytic and neutrophilic infiltrations and mucosal ulceration with varying degrees of severity. The cumulative histological scores (proximal and distal segments) showed enhanced inflammation in IL-10−/− mice at 8 weeks compared to WT mice (8.6±1.89 vs. 1.1±0.4, p<0.001). Renal Klotho mRNA (Fig. 2B) and protein expression (Fig. 2C) was decreased by 60% in IL-10−/− mice 8 weeks post-colonization.
Figure 2. Progressing colitis in SPF-colonized germ-free IL-10−/− mice results in decreased renal Klotho expression.
(A) Representative microendoscopy picture of the distal colonic mucosa of WT mice, or IL-10−/− mice 8 weeks after colonization with SPF microflora. (B) Real-time RT-PCR analysis of renal expression of Klotho mRNA in WT and colitic IL-10−/− mice. Values referred to age-matched wild-type (WT) mice maintained in SPF conditions (n=5–9; * p<0.05 t-test WT vs. IL-10−/−). (C) Representative western blot analysis of Klotho protein in the renal lysates of WT and IL-10−/− mice 8 weeks post-colonization.
Downregulation of Klotho in adoptive T-cell transfer colitis
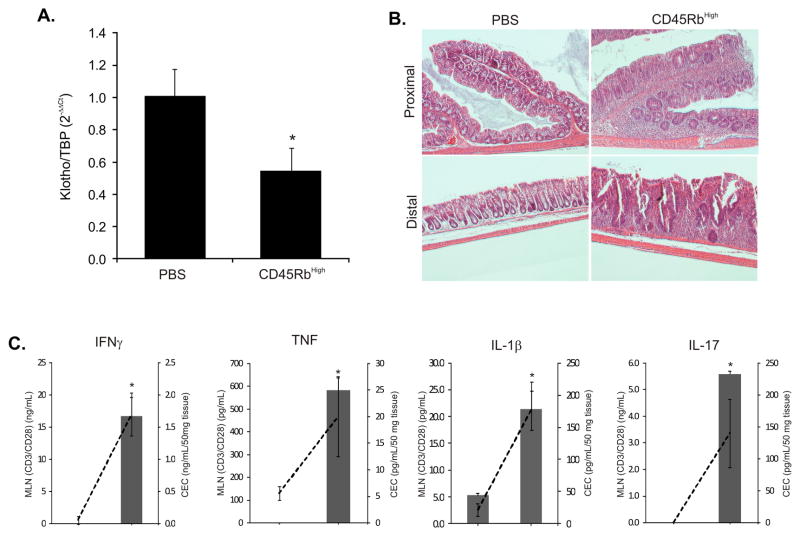
Adoptive transfer of CD4+CD45RBhigh T-cells (naïve T-cells) from healthy wild-type mice into syngeneic recipients that lack T- and B-cells induces pancolitis and small bowel inflammation within 5–8 wk with symptoms resembling human Crohn’s disease. The CD45RBhigh T-cell transfer led to significantly reduced renal Klotho expression (Fig. 3A) coinciding with moderate-to-severe chronic colitis, characterized by increased mucosal thickness and transmural infiltration of lymphocytes, monocytes, macrophages, and granulocytes, affecting both proximal and distal colon (Fig. 3B). Production of selected cytokines (IFN-γ, TNF, IL-1β, and IL-17) was significantly elevated in the supernatants obtained from unstimulated colonic explants cultures as well as from CD3/CD28-stimulated MLN lymphocyte culture (Fig. 3C).
Figure 3. Renal Klotho expression is downregulated in the adoptive T-cell transfer model of Crohn’s disease.
0.5×106 naïve CD4+CD45RBhigh lymphocytes were transferred into Rag-2−/− host. Control (PBS-injected) and colitic mice were sacrificed 8 weeks after transfer. (A) Real-time RT-PCR analysis of renal Klotho mRNA expression in control and adoptively transferred mice (n=4). (B) Representative H&E-stained sections of the proximal and distal colon in control (PBS) mice and mice transferred with CD45RBhigh lymphocytes. (C) Secretion of IFN-γ, TNF, IL-1β, and IL-17 by the MLN cells cultured in the presence of CD3/CD28 antibodies (bars, left axis) and by the colonic explant cultures (dashed line, right axis). Asterisks indicate statistical differences between groups (p<0.05 Student t-test; n=4).
In vitro, IFN-γ and TNF potently reduce Klotho expression in mpkDCT4 cells
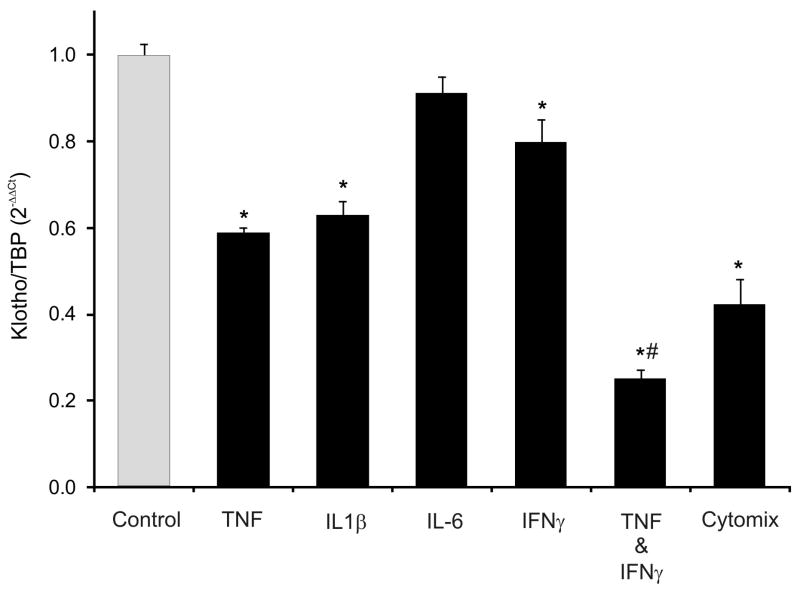
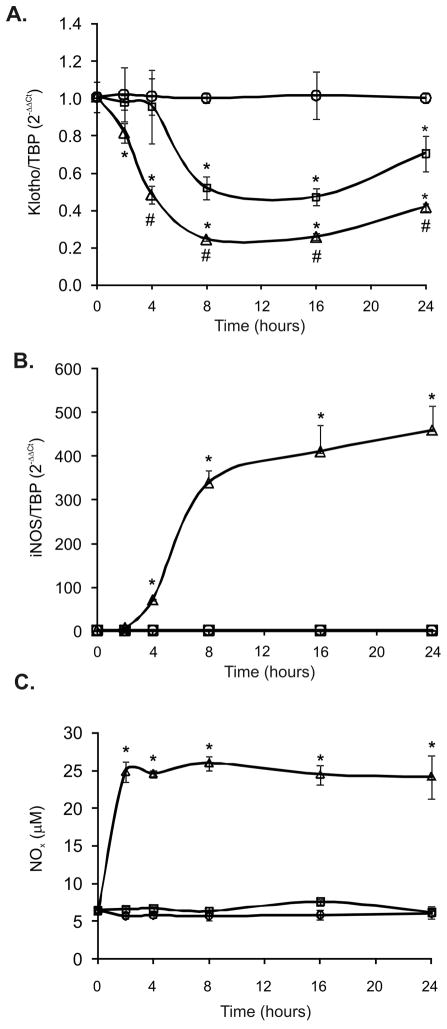
To further examine the possible role of proinflammatory cytokines in the downregulation of Klotho, immortalized mouse epithelial cells of distal convoluted tubule origin, mpkDCT4, were treated with selected proinflammatory cytokines (TNF, IL-1β, IL-6, IFN-γ) alone or in combination. All but IL-6 were able to significantly inhibit KL mRNA expression (data not shown). A combination treatment of all cytokines (cytomix) resulted by the most significant decrease of approximately 60% (p<.001), which could be mimicked by selective combination of TNF and IFN-γ (data not shown and Fig. 4). To examine the dynamics of these two cytokines, Klotho expression was analyzed over time by real-time RT-PCR (0 –24hr). We observed some latency of the TNF effect with a significant decrease in Klotho mRNA at 4–8 hours after treatment and nadir at 8–16 hours (Fig. 5A). A combination of TNF and IFN-γ not only further decreased the expression of Klotho, but also accelerated the decline which manifested at just 2 hours following treatment.
Figure 4. The effect of selected cytokines alone and in combination on Klotho mRNA expression mpkDCT4 cells.
Immortalized mouse distal convoluted tubule epithelial cells were treated with TNF (20ng/mL), IL1β (10 ng/mL), IL-6 (10 ng/mL), IFN-γ (100U/mL), combination of TNF and IFN-γ, or all four cytokines (Cytomix) for 24 hours. Klotho mRNA was analyzed by real-time RT-PCR. (* p<0.05 control vs. cytokine treatment; # p<0.05 TNF vs. TNF/IFN-γ; ANOVA followed by Fisher PLSD test; n=4).
Figure 5. Time course analysis of the effects of TNF and TNF/IFN-γcombination on the expression of Klotho, iNOS, and NO production in mpkDCT4 cells.
Cells were treated for 2–24 hours with TNF (20ng/mL; open squares) or TNF/IFN-γ (20ng/mL and 100U/mL, respectively; open triangles) and expression of Klotho (A) and iNOS (B) was measured by real-time RT-PCR. (C) NO in the media was measured after conversion of nitrate to nitrite using nitrite reductase and colorimetric Griess reaction (Active Motif Nitric Oxide Quantitation Kit). (* p<0.05 control vs. cytokine treatment; # p<0.05 TNF vs. TNF/IFN-γ; ANOVA followed by Fisher PLSD test; n=4).
Nitric Oxide mediates the downregulation of Klotho by IFN-γ
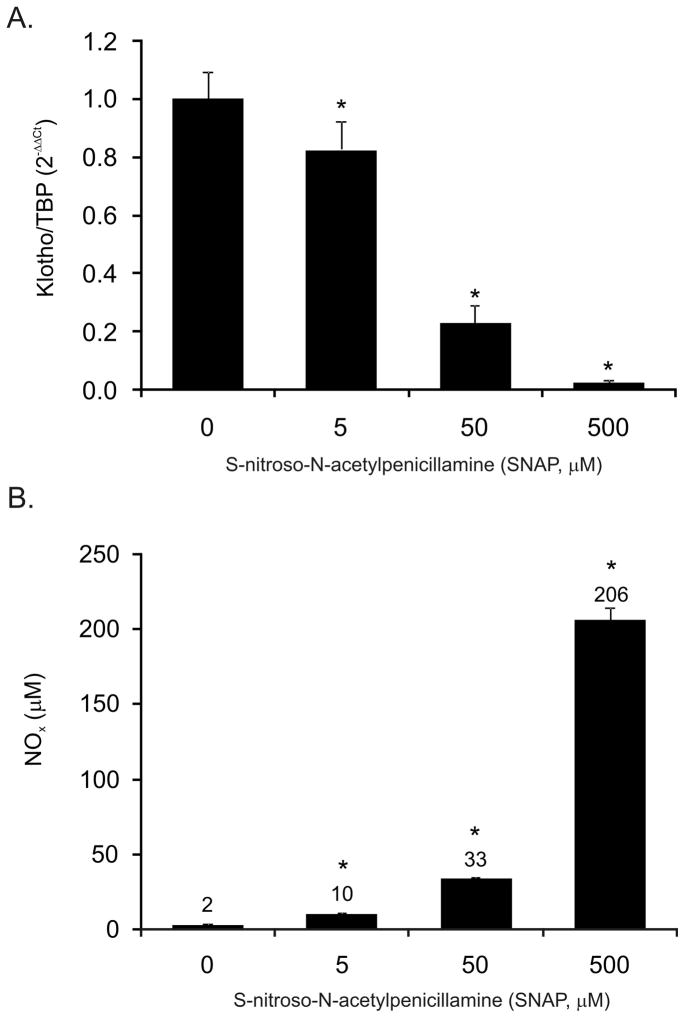
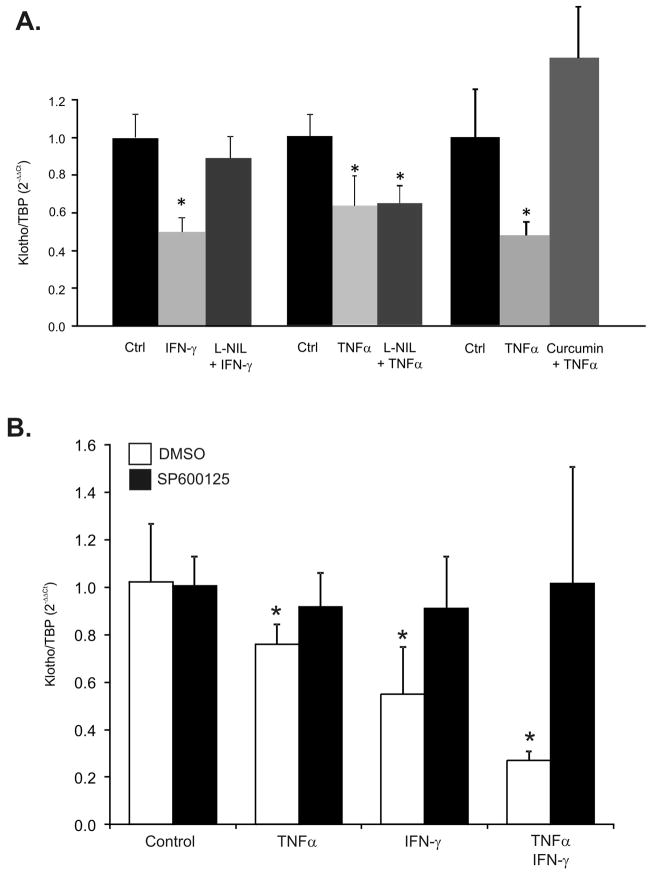
The potentiating effects of IFN-γ on the TNF-mediated inhibition of Klotho gene expression in mpkDCT4 cells coincided with a dramatic increase in expression of iNOS (Fig. 5B) and NO production, which level remained stably elevated between 2–24 hours of treatment (Fig. 5C). To determine whether nitric oxide was sufficient in downregulating Klotho expression, mpkDCT4 cells were treated with increasing concentrations of a NO donor (SNAP) for 20 hours at increasing concentrations. We observed a concentration-dependent decrease of Klotho mRNA expression (Fig. 6A). 50 μM of SNAP resulted in cumulative NO production reaching 33 μM in medium (Fig. 6B), comparable with ~25μM NO observed after TNF/IFN-γ treatment, and resulting in 77% reduction of Klotho expression (Fig. 6A). Furthermore, in mpkDCT4 cells, the iNOS inhibitor, L-NIL, effectively reversed the IFN-γ-induced, but not TNF-induced inhibition of Klotho expression (Fig. 7A). The effect of TNF was reversible with curcumin, a broad spectrum inhibitor of NF-κB and MAPK pathways (Fig. 7A).
Figure 6. The effects of nitric oxide on Klotho expression in mpkDCT4 cells.
Cells were treated with SNAP for 20 hours with medium changed every 5 hours. Klotho expression was measured with real-time PCR (A) and the corresponding NO production was measured in medium using Nitric Oxide Quantitation Kit (Active Motif) (B). (* p<0.05 control vs. SNAP treatment; values above bars in (B) indicate the mean NOx concentration in medium).
Figure 7. iNOS inhibitor L-NIL restores Klotho expression in IFN-γ-treated cells, while JNK inhibition abrogates the effects of TNF, IFN-γ, or combination of the two cytokines.
(A) Cells were pre-treated with 1mM L-NIL prior to addition of IFN-γ (100U/mL) or TNF (20ng/mL) to the medium. Cells were harvested 24 hours later and Klotho expression was analyzed by real-time RT-PCR. Curcumin (20μM) used as a non-specific inhibitor of NF-κB and AP-1 effectively reversed both TNF-induced downregulation of Klotho expression. (* p<0.05 control vs. cytokine treatment; n=4). (B). Cells were pretreated with a selective inhibitor of JNK, 1,9-pyrazoloanthrone (SP600125; 20 μM) prior to 24-hr cytokine treatment (TNF at 20ng/mL, IFN-γ at 100U/mL) and Klotho mRNA expression was analyzed by real-time RT-PCR (* p<0.05 control vs. cytokine treatment; n=4).
JNK kinase mediates the inhibitory effects of TNF and IFN-γ
To follow up on the protective effects of curcumin we tested the role of NF-κB in the cytokine-mediated reduction of Klotho expression. Inhibition of NF-κB signaling in mpkDCT4 cells with BAY11–7082 ((E)3-[(4-Methylphenyl)sulfonyl]-2-propenenitrile), a potent and irreversible inhibitor of TNF-induced IκBα phosphorylation, remained without effect on both the TNF and IFN-γ-induced decrease of Klotho (data not shown). However, selective inhibition of c-Jun N-terminal kinase (JNK) with 1,9-pyrazoloanthrone (SP600125) reversed the negative effects of TNF and IFN-γ used alone or in combination (Fig. 7B).
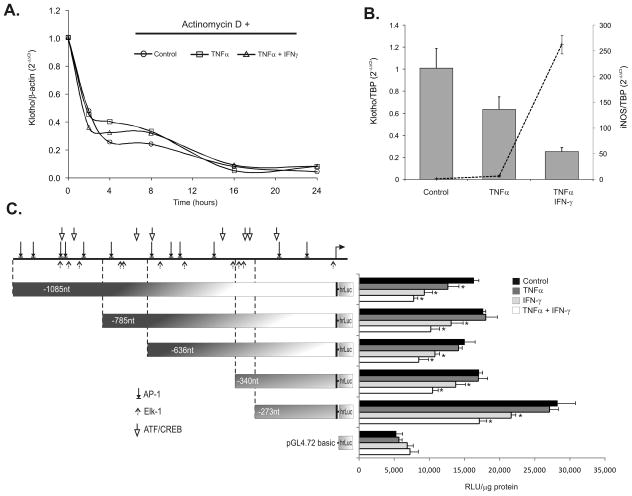
TNF and IFN-γ affect Klotho at the level of gene transcription
To further dissect the effects of TNF and IFN-γ on Klotho mRNA expression, we first determined the effect of the cytokines on the Klotho transcript RNA stability. De novo gene transcription was inhibited in mpkDCT4 with ActD prior to and during cytokine treatment and the decline of Klotho transcript (as a measure of mRNA degradation) was assessed by real-time RT-PCR. Neither cytokine (nor their combination) affected the rate of Klotho transcript degradation (Fig. 8A), thus implicating a transcriptional mechanism. We therefore cloned the 5′ regulatory region of the murine Klotho gene and generated several 5′ deletion constructs to test their responsiveness to the two cytokines in transiently transfected renal epithelial cells. Immortalized mpkDCT4 cells were very difficult to transfect (including the Amaxa nucleofection technique) and yielded poorly reproducible and low reporter activity values. mIMCD-3 cells, originally isolated from the mouse medullary collecting ducts, expressed lower levels of Klotho, but reacted in a similar fashion to TNF, and IFN-γ in terms of Klotho and iNOS gene expression (Fig. 8B), and were more amenable to transient transfection techniques. In mIMCD-3 cells transfected with Klotho promoter constructs, TNF effectively reduced the activity of the construct spanning −1085/+14nt, but not the −785/+14 nt of the mKL promoter (Fig. 8C), thus suggesting an important regulatory element within −1085/−786nt region driving the effects of TNF. All shorter constructs reacted to IFN-γ alone or the combination of the cytokines. The construct containing -273nt upstream of the transcription start site was the most active, and appeared to contain the necessary cis-elements required for INF-γ-mediated inhibition of gene transcription. The visible additive effects of IFN-γ and TNF in the constructs containing -785nt and shorter may be related to TNF-induced expression of IFN-γ receptor subunits reported in other systems33. Fig. 8C also depicts prediction analysis of the transcription factor binding sites within the -1085nt fragment of the murine Klotho promoter, with a particular emphasis on prototypical JNK pathways targets, AP-1, Elk-1 and ATF transcription factors (Match™ software and Transfac transcription factor binding site matrices).
Figure 8. Klotho is regulated by TNF and/or IFN-γ via a transcriptional mechanism.
(A) De novo transcription was inhibited in mpkDCT4 cells with Actinomycin D (ActD) and Klotho transcript decay rate was followed over time by real-time RT-PCR analysis. No significant differences were observed in the rate of mRNA degradation in control (open circles), TNF-treated (open squares) or TNF/IFN-γ co-treated cells (open triangles). (B) The effects of the two cytokines on Klotho (bars) and iNOS (line) expression in mIMCD-3 cells mirrors the effects observed in the mpkDCT4 cells. (C) The effects of TNF, IFN-γ, and their combination on murine Klotho gene promoter activity in transiently transfected mIMCD-3 cells. 5′-deletion constructs spanning -1085nt to -273nt upstream of the transcription start site are depicted with predicted binding sites for the three prototypical JNK-regulated transcription factors, AP- 1,Elk-1, and ATF/CREB (* p<0.05 control vs. cytokine treatment, n=4). Transcription start site, mapped by Shiraki-Iida et al.32 is indicated by a right-pointing arrow.
Discussion
Extraintestinal manifestations occur in approximately one-third of the IBD patients with joints, skin, eyes and biliary tract most commonly involved. Many extraintestinal disorders may be direct inflammatory and metabolic complications of the intestinal inflammation (i.e., osteoporosis, growth retardation, nephrolithiasis, or thromboembolic disease). Despite the rapid progress in understanding the pathogenesis and consequences of IBD, the line between direct and indirect systemic effects of chronic intestinal inflammation remains less defined. In this study, we provide the first evidence of experimental colitis negatively affecting expression of Klotho, a gene involved not only in the pathogenesis of aging, but also involved in mineral homeostasis and vascular protection. Since renal biopsy is not indicated in IBD, and since no single animal model of colitis is fully representative of human IBD, we employed three distinct models of the disease to demonstrate decreased expression of Klotho. These include the haptenizing model of TNBS colitis, IL-10 deficiency colitis, and the immune model of adoptive T-cell transfer into Rag2−/− mice. We showed that the degree of Klotho inhibition is related to the severity of colitis and that attenuation of inflammation with a neutralizing anti-TNF antibody prevented this inhibition. A combination of in vivo and in vitro approaches further demonstrated that IFN-γ potentiates the effects of TNF via induction of iNOS and via the suppressive effect of NO. These effects were JNK-dependent and involved transcriptional repression of the Klotho gene promoter.
In another report, a similar inhibition of Klotho was demonstrated in vivo in a rat model of endotoxemia17, although the results could not be confirmed in vitro in embryonic HEK239 kidney cells or in human renal proximal tubular epithelial cells (RPTEC). The interpretation of these results is difficult, since Klotho is normally not expressed in the proximal tubules, and our own observations indicate that expression of Klotho in HEK293 cells is below detection limit (real-time RT-PCR, unpublished data).
Transcriptional inhibition of Klotho expression in the kidney, an organ considered to be the major site of Klotho expression and the primary source of the circulating protein, would likely result in diminished circulating levels of Klotho. As discussed below, the consequences of this decrease may be wide and significant, and will have to be addressed experimentally. At this point, we can only predict these consequences based on data from the documented in vitro effects of Klotho, and from the phenotype of the Klotho hypomorphic mice. The latter may be difficult to compare, however, due to different degrees of inhibition (ca. 50% in colitis vs. 90% in Klotho mice), and the developmental complications due to early onset of Klotho deficiency in Kl/Kl mice.
Klotho has been shown to affect Wnt signaling by inhibition of various Wnt family members34. Disinhibition of Wnt signaling has been shown to contribute to stem and progenitor cell senescence and chronically reduced Klotho expression may influence the rate of cellular aging and negatively affect tissue repair mechanisms. Klotho protein also protects cells and tissues from oxidative stress, possibly through de-repression of FOXO transcription factors35. Klotho insufficiency may therefore adversely affect not only vascular endothelium, but also colonic epithelium in IBD. Recent data demonstrates that FOXO-4 not only protects from the development of experimental colitis, but also participates in the inhibition of NF-κB activity in colonic epithelial cells36. Moreover, Klotho was also recently reported to inhibit TNF-induced NF-κB and vascular adhesion molecule expression in endothelial cells5. It is, therefore, not inconceivable, that diminished expression of Klotho could feed back to further exacerbate the inflammation.
Perhaps the most fascinating aspect of Klotho physiology is its role in mineral homeostasis. Similar to a significant number of IBD patients who develop osteopenia and osteoporosis37, Klotho hypomorphs also develop osteoporosis, likely secondary to elevated levels of 1,25(OH)2D314. Interestingly, despite the overall consensus that hypovitaminosis D is prevalent in IBD patients (based on 25(OH)D3 levels as an indication of vitamin D nutritional status)38, as much as 42% of Crohn’s patients have been reported to have abnormally elevated levels of 1,25(OH)2D3, correlating with increased expression of 1α-OHase in the inflamed gut39. It is important to note that under limited Ca2+ and Pi availability (impaired intestinal absorption), and particularly in the presence of inflammatory cytokines promoting osteoclast differentiation, 1,25(OH)2D3 may act to stimulate bone turnover to maintain serum Ca2+ at the expense of bone mineral density. Such impaired intestinal absorption has been long postulated and reduced expression of key intestinal Pi and Ca2+ transporters in experimental IBD has been recently confirmed by our group21 and by others38. It is tempting to speculate that decreased Klotho contributes to the de-repression of 1α-OHase expression and increased 1,25(OH)2D3 production in active IBD due to a defective FGF23 signaling mechanism. On the other hand, impaired FGF23 function in Klotho insufficiency could be viewed as beneficial, since it would lead to inhibition of phosphaturic effects of FGF23. These are normally mediated by inhibition of NaPi-IIa, which along with NaPi-IIc provides the major route for renal Pi re-absorption. This phosphate sparing mechanism is unlikely, however, due to the observed concomitant increase of circulating FGF23, and significant decrease in renal expression of NaPi-IIa and NaPi-IIc in TNBS and T-cell transfer colitis (own unpublished observations).
Decreased Klotho expression could also have a profound effect on Ca2+ homeostasis through impaired [Ca2+]o sensing and PTH release from the parathyroid gland and through potentially impaired function of TRPV5 in the distal convoluted tubules, where Klotho acts to stabilize TRPV5 on the apical membrane through removal of sialic acid and enabling the interaction with galectin-1 6. Recent data confirms renal Ca2+ wasting in Klotho-deficient mice40.
Conclusions
We provide novel data describing inhibition of Klotho expression in mouse models of inflammatory bowel disease. This finding may have broad implications not only for IBD and the associated changes in mineral homeostasis, but also for other systemic complications of this and other chronic inflammatory conditions. While the pathophysiological consequences and causative relationship of decreased Klotho will have to be experimentally addressed, our findings lay the foundation for future work related to the contribution of Klotho to chronic inflammatory diseases in human patients.
Supplementary Material
Acknowledgments
Grant support: R37DK033209 (To F.K. Ghishan), 5R01DK067286 (to P.R. Kiela), 2R56DK047700 (to C. Jobin)
We would like to thank Dr. Salvatore Albani and Nicole Schechter at the Arizona Arthritis Center for their assistance in flow sorting of CD4+CD45RBhigh lymphocytes.
Abbreviations
- KL
Klotho
- IBD
inflammatory bowel disease
- TNBS
trinitrobenzene sulfonic acid
- iNOS
inducible nitric oxide synthase
- NO
nitric oxide
- SNAP
S-Nitroso-N-acetyl-DL-penicillamine
- FGF23
fibroblast growth factor 23
Footnotes
Financial disclosures: All authors declare that there is no conflict of interest to disclose.
Author contributions
Robert D. Thurston - acquisition of data, analysis and interpretation of data, drafting and revision of manuscript
Claire B. Larmonier - acquisition of data
Pawel M. Majewski - acquisition of data
Rajalakshmy Ramalingam- acquisition of data
Monica Midura-Kiela – acquisition of data
Daniel Laubitz - acquisition of data
Alain Vandewalle - material support, critical revision of the manuscript for important intellectual content
David G. Besselsen - acquisition of data
Marcus Mühlbauer - acquisition of data
Christian Jobin - material support, critical revision of the manuscript for important intellectual content, obtained funding
Pawel R. Kiela -study supervision, study concept and design, analysis and interpretation of data, drafting of the manuscript; critical revision of the manuscript for important intellectual content; statistical analysis, obtained funding
Fayez K. Ghishan- study supervision, study concept and design, critical revision of the manuscript for important intellectual content, obtained funding
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 2.Tohyama O, Imura A, Iwano A, et al. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem. 2004;279:9777–84. doi: 10.1074/jbc.M312392200. [DOI] [PubMed] [Google Scholar]
- 3.Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–7. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maekawa Y, Ishikawa K, Yasuda O, et al. Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine. 2009;35:341–6. doi: 10.1007/s12020-009-9181-3. [DOI] [PubMed] [Google Scholar]
- 6.Cha SK, Ortega B, Kurosu H, et al. Removal of sialic acid involving Klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A. 2008;105:9805–10. doi: 10.1073/pnas.0803223105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang Q, Hoefs S, van der Kemp AW, et al. The beta-glucuronidase klotho hydrolyzes and activates the TRPV5 channel. Science. 2005;310:490–3. doi: 10.1126/science.1114245. [DOI] [PubMed] [Google Scholar]
- 8.Razzaque MS. Klotho and Na+,K+-ATPase activity: solving the calcium metabolism dilemma? Nephrol Dial Transplant. 2008;23:459–61. doi: 10.1093/ndt/gfm702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imura A, Tsuji Y, Murata M, et al. alpha-Klotho as a regulator of calcium homeostasis. Science. 2007;316:1615–8. doi: 10.1126/science.1135901. [DOI] [PubMed] [Google Scholar]
- 10.Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–3. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Urakawa I, Yamazaki Y, Shimada T, et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–4. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 12.Perwad F, Zhang MY, Tenenhouse HS, et al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal Physiol. 2007;293:F1577–83. doi: 10.1152/ajprenal.00463.2006. [DOI] [PubMed] [Google Scholar]
- 13.Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–35. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- 14.Ohnishi M, Nakatani T, Lanske B, et al. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–72. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baum M, Schiavi S, Dwarakanath V, et al. Effect of fibroblast growth factor-23 on phosphate transport in proximal tubules. Kidney Int. 2005;68:1148–53. doi: 10.1111/j.1523-1755.2005.00506.x. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto K, Ito M, Kuwahata M, et al. Inhibition of intestinal sodium-dependent inorganic phosphate transport by fibroblast growth factor 23. Ther Apher Dial. 2005;9:331–5. doi: 10.1111/j.1744-9987.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 17.Ohyama Y, Kurabayashi M, Masuda H, et al. Molecular cloning of rat klotho cDNA: markedly decreased expression of klotho by acute inflammatory stress. Biochem Biophys Res Commun. 1998;251:920–5. doi: 10.1006/bbrc.1998.9576. [DOI] [PubMed] [Google Scholar]
- 18.Okada S, Yoshida T, Hong Z, et al. Impairment of B lymphopoiesis in precocious aging (klotho) mice. Int Immunol. 2000;12:861–71. doi: 10.1093/intimm/12.6.861. [DOI] [PubMed] [Google Scholar]
- 19.Witkowski JM, Soroczynska-Cybula M, Bryl E, et al. Klotho--a common link in physiological and rheumatoid arthritis-related aging of human CD4+ lymphocytes. J Immunol. 2007;178:771–7. doi: 10.4049/jimmunol.178.2.771. [DOI] [PubMed] [Google Scholar]
- 20.Ardizzone S, Puttini PS, Cassinotti A, et al. Extraintestinal manifestations of inflammatory bowel disease. Dig Liver Dis. 2008;40 (Suppl 2):S253–9. doi: 10.1016/S1590-8658(08)60534-4. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, Xu H, Dong J, et al. Tumor necrosis factor-alpha impairs intestinal phosphate absorption in colitis. Am J Physiol Gastrointest Liver Physiol. 2009;296:G775–81. doi: 10.1152/ajpgi.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uno JK, Kolek OI, Hines ER, et al. The role of tumor necrosis factor alpha in down-regulation of osteoblast Phex gene expression in experimental murine colitis. Gastroenterology. 2006;131:497–509. doi: 10.1053/j.gastro.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Billerey-Larmonier C, Uno JK, Larmonier N, et al. Protective effects of dietary curcumin in mouse model of chemically induced colitis are strain dependent. Inflamm Bowel Dis. 2008;14:780–93. doi: 10.1002/ibd.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ostanin DV, Bao J, Koboziev I, et al. T cell transfer model of chronic colitis: concepts, considerations, and tricks of the trade. Am J Physiol Gastrointest Liver Physiol. 2009;296:G135–46. doi: 10.1152/ajpgi.90462.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karrasch T, Kim JS, Muhlbauer M, et al. Gnotobiotic IL-10−/−;NF-kappa B(EGFP) mice reveal the critical role of TLR/NF-kappa B signaling in commensal bacteria-induced colitis. J Immunol. 2007;178:6522–32. doi: 10.4049/jimmunol.178.10.6522. [DOI] [PubMed] [Google Scholar]
- 26.Kiela PR, Midura AJ, Kuscuoglu N, et al. Effects of Boswellia serrata in mouse models of chemically induced colitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G798–808. doi: 10.1152/ajpgi.00433.2004. [DOI] [PubMed] [Google Scholar]
- 27.Joo YE, Karrasch T, Muhlbauer M, et al. Tomato lycopene extract prevents lipopolysaccharide-induced NF-kappaB signaling but worsens dextran sulfate sodium-induced colitis in NF-kappaBEGFP mice. PLoS One. 2009;4:e4562. doi: 10.1371/journal.pone.0004562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larmonier CB, Uno JK, Lee KM, et al. Limited effects of dietary curcumin on Th-1 driven colitis in IL-10 deficient mice suggest an IL-10-dependent mechanism of protection. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1079–91. doi: 10.1152/ajpgi.90365.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Diepens RJ, den Dekker E, Bens M, et al. Characterization of a murine renal distal convoluted tubule cell line for the study of transcellular calcium transport. Am J Physiol Renal Physiol. 2004;286:F483–9. doi: 10.1152/ajprenal.00231.2003. [DOI] [PubMed] [Google Scholar]
- 30.Rauchman MI, Nigam SK, Delpire E, et al. An osmotically tolerant inner medullary collecting duct cell line from an SV40 transgenic mouse. Am J Physiol. 1993;265:F416–24. doi: 10.1152/ajprenal.1993.265.3.F416. [DOI] [PubMed] [Google Scholar]
- 31.Kiela PR, Laubitz D, Larmonier CB, et al. Changes in Mucosal Homeostasis Predispose NHE3 Knockout Mice to Increased Susceptibility to DSS-Induced Epithelial Injury. Gastroenterology. 2009;137(3):965–75. 975.e1–10. doi: 10.1053/j.gastro.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shiraki-Iida T, Aizawa H, Matsumura Y, et al. Structure of the mouse klotho gene and its two transcripts encoding membrane and secreted protein. FEBS Lett. 1998;424:6–10. doi: 10.1016/s0014-5793(98)00127-6. [DOI] [PubMed] [Google Scholar]
- 33.Shirey KA, Jung JY, Maeder GS, et al. Upregulation of IFN-gamma receptor expression by proinflammatory cytokines influences IDO activation in epithelial cells. J Interferon Cytokine Res. 2006;26:53–62. doi: 10.1089/jir.2006.26.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Fergusson MM, Castilho RM, et al. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–6. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem. 2005;280:38029–34. doi: 10.1074/jbc.M509039200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou W, Cao Q, Peng Y, et al. FoxO4 Inhibits NF-kappaB and Protects Mice Against Colonic Injury and Inflammation. Gastroenterology. 2009 doi: 10.1053/j.gastro.2009.06.049. In Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tilg H, Moschen AR, Kaser A, et al. Gut, inflammation and osteoporosis: basic and clinical concepts. Gut. 2008;57:684–94. doi: 10.1136/gut.2006.117382. [DOI] [PubMed] [Google Scholar]
- 38.Huybers S, Apostolaki M, van der Eerden BC, et al. Murine TNF(DeltaARE) Crohn’s disease model displays diminished expression of intestinal Ca2+ transporters. Inflamm Bowel Dis. 2008;14:803–11. doi: 10.1002/ibd.20385. [DOI] [PubMed] [Google Scholar]
- 39.Abreu MT, Kantorovich V, Vasiliauskas EA, et al. Measurement of vitamin D levels in inflammatory bowel disease patients reveals a subset of Crohn’s disease patients with elevated 1,25-dihydroxyvitamin D and low bone mineral density. Gut. 2004;53:1129–36. doi: 10.1136/gut.2003.036657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alexander RT, Woudenberg-Vrenken TE, Buurman J, et al. Klotho Prevents Renal Calcium Loss. J Am Soc Nephrol. 2009 doi: 10.1681/ASN.2008121273. In Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.