Abstract
Polystyrene (PS), a standard material for cell culture consumable labware, was molded into microstructures with high fidelity of replication by an elastomeric polydimethylsiloxane (PDMS) mold. The process was a simple, benchtop method based on soft lithography using readily available materials. The key to successful replica molding by this simple procedure relies on the use of a solvent, for example, gamma-butyrolactone, which dissolves PS without swelling the PDMS mold. PS solution was added to the PDMS mold, and evaporation of solvent was accomplished by baking the mold on a hotplate. Microstructures with feature sizes as small as 3 µm and aspect ratios as large as 7 were readily molded. Prototypes of microfluidic chips made from PS were prepared by thermal bonding of a microchannel molded in PS with a flat PS substrate. The PS microfluidic chip displayed much lower adsorption and absorption of hydrophobic molecules (e.g. rhodamine B) compared to a comparable chip created from PDMS. The molded PS surface exhibited stable surface properties after plasma oxidation as assessed by contact angle measurement. The molded, oxidized PS surface remained an excellent surface for cell culture based on cell adhesion and proliferation. The micromolded PS possessed properties that were ideal for biological and bioanalytical needs, thus making it an alternative material to PDMS and suitable for building lab-on-a-chip devices by soft lithography methods.
INTRODUCTION
Polystyrene (PS) has numerous advantages for biological applications including low cost, optical clarity, biocompatibility, chemical inertness, chemical stability, and rigidity. The polymer is also readily chemically functionalized to yield new surface or bulk properties. These advantages make PS the material of choice for the fabrication of cell-culture labware including tissue-culture dishes, flasks, and multiwell plates.1 By virtue of its properties and advantages, PS would be an ideal material for creating miniaturized lab-on-a-chip devices, which are anticipated to have wide-ranging applications in diagnostics, therapeutics, and bio-analytical assays.2 To fabricate such miniaturized devices, a convenient and repeatable microfabrication process is needed for creating intricate microscale features in a PS substrate with high resolution and reproducibility.
Conventional microfabrication processing steps, e.g. etching, deposition, and lithography, originally developed for inorganic substrates such silicon, glass, and quartz, are generally not suitable for microfabrication of PS due to its physical properties.3, 4 Microstructures have been fabricated on PS films by a combination of reactive ion etching (RIE) and a physical mask on a shrinkable PS film.5 Heating was used to shrink the patterned surface resulting in a 100-fold increase in the aspect ratio of the patterned microstructures in the PS film.5 This methodology requires an expensive tool (RIE) and an oriented polystyrene film. The thermal shrinkage is also a non-conventional process that is difficult to control with accuracy.
Hot embossing, injection molding, thermal forming, and indentation against a rigid master can be used to create small patterns into thermoplastics such as PS.6, 7 These methods are commonly employed for thermoplastics such as poly(methyl methacrylate) (PMMA) and cyclic olefin copolymer (COC).8, 9 More recently, embossing was optimized for PS to build microfluidic systems.10 These fabrication methods require dedicated tools such as a hot-press or injection molding machines as well as expensive master molds. In these processes, both the formed polymers and the master are rigid; therefore, it is difficult to detach the molded polymer from the master without damaging the intricate micropatterns, especially for high-aspect-ratio microstructures. As a result, these processing methods have not gained favor in creating lab-on-a-chip devices from PS, although a few examples have been described in fabricating PS microfluidic devices.10–12 A non-conventional fabrication method called “Shrinky-Dink” was used to fabricate PS microfluidics from biaxially pre-stressed PS sheets.13 The features were drawn on the sheets by mechanical scribing or ink jet printing. Heating caused the inscribed films to shrink to their original size, while the drawn features became narrower and more raised, creating a microstructured surface. Although this method is a rapid benchtop process, it is not possible to control the accuracy and resolution of the microstructures formed.
Soft lithography is a technique for fabricating or replicating small structures using elastomeric polydimethylsiloxane (PDMS) stamps and molds.14, 15 It is a simple benchtop process that does not require sophisticated tools, and therefore has gained widespread acceptance for rapid prototyping of lab-on-a-chip devices.16, 17 For replica molding, a liquid polymer precursor is poured onto a PDMS mold and the polymer is allowed to cure. The PDMS mold is then detached from the solidified polymer with the microstructure pattern on the PDMS mold being replicated into the polymer. Polymer materials that can be molded by soft lithography include PDMS itself, UV-curable polyurethane, thermally curable epoxy, as well as a number of other polymers.14, 16 PDMS is the most popular material for soft lithography due to the ease of the liquid molding process and its numerous advantages.18, 19 On the other hand, PDMS when used not as the mold, but for actual lab-on-a-chip devices, has intrinsic limitations, for example, unstable surface properties (post-oxidation hydrophobic recovery),20, 21 leaching of low molecular weight species,22 undesired surface adsorption of molecules, diffusion of hydrophobic molecules into the PDMS bulk,23 low rigidity, and high gas solubility.24
For the various reasons discussed above, it would be a great advantage to apply soft lithography processing to the microfabrication of PS by combining both the advantages of PS as a material with the convenience of this rapid prototyping procedure. Since PS is complementary in many of its properties to PDMS, for example, rigidity, lack of non-specific adsorption of hydrophobic molecules, stable surface property after oxidation, no leaching of low molecular species, high biocompatibility, and low solubility of gases (i.e. low gas permeability), it would be an ideal alternative material for building lab-on-a-chip devices. However, to be processed by soft lithography, PS must be deformable in order to be replicated by a PDMS mold. PS can be heated to a melting (T > Tm) or rubber-like (T > Tg) state for replica molding. For example, PS has been hot embossed against a PDMS master at 180°C to create a microstructured chip for culture of single cells.25 The elasticity and low surface energy of the PDMS mold allowed it to be released easily from PS, but it was not possible to emboss features with aspect ratios higher than 2 because of the deformation of the PDMS master during the embossing process.25 Alternatively, a liquid precursor of PS (e.g. styrene monomer), or a PS solution in an organic solvent could be used for replica molding; however, it has been found that the monomer styrene quickly swells and distorts a PDMS mold, as do typical organic solvents used for dissolving PS.26 Highly fluorinated perfluoropolyether (PFPE) elastomer has excellent solvent resistance and has been demonstrated to be an appropriate molding material for soft lithography, but the precursors of this elastomer are not commercially available for replica molding.27, 28 Therefore, the search for a solvent that can dissolve PS, but that does not swell the PDMS mold, is crucial in the application of soft lithography for PS. The focus of this report was the identification of solvents compatible with both PS and PDMS. Once such a solvent was identified, PS microdevices were readily fabricated by replica molding on a PDMS master. High aspect ratio microstructures were created with high reproducibility and fidelity demonstrating that microstructured PS can be fabricated by benchtop soft lithography with readily available materials. These PS microdevices will be of high utility for a wide array of biological applications.
MATERIALS AND METHODS
Materials
The organic solvents screened for PS solubility and PDMS swelling (Table S1), rhodamine B, octyltrichlorosilane, propylene glycol monomethyl ether acetate (PGMEA), α-cyano-4-hydroxycinnamic acid, Hoechst 33342 and glutaraldehyde were purchased from Sigma Aldrich (St. Louis, MO). The PS used in these studies was obtained by cutting Falcon™ petri dishes (100 × 15 mm Style) (BD, Franklin Lakes, NJ) into small pieces (2–4 cm) that were then dissolved in the solvent. SU-8 photoresist was purchased from MicroChem Corp. (Newton, MA). PDMS was prepared from the Sylgard 184 silicone elastomer kit (Dow Corning, Midland, MI). Dulbecco's Modified Eagle Medium (DMEM), fetal bovine serum (FBS), and penicillin/streptomycin were obtained from Invitrogen (Carlsbad, CA). All other reagents were from Fisher Scientific (Pittsburgh, PA).
Fabrication of the PDMS mold
The PDMS mold was fabricated by casting PDMS on an SU-8 master. The SU-8 master was fabricated by standard photolithography on a glass slide spin-coated with an SU-8 layer of 10 – 250 µm thickness. The surface of the master mold was spin coated with 1 vol% octyltrichlorosilane in PGMEA at 2000 rpm for 30 s, followed by baking at 120 °C on a hotplate for 10 min. PDMS prepolymer (10:1 mixture of base:curing-agent in the Sylgard 184 kit) was spread on the master mold, and degassed under vacuum to remove trapped air bubbles. The PDMS was cured by baking the master at 100 °C on a hotplate for 30 min. The PDMS mold forming various microstructures was then obtained by peeling it from the master.
Measurement of solvent compatibility of PDMS
The solvent compatibility of PDMS was assessed by measuring the swelling of PDMS before and after immersion in a solvent as described previously.26 Briefly, the swell ratio was measured by comparing the lengths of PDMS strips (50 mm × 5 mm × 1 mm) before and after incubation in a solvent for 30 min.
Assessment of PS solubility in organic solvents
One gram of PS solid was added to 9 mL of each of the organic solvents listed in Table 1 in a 15 mL centrifuge tube. The tubes were rotated on a tube shaker for 7 days at room temperature. Determination of PS solubility in the solvent was based on complete dissolution of the PS solid with the creation of a clear, homogenous, single-phase solution.
Table 1.
Screening of solvents for PDMS swelling and PS solubility
| Solvents | PDMS Swell Ratio | PS Solubility | |
|---|---|---|---|
| # | Name | ||
| 1 | water | 1.00 * | |
| 2 | glycerol | 1.00 * | |
| 3 | ethylene glycol | 1.00 * | |
| 4 | perfluorotributylamine | 1.00 * | |
| 5 | perfluorodecalin | 1.00 * | |
| 6 | gamma-butyrolactone | 1.00 | + |
| 7 | dimethyl sulfoxide | 1.00 * | |
| 8 | delta-valerolactone | 1.00 | + |
| 9 | gamma-valerolactone | 1.00 | + |
| 10 | nitromethane | 1.00 * | |
| 11 | tetramethylene sulfone | 1.00 * | |
| 12 | acetonitrile | 1.01 * | |
| 13 | propylene carbonate | 1.01 * | |
| 14 | trifluoroethanol | 1.01 * | |
| 15 | epsilon-caprolactone | 1.01 | + |
| 16 | dimethylformamide | 1.02 * | + |
| 17 | methanol | 1.02 * | |
| 18 | 1,1,3,3-tetramethylurea | 1.02 * | + |
| 19 | 1-methyl-2-pyrrolidone | 1.03 * | + |
| 20 | dimethyl carbonate | 1.03 * | + |
| 21 | 1-methoxy-2-propanol | 1.03 | |
| 22 | ethanol | 1.04 * | |
| 23 | 1-butanol | 1.05 | |
denotes the swell ratio data obtained from reference 26.
Replica micromolding of PS by soft lithography
PS was dissolved in gamma-butyrolactone (GBL) with the concentration of solid being 25 wt%. Fig. 1A shows the soft lithography process for micromolding PS. To prevent the PS solution from dewetting from the mold during baking, a PDMS wall (6 mm depth) was glued around the edge of the PDMS mold (Fig. 1Ai). PS solution was added to the PDMS mold (Fig. 1Aii). The trapped air bubbles were removed by degassing under vacuum for 1 min. For the microchannel patterns, no degassing under a vacuum was necessary). The PDMS mold was then placed on a hotplate at 150 °C inside a fume hood for 16 h to allow complete evaporation of the GBL solvent from the molded material. Finally, the PDMS mold was slowly peeled from the solidified PS leaving the micropatterns imprinted on the PS piece with a high fidelity of replication (Fig. 1Aiii). The uneven edges of the PS piece were trimmed to yield the final device.
Fig. 1.
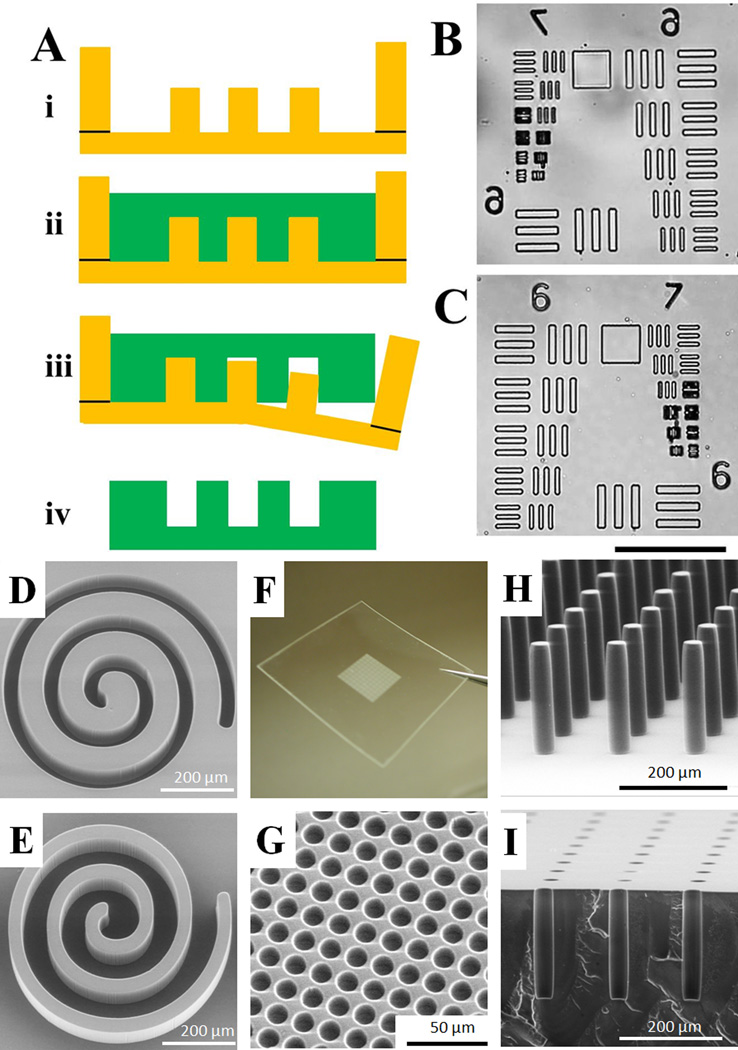
Micromolding PS by soft lithography. (A) Schematic of the process. i) A PDMS mold bounded by a raised PDMS well. ii) The PS solution is poured onto the PDMS mold, degassed, and baked to evaporate the GBL solvent. iii) The PDMS mold is released from the solidified PS leaving iv) the micromolded PS part. (B–C) Brightfield microscopy images of the standard USAF 1951 resolution test pattern on the PDMS mold (B) and the replicated PS (C). The scale bar is 100 µm. (D–E) SEM images of a spiral pattern on the PDMS mold (D) and the replicated PS (E). (F–I) A variety of microstructures created in PS by soft lithography. (F) A region of an array possessing 550,000 microwells. (G) SEM image of a section of the array in “F” revealing the individual microwells (12.5 µm diameter, 5 µm inter-well gap, 20 µm depth). (H) Pillars with an aspect ratio >7 (30 µm diameter, 215 µm height). (I) A coronal section of an array composed of microwells with high aspect ratio (30 µm diameter, 215 µm depth).
Fabrication of PS microfluidic chip
An open PS microchannel was fabricated by the replica micromolding procedure described above. To enclose the channel, a flat PS sheet was bonded on the top of the channel via thermal bonding. The flat PS sheet (~0.5 mm thickness) was obtained by casting PS solution on a flat PDMS sheet (20 mm × 20 mm × 6 mm) and baking at 150°C for 16 h. Holes (2 mm diameter) on the PS flat sheet were drilled as inlets and outlets by a hand drill or a CNC machine. The PS piece with imprinted channels and the PS sheet were oriented en face and placed on a hotplate (95 °C). The bonding was accomplished by simply pressing with manual pressure onto the PS pieces for 10 – 20 seconds.
Assessment of properties of molded PS
Three properties of molded PS were assessed in comparison with those of PDMS:
Adsorption/absorption of hydrophobic species. The microfluidic channel (50 µm width, 50 µm depth) was incubated with 100 µM rhodamine B for 15 min, and imaged by a Nikon Eclipse TE300 inverted fluorescent microscope equipped with a CY3 filter set. The channel was rinsed with deionized (DI) water and imaged again.
Hydrophobic recovery of plasma-oxidized surface. Flat samples of PDMS and PS were treated in an air plasma cleaner (Harrick Plasma, Ithaca, NY) for 1 min. The surface hydrophobicity was characterized by the contact angle of a 10-µL drop of deionized water on its surface at various time points.
Cell proliferation on oxidized surfaces. Flat samples (75 mm × 50 mm) were oxidized with plasma for 1 min, and then incubated in air at room temperature for 7 days. The samples were sterilized with ethanol, and lung carcinoma cell line H1299 cells (ATCC, Manassas, VA) were plated on the surface at a density of 25 cells/mm2. The cells were cultured in DMEM supplemented with FBS (10%), and L-glutamine (584 mg/L) at 37 °C in a humidified, 5% CO2 atmosphere. Penicillin (100 units/mL) and streptomycin (100 µg/mL) were added to the media to inhibit bacterial growth. At various times after cell plating, the samples were rinsed with PBS, and the cells were fixed with 4% glutaraldehyde in PBS for 1 min, then fixed in a PBS solution containing 0.25% glutaraldehyde and 0.1% Triton-100 for 10 min, and finally stained with Hoechst 33342 (1 µg/mL, 15 min), and imaged with a fluorescent microscope equipped with a DAPI filter set. Cell density was obtained by counting the cell number per 10× image field using ImageJ software (NIH, Bethesda, MD). Cells grown on a commercial polystyrene Petri dish optimized for cell culture (CELLSTAR Greiner Bio-One) was used as a control in these studies.
Detection of oligomer leaching from a PS microfluidic chip
A PS microfluidic chip (channel dimension: length × width × depth = 120 mm × 0.65 mm × 0.2 mm) was prepared as above. The channel was filled with 15 µL deionized water and incubated for 24 hours at 37°C. The contents of the channel were aspirated and analyzed by matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) (Applied Biosystems 4800, Life Technologies Corporation; Carlsbad, CA). Aqueous samples were diluted 1:1 with acetonitrile plus 0.1% trifluoroacetic acid (TFA), then 0.8 µL of each sample was spotted on a 384-well MALDI plate (Opti-TOF, Applied Biosystems), followed by 0.35 µL of saturated matrix solution (α-cyano-4-hydroxycinnamic acid in 1:1 acetonitrile/water plus 0.1% TFA). All samples were analyzed in linear mode between 1500–6000 Da, and reflectron mode from 300–3000 Da.
Measurement of residual GBL following baking
Three grams of a PS solution (25% in GBL) was added to a PDMS mold and baked at various temperatures (100 °C, 150 °C and 200 °C). The remaining weight (expressed as % of initial weight) was measured using an analytical balance.
Scanning electron microscopy
The various samples were sputtered with a 5-nm thick gold layer and the surfaces imaged by scanning electron microscopy (SEM) (FEI Quanta 200 ESEM, FEI Company, Hillsboro, Oregon).
RESULTS AND DISCUSSION
Screening of organic solvents for soft lithography of PS
The ideal solvent suitable for soft lithography should completely dissolve PS, but be compatible with PDMS (e.g. minimal swelling and leaching of oligomer). The problem to be overcome is that swelling of the PDMS mold will lead to its distortion and unsuitability for replication. For example, toluene is a well-known solvent for PS, but it quickly swells PDMS. When 2 mL PS solution (25 wt% in toluene) was added to a PDMS sheet (75 mm × 50 mm × 0.5 mm), within 5 min the PDMS sheet curled losing its ability to serve as a mold (supplementary information Fig. S1A). The solvent compatibility of PDMS has also been studied by others with 38 different organic solvents including aliphatic hydrocarbons, aromatic hydrocarbons, fluorocarbons, chlorides, alcohols, ethers, esters, acids, and amines measured for their ability to swell PDMS.26 We screened an additional 13 organic solvents including GBL, gamma-valerolactone (GVL), and delta-caprolactone for their ability to swell PDMS. Among the 51 organic solvents, 28 solvents were excluded because they were known to or found to cause >5% swelling of the PDMS (supplementary information Table S1A).
The solvents compatible with PDMS (Table 1) were screened for their ability to solubilize PS. Of the solvents tested, only 8 were found that fully dissolved PS (Table 1). Of these, GBL, GVL and delta-valerolactone had swell ratios for PDMS of <1%, and were therefore felt to serve as the most suitable solvents for PS molding. 1-Methyl-2-pyrrolidone was not deemed suitable since PS molded from 1-methyl-2-pyrrolidone became discolored. Dimethylformamide (DMF) and dimethyl carbonate were unsuccessful since PS molded from these solvents formed surface bubbles mostlikely due to the baking temperature being close to or higher than the boiling points of DMF and dimethyl carbonate. GVL was particularly attractive since it is a naturally occurring chemical in fruits, and is a promising "green" solvent. Thus it is a solvent that merits future study.29 However, GBL possessed the smallest swell ratio among the solvents that dissolved PS. In testing, it was found that PS completely dissolved in GBL at concentrations greater than 40 wt%, although at these higher concentrations the resulting solution was too viscous for efficient molding. In further testing, when PS solution (25 wt% in 2 mL of GBL) was added to a PDMS sheet (75 mm × 50 mm × 0.5 mm) and incubated for 2 h at room temperature, the PDMS sheet did not show distortion (supplementary information Fig. S1B). GBL thus showed the unique capacity of solubilizing PS while not swelling PDMS during molding. Once dissolved in GBL, the PS solution was found to be stable (up to 6 months) at room temperature. While GBL appeared to be an ideal candidate solvent for soft lithography of PS and was used in the current studies, the two alternate solvents (GVL and delta-valerolactone) have also been tested with similar results (data not shown).
Replica micromolding of PS by soft lithography
To replicate PS on a PDMS mold, approximately 3 g of PS solution (25 wt% in GBL) was placed onto the PDMS mold (chamber area of 40 mm × 40 mm). The solution was then degassed under a vacuum with the PS solution remaining transparent. Since GBL possesses a high boiling point (204°C), it evaporated very slowly at room temperature and did not evaporate during pre-molding steps (e.g. spreading, degassing). The mold was heated on a hotplate to evaporate the GBL solvent. At 150°C, 96% of GBL was evaporated from the solution within 1 h (Supplementary data Fig. S2). An extensive baking time (16 h) evaporated 99.4% of the GBL. Baking at a higher temperature (200°C) led to complete evaporation of the GBL (supplementary information Fig. S2), but was not employed due to concerns of polymer degradation at this temperature. Under these conditions, a film of approximately 0.3 mm thickness was present after the baking step. The PDMS/PS was cooled to room temperature and the PDMS mold was slowly peeled from the solidified PS. Since PS has no adhesion to PDMS, it was easily peeled from the PS, even over a large area (e.g. 650 mm2). A single PDMS mold was repeatedly used to produce a number of PS devices (up to 10 moldings in our hands) without degrading the fidelity of replication.
To assess the resolution of PS micromolding by this method, a standard resolution test target (USAF 1951) was used to create 10-µm height micropatterns on SU-8, and then replicated to a PDMS mold (Fig. 1B).30 The best resolution obtained was 3 µm and was limited by the resolution of the SU-8 master mold made to form the PDMS mold. The molded PS possessed the same resolution as the PDMS mold (Fig. 1C). It is possible that submicron resolution may be achieved for molded PS since soft lithography can achieve 6-nm resolution for other materials.14
To demonstrate the high fidelity of replica micromolding, a spiral pattern (line width 25 µm, height 50 µm) was created on a PDMS mold (Fig. 1D), and the pattern transferred to PS without distortion (Fig. 1E). Fig. 1F–I show a variety of microstructures created by soft lithography of PS. An array of 550,000 microwells was molded on a PS sheet, with an array area of 13 mm × 13 mm (Fig. 3F). The microwells (12.5 µm diameter, 5 µm inter-well gap, 20 µm depth) showed high uniformity across the array (Fig. 3G). The soft lithography yielded PS microstructures with a high aspect ratio. For example, a pillar array with an aspect ratio >7 (30 µm diameter, 215 µm height) was micromolded without twisting or bending of the pillars (Fig. 3H). Similarly, an array of deep microwells (30 µm diameter, 215 µm depth) with an aspect ratio >7 was molded (Fig. 3I). It is generally very difficult for other fabrication methods (e.g. microinjection molding, hot embossing) to create microstructures with such high resolution and aspect ratio due to problems associated with demolding from a rigid master (typically nickel, stainless steel, silicon, or epoxy). As an example, molding PS on a rigid SU-8 template yielded microstructures that were detached or broken during the demolding process (data not shown). To the best of our knowledge, PS microstructures with such high-aspect-ratio have not been realized using hot embossing, liquid microcontact printing, or injection molding.
Fig. 3.
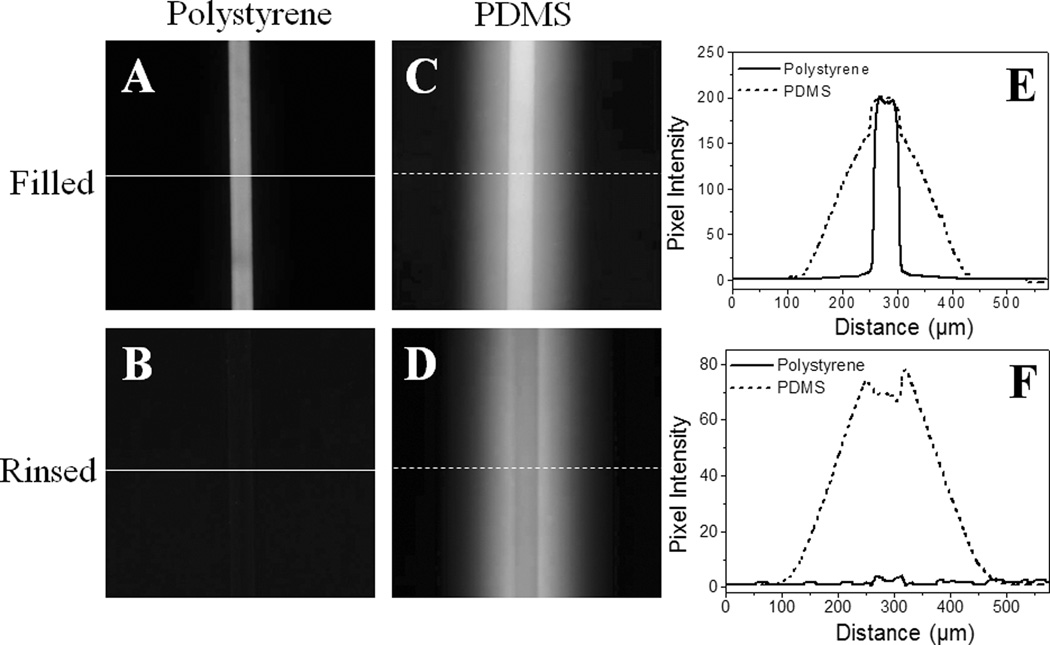
Rhodamine B adsorption/absorption tests. Fluorescence images of PS (A and B) and PDMS (C and D) devices are shown. The channels on these devices are filled with 100 µM rhodamine B in water for 15 min, and then rinsed with water. Fluorescent profiles of the PS and PDMS channels are also shown in E and F, respectively. These profiles were taken along the white lines in images A–D.
PS microfluidic chip
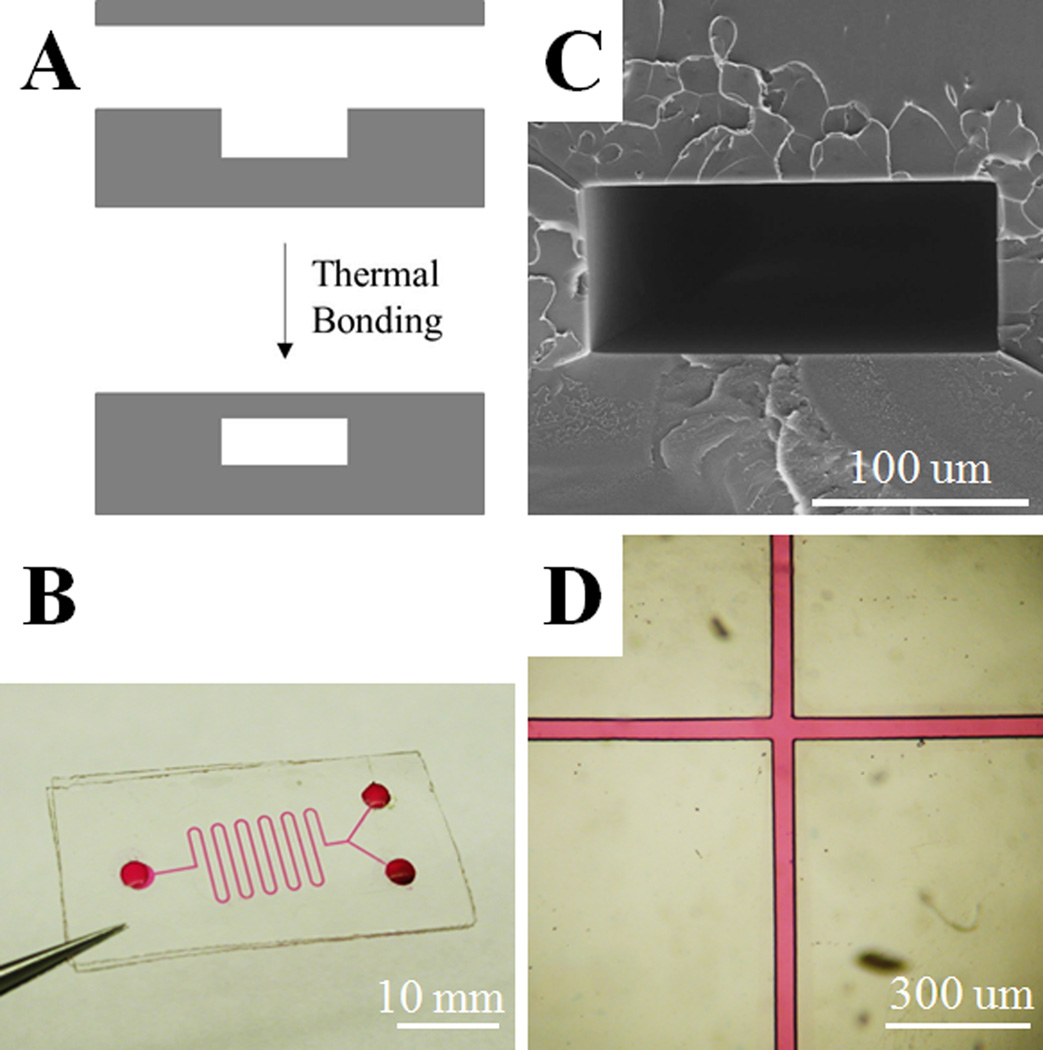
One of the most important applications for lab-on-a-chip devices is in the area of microfluidics. To evaluate PS for this application, we fabricated microfluidic chips from PS using soft lithography (Fig. 2A). An open microchannel was fabricated by replica micromolding of PS. A flat PS plate was then mated with the PS structure housing the channel. Initial testing demonstrated that heating at 95 °C under manual pressure generated excellent bonding for a prototype microfluidic chip possessing a channel width of 200 µm and height of 50 µm (Fig. 2B). An SEM image of the channel cross-sectional demonstrated that the two parts of the device were bonded seamlessly without channel distortion (Fig. 2C). No leakage was observed from the microfluidic channel, as shown by filling the channel (50 µm width, 50 µm depth) with 10 mM rhodamine B in water (Fig. 2D).
Fig. 2.
PS microfluidic chip. (A) Schematic of thermal bonding to form an enclosed microfluidic channel. (B) A prototype of a PS microfluidic chip. Rhodamine B (10 mM) was used for visualization of the microchannel (200 µm width, 50 µm depth). (C) Cross section of the channel (200 µm width, 50 µm depth). (D) Microfluidic channel (50 µm width, 50 µm depth) filled with 10 mM rhodamine B.
Absorption of hydrophobic species on the surface
An intrinsic weakness of PDMS is that hydrophobic molecules are adsorbed onto its surface and migrate into the bulk PDMS due to its hydrophobic surface and porous structure.23, 31 These properties present significant limitations. For example, adsorption and absorption of biomolecules can lead to carryover between repetitive biological or biochemical assays performed on a PDMS device. In the case of a PDMS device used for chemical separations, adsorption leads to a variety of problems and limits the analytes that can be chemically separated.32 As an illustration of this problem, a PDMS microfluidic channel (50 µm width, 50 µm depth) was incubated with 100 µM rhodamine B for 15 min, after which significant absorption of the dye into the bulk of the PDMS microchip was observed (Fig. 3C). Even after extensive rinsing, the channel exhibited strong fluorescence due to the retention of the dye (Fig. 3D) consistent with prior reports.32 To address this issue, the PDMS surface must be coated with an impermeable barrier (e.g. silicon oxide) using a sol-gel method.32, 33 In contrast, when using a microfluidic device composed of PS, rhodamine B did not diffuse into the bulk of the PS when the dye was loaded into the channel (50 µm width, 50 µm depth) in an identical manner to that described for the PDMS channel above (Fig. 3A). After rinsing with water, only a very weak adsorption of rhodamine B was observed on the surface and no fluorescent signal from absorbed dye was detected in the bulk material (Fig. 3B). Fig. 3E and F show the quantitative comparison of these results on PDMS and PS chips. These data show that compared to PDMS, the PS microchip exhibited dramatically reduced non-specific adsorption and absorption of hydrophobic analytes, which can be expected to result in higher detection sensitivity and separation efficiencies.
Cell biocompatibility
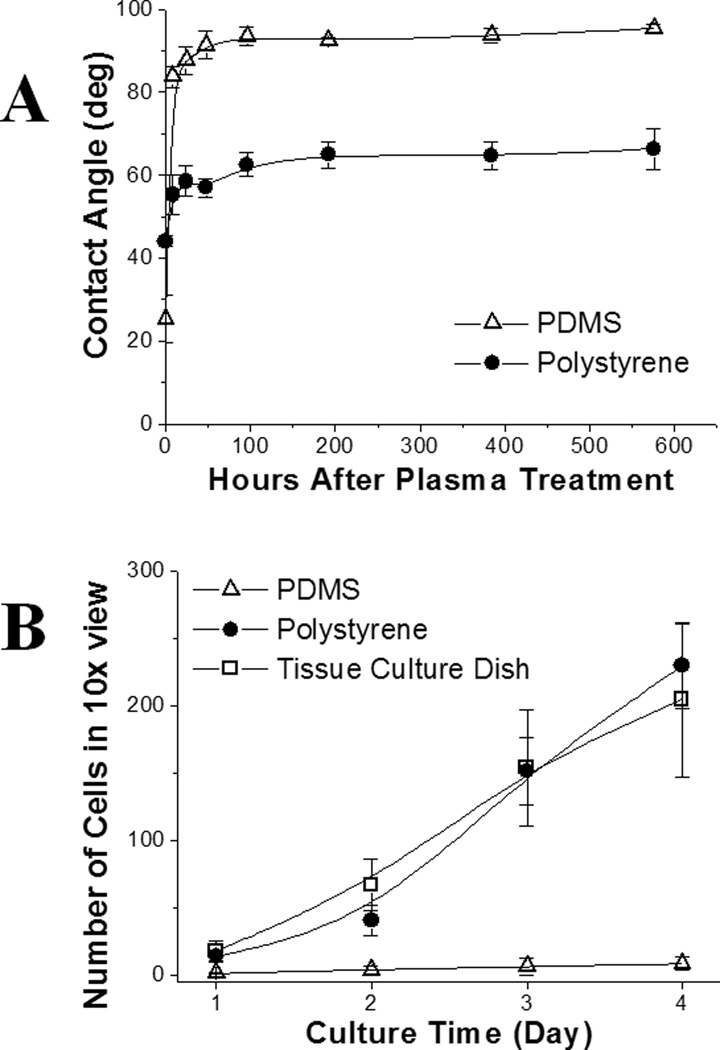
One limitation of PDMS microdevices for cell-based applications is the fast hydrophobic recovery after surface oxidation by plasma treatment.20, 21 After treatment in an air plasma for 1 min, the oxidized surface of PDMS recovered to its hydrophobic state (contact angle >90 °) in 48 h (Fig. 4A). This behavior has been attributed to the low glass transition temperature of PDMS (−120 °C) such that hydrophobic groups in PDMS migrate up to the surface.34, 35 This phenomenon results in unstable coating of extracellular matrix (ECM) on the PDMS surface, and thus poor cell attachment, which negatively impacts the performance of PDMS devices for cell-based assays.36, 37 In contrast, PS has a high glass transition temperature (95 °C). Once oxidized, the surface properties remain stable (e.g. the long shelf life of cell culture grade Petri dishes which are surface-treated by the manufacturer). Molded PS remained hydrophilic after plasma oxidization with a contact angle of 66° after 24 days of storage in contact with air (Fig. 4A).
Fig. 4.
Surface stability and cell biocompatibility. (A) Surface hydrophobic recovery after plasma oxidation followed by exposure to air. The contact angle was measured at varying times after plasma treatment. (B) Growth kinetics of H1299 cells on the PS, PDMS and a standard cell culture Petri dish. Each cell count was averaged on 3 samples. The error bars represent the standard deviation.
PS molded from a flat PDMS surface was assessed for its biocompatibility and support of cell attachment. After replica molding, the PS surface possessed the native hydrophobicity of PS, i.e. inert and hydrophobic as assessed by the contact angle of water (80°) on the treated surface. Plasma treatment was required to re-generate a hydrophilic and ionic surface suitable for cell culture. A molded PS surface and a PDMS surface were subjected to a 1 min plasma treatment, and placed in contact with air at room temperature for 7 days. H1299 cells were cultured on the molded PS surface, a PDMS surface, and a commercial polystyrene Petri dish optimized for cell culture. Within 4 days, the cells spread, attached, and proliferated equally well on both the polystyrene tissue culture dish and molded PS surfaces demonstrating that the molded PS was equivalent to the tissue culture dish in terms of cell adhesion and growth. In contrast, the cell attachment and proliferation on the PDMS surface were dramatically reduced compared to that seen on the control polystyrene and molded PS surfaces. These differences are likely due to the post-oxidation hydrophobic recovery of the PDMS,20 since a hydrophobic PDMS surface generally does not favor cell attachment and proliferation.38, 39
Leaching of low molecular weight species has been shown to be non-toxic to cells in an open culture system in contact with a large volume of media (typically milliliters).40 However, leaching has been shown to be problematic in microfluidic systems due to the high surface-area-to-volume ratio.22 In the PDMS microchannel where a small volume (typically microliters) of media is used, leaching of PDMS oligomers was detectable in the media by MALDI MS and even on the cell membranes when cells were cultured in the media.22 In the current study, leaching of oligomers into water incubated under tissue culture conditions within PS microfluidic chips was not detectable by MALDI MS (data not shown). Given the hydrophilic stability of the PS surface and its compatibility with cell culture, PS microdevices created by soft lithography are likely to prove a valuable alternative to PDMS for the biologic research community.
CONCLUSIONS
Through a rigorous screening process, GBL together with two additional solvents (GVL and delta-valerolactone) were found to serve as appropriate solvents based on their ability to dissolve PS without swelling PDMS; therefore, serving as ideal solvents that enable micromolding of PS by soft lithography methods. Micromolding of a variety of PS microstructures with high resolution and high fidelity demonstrated the broad capabilities of this approach for rapid prototyping. Importantly, high resolution and high-aspect-ratio structures were easily fabricated using this micromolding process, which would be very difficult to accomplish using other methods. In another example, a prototype PS microfluidic chip showed much lower non-specific absorption of hydrophobic molecules and greater surface stability than a device composed of PDMS. In summary, this study described a process for creating PS structures that does not require any dedicated or costly instrumentation and can be performed on a bench top. All of the raw materials are readily available from standard commercial sources. With the numerous advantageous characteristics of PS as a material for biological applications, including stable surface properties after oxidation, non-leaching of low molecular weight species and high biocompatibility, PS represents a superlative material for building lab-on-a-chip devices. The process described in this study is particularly useful for rapid prototyping of microdevices for biological applications.
Supplementary Material
figure 5.
ACKNOWLEDGEMENT
This research was supported by the NIH (R01HG004843, R01HG004843S1, and R01EB012549). We thank Michelle Kovarik for helpful discussions.
REFERENCES
- 1.Curtis ASG, Forrester JV, McInnes C, Lawrie F. Journal of Cell Biology. 1983;97:1500–1506. doi: 10.1083/jcb.97.5.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin CD, Linder V, Sia SK. Lab on a Chip. 2007;7:41–57. doi: 10.1039/b611455e. [DOI] [PubMed] [Google Scholar]
- 3.Madou M. Fundamentals of microfabrcation. New York: CRC; 2002. [Google Scholar]
- 4.Becker H, Gartner C. Electrophoresis. 2000;21:12–26. doi: 10.1002/(SICI)1522-2683(20000101)21:1<12::AID-ELPS12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Zhao XM, Xia YN, Schueller OJA, Qin D, Whitesides GM. Sensors and Actuators a-Physical. 1998;65:209–217. [Google Scholar]
- 6.Soper SA, Ford SM, Qi S, McCarley RL, Kelly K, Murphy MC. Analytical Chemistry. 2000;72:642A–651A. doi: 10.1021/ac0029511. [DOI] [PubMed] [Google Scholar]
- 7.Becker H, Gartner C. Analytical and Bioanalytical Chemistry. 2008;390:89–111. doi: 10.1007/s00216-007-1692-2. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Zhang LY, Chen G. Electrophoresis. 2008;29:1801–1814. doi: 10.1002/elps.200700552. [DOI] [PubMed] [Google Scholar]
- 9.Steigert J, Haeberle S, Brenner T, Muller C, Steinert CP, Koltay P, Gottschlich N, Reinecke H, Ruhe J, Zengerle R, Ducree J. Journal of Micromechanics and Microengineering. 2007;17:333–341. [Google Scholar]
- 10.Young EW, Berthier E, Guckenberger DJ, Sackmann E, Lamers C, Meyvantsson I, Huttenlocher A, Beebe DJ. Analytical Chemistry. 2011;83:1408–1417. doi: 10.1021/ac102897h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker SLR, Ross D, Tarlov MJ, Gaitan M, Locascio LE. Analytical Chemistry. 2000;72:5925–5929. doi: 10.1021/ac0008690. [DOI] [PubMed] [Google Scholar]
- 12.Barker SLR, Tarlov MJ, Canavan H, Hickman JJ, Locascio LE. Analytical Chemistry. 2000;72:4899–4903. doi: 10.1021/ac000548o. [DOI] [PubMed] [Google Scholar]
- 13.Chen CS, Breslauer DN, Luna JI, Grimes A, Chin WC, Leeb LP, Khine M. Lab on a Chip. 2008;8:622–624. doi: 10.1039/b719029h. [DOI] [PubMed] [Google Scholar]
- 14.Xia YN, Whitesides GM. Annual Review of Materials Science. 1998;28:153–184. [Google Scholar]
- 15.Qin D, Xia YN, Whitesides GM. Nature Protocols. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 16.Whitesides GM, Ostuni E, Takayama S, Jiang XY, Ingber DE. Annual Review of Biomedical Engineering. 2001;3:335–373. doi: 10.1146/annurev.bioeng.3.1.335. [DOI] [PubMed] [Google Scholar]
- 17.Kim P, Kwon KW, Park MC, Lee SH, Kim SM, Suh KY. Biochip Journal. 2008;2:1–11. [Google Scholar]
- 18.McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu HK, Schueller OJA, Whitesides GM. Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 19.Sia SK, Whitesides GM. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 20.Fritz JL, Owen MJ. Journal of Adhesion. 1995;54:33–45. [Google Scholar]
- 21.Occhiello E, Morra M, Cinquina P, Garbassi F. Polymer. 1992;33:3007–3015. [Google Scholar]
- 22.Regehr KJ, Domenech M, Koepsel JT, Carver KC, Ellison-Zelski SJ, Murphy WL, Schuler LA, Alarid ET, Beebe DJ. Lab on a Chip. 2009;9:2132–2139. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toepke MW, Beebe DJ. Lab on a Chip. 2006;6:1484–1486. doi: 10.1039/b612140c. [DOI] [PubMed] [Google Scholar]
- 24.Merkel TC, Bondar VI, Nagai K, Freeman BD, Pinnau I. Journal of Polymer Science Part B-Polymer Physics. 2000;38:415–434. [Google Scholar]
- 25.Dusseiller MR, Schlaepfer D, Koch M, Kroschewski R, Textor M. Biomaterials. 2005;26:5917–5925. doi: 10.1016/j.biomaterials.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 26.Lee JN, Park C, Whitesides GM. Analytical Chemistry. 2003;75:6544–6554. doi: 10.1021/ac0346712. [DOI] [PubMed] [Google Scholar]
- 27.Truong TT, Lin RS, Jeon S, Lee HH, Maria J, Gaur A, Hua F, Meinel I, Rogers JA. Langmuir. 2007;23:2898–2905. doi: 10.1021/la062981k. [DOI] [PubMed] [Google Scholar]
- 28.Gratton SEA, Williams SS, Napier ME, Pohlhaus PD, Zhou ZL, Wiles KB, Maynor BW, Shen C, Olafsen T, Samulski ET, Desimone JM. Accounts of Chemical Research. 2008;41:1685–1695. doi: 10.1021/ar8000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horvath IT, Mehdi H, Fabos V, Boda L, Mika LT. Green Chemistry. 2008;10:238–242. [Google Scholar]
- 30.Desai SP, Taff BA, Voldman J. Langmuir. 2008;24:575–581. doi: 10.1021/la702827v. [DOI] [PubMed] [Google Scholar]
- 31.Huang B, Wu HK, Kim S, Zare RN. Lab on a Chip. 2005;5:1005–1007. doi: 10.1039/b509251e. [DOI] [PubMed] [Google Scholar]
- 32.Roman GT, Hlaus T, Bass KJ, Seelhammer TG, Culbertson CT. Analytical Chemistry. 2005;77:1414–1422. doi: 10.1021/ac048811z. [DOI] [PubMed] [Google Scholar]
- 33.Gomez-Sjoberg R, Leyrat AA, Houseman BT, Shokat K, Quake SR. Analytical Chemistry. 2010;82:8954–8960. doi: 10.1021/ac101870s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim J, Chaudhury MK, Owen MJ, Orbeck T. Journal of Colloid and Interface Science. 2001;244:200–207. [Google Scholar]
- 35.Eddington DT, Puccinelli JP, Beebe DJ. Sensors and Actuators B-Chemical. 2006;114:170–172. [Google Scholar]
- 36.Mehta G, Kiel MJ, Lee JW, Kotov N, Linderman JJ, Takayama S. Advanced Functional Materials. 2007;17:2701–2709. [Google Scholar]
- 37.Zhou JW, Ellis AV, Voelcker NH. Electrophoresis. 2010;31:2–16. doi: 10.1002/elps.200900475. [DOI] [PubMed] [Google Scholar]
- 38.Lee JN, Jiang X, Ryan D, Whitesides GM. Langmuir. 2004;20:11684–11691. doi: 10.1021/la048562+. [DOI] [PubMed] [Google Scholar]
- 39.Fuard D, Tzvetkova-Chevolleau T, Decossas S, Tracqui P, Schiavone P. Microelectronic Engineering. 2008;85:1289–1293. [Google Scholar]
- 40.Wlodkowic D, Faley S, Skommer J, McGuinness D, Cooper JM. Analytical Chemistry. 2009;81:9828–9833. doi: 10.1021/ac902010s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.