Abstract
The extensive adaptability of dendrimer-based contrast agents is ideal for the molecular imaging of organs and other target-specific locations. The ability of literally atom-by-atom modification on cores, interiors, and surface groups, permits the rational manipulation of dendrimer-based agents in order to optimize their physical characteristics, biodistribution, receptor-mediated targeting, and controlled release of the payload. Such modifications enable agents to localize preferentially to areas or organs of interest for facilitating target-specific imaging as well as assume excretion pathways that do not interfere with desired applications. Recent innovations in dendrimer research have increased agent directibility and new synthetic chemistry approaches have increased efficiency of production as well as led to the creation of novel dendrimer-based contrast agents. In addition, by taking advantage of the numerous attachment sites available on the surface of a single dendrimer molecule, new synthetic chemistry techniques have led to the development of multimodality magnetic resonance, radionuclide, and fluorescence imaging agents for molecular imaging. Herein we discuss advances in dendrimer-based contrast agents for molecular imaging focusing mainly on the chemical design as applied to optical, magnetic resonance, computer tomography, radionuclide, and multi-modality imaging.
Keywords: Dendrimer, contrast agent, molecular imaging, nanomedicine, magnetic resonance imaging, optical imaging, radionuclide imaging, multiple modalities
INTRODUCTION
The ideal molecular imaging contrast agent would contain a targeting moiety and deliver an imaging payload, while allowing the in vivo imaging properties to be manipulated to improve bio-distribution and excretion of the agent, thus customizing the agent for the desired imaging application. Conventional imaging agents for magnetic resonance (MR) and computer tomography (CT) are low in molecular weight (LMW) and thus, their in vivo behavior is difficult to control. LMW agents exhibit rapid clearance from vascular circulation and rapid renal excretion, thus limiting their availability for time-dependent imaging studies stretching over hours. Unlike LMW agents however, dendrimer-based contrast agents offer much greater flexibility for in vivo applications. For example, even nanoscale changes in dendrimer size result in changes in biodistribution, pharmacokinetics, and excretion properties allowing such agents to be custom designed for specific purposes. Dendrimer-based contrast agents offer a sufficient number of binding sites to which numerous imaging and targeting moieties can be conjugated.
Dendrimers are a group of highly branched spherical polymers that are synthesized with structural control rivaling traditional biomolecules such as DNA/RNA and are often referred to as ‘artificial proteins’. Yet, their nanoscale scaffolding, nanocontainer properties, and tunable variation in size, surface chemistry, and interior void space make dendrimers unique nano-materials that are more versatile than traditional biomolecules [1]. The application of dendrimer chemistry to molecular imaging represents the fusion of two rapidly advancing areas of science [2].
Several properties make dendrimers unique nano-structures. The interior-surface architecture, consists of a core concentrically surrounded by annular shells, or generations (referred to as G1, G2, G3, etc), of covalently linked branched interiors [2]. This creates a layered structure upon which terminal surface groups are presented and geometrically amplified as the dendrimer generation increases [1]. The final dendrimer structure, consisting of a core, branched interiors, and numerous surface functional groups serves as a platform to which additional substrates can be added to this spherical molecule in a highly controlled manner (Fig. 1). The chemical properties of the core and the surface create a potential space within the dendrimer particle referred to as “interior void space” that can be utilized to carry potential therapeutic payloads. This nano-space represents an isolated environment, thus decreasing toxicity associated with the payload [2] while protecting the drug from enzymatic degradation before its release at the target. “Controlled release” of these payload molecules can be achieved after targeting of the molecules to organ or cell-specific locations.
Fig. (1).
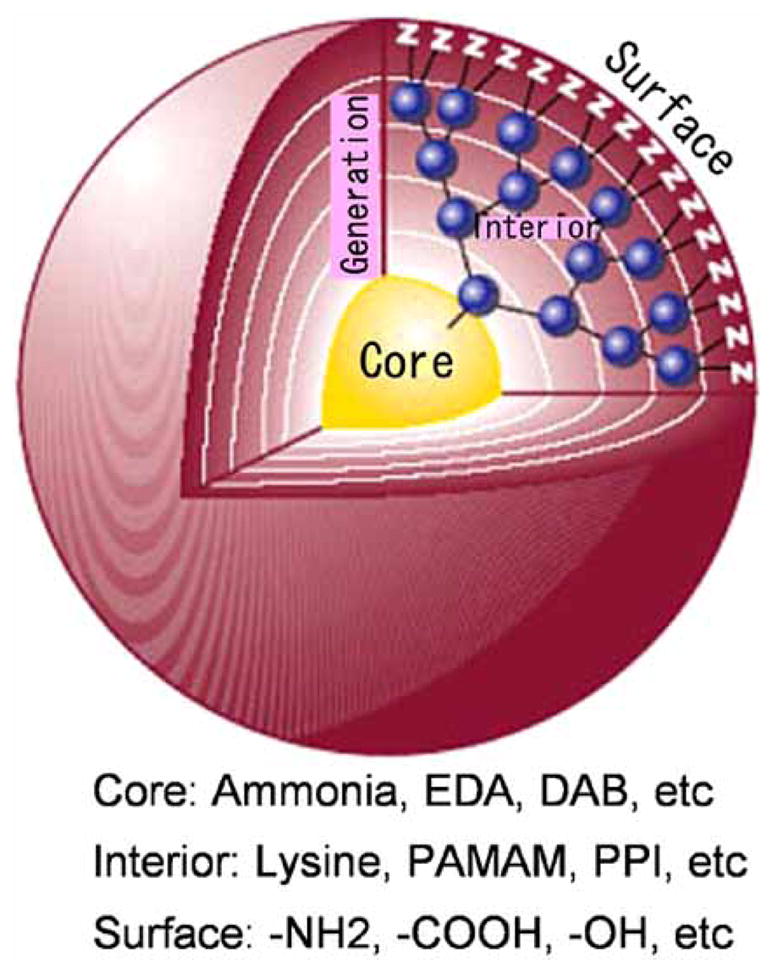
Schematic representation of the dendrimer structure.
The ability to modify and optimize dendrimer surface groups, permits the rational manipulation of dendrimer agents by changing their diameter, biodistribution, receptor-mediated targeting, and controlled release of the payload. The nanoscaffold property of dendrimers derives from the ability to attach molecules in a controlled and well-defined manner to the dendritic surface of the outer shell. The abundance of functional groups on the outer shell serves as a platform for attachment and presentation of cell-specific targeting groups, solubility modifiers, and imaging agents [1]. These tunable properties make dendrimers more attractive agents for biomedical applications compared to other nano-vectors such as micelles, liposomes, or emulsion droplets whose properties are more difficult to control [1].
Dendrimers are composed of combinations of core types, including ethylenediamine (EDA), diaminobutyl (DAB), polyamidoamine (PAMAM) and polypropylimine (PPI). In addition, surface residues such as amine, carboxyl, and alcohol groups contribute to the chemical signature of the specific agent. Those that have been used as platforms for imaging agents, such as PAMAM or PPI dendrimers, are highly soluble in aqueous solution and possess a primary amine rich surface that is uncharged at pH greater than 9.0 [3, 4]. The abundance of primary amine functional groups on the exterior shell serve as optimal attachment sites for chelating agents in the development of macromolecular contrast agents, or for antibody molecules for site-specific targeting [3]. The ability to attach both targeting and chelating moieties to DAB/PPI and PAMAM dendrimers has propelled their use as contrast agents for molecular imaging. In comparison to DAB/PPI and PAMAM dendrimer cores, which have polyamine and amide core components respectively, newer families of dendrimers possess a cystamine functional group core component, which, when subject to traditional redox reactions, permits the use of maleimide and sulfohydryl coupling reactions, for the conjugation of peptides, monoclonal antibodies, biotin, or other targeting moieties [5, 6].
The biocompatibility profile of dendrimers contributes to their utility in molecular imaging. In addition, the biocompatibility can be increased via functionalization with small molecules or by PEGylating the surface groups, both of which further decrease immunogenicity [2]. Increased biocompatibility is also associated with lower generation branch cells with anionic or neutral groups compared to similar branch cells of higher generations which have cationic surface groups [1].
The use of dendrimers for diagnostic imaging was proposed in the patent literature nearly a decade ago, stemming from anticipated applications of work by Tomalia et al. [1]. Early applications of dendrimers in molecular imaging involved ‘passive targeting,’ wherein variations in the physical attributes of dendrimers, such as size and charge, were utilized to achieve organ-specific imaging.
Passive organ-specific targeting was achieved using discrete differences in dendrimer generation number, and thus agent diameter. Excretion routes of each agent were observed to be affected by generation number [1]. This was an important discovery as it suggested that the physical properties of dendrimers can be modified for organ-specific imaging and that dendrimers can act as “platforms for designer imaging agents” that can be adapted to obtain desirable distribution and excretion routes that do not interfere with the intended imaging application. Non-passive, cell-specific targeting, was achieved by Wiener and colleagues. By functionalizing dendrimer-based imaging agents with folic acid, the group created an imaging agent that specifically targeted cells with increased expression of folic acid receptors, a characteristic of certain cancer types.
There is continued effort to establish the relationship between the size and chemical properties of dendrimers and their in vivo behavior. One example of the effect of chemical characteristics on in vivo behavior is the hepatocellular uptake and biliary excretion observed with agents possessing hydrophobic interior cores. In addition, the relationship between size and behavior is exemplified by the observation that simply increasing dendrimer diameter increases vascular compartment retention time and the observation that smaller macromolecular agents transiently concentrate in the renal tubules [7]. Further clarification of such relationships will allow researchers to harness the potential of these agents for imaging applications. In addition, functionalizing dendrimer scaffolds with target-specific ligands is a rapidly growing area of research that may represent the next frontier of molecular imaging in diagnostic radiology.
MRI
Since the observation that paramagnetic chelates such as Gd(III)-diethylenetriaminepentaacetic acid [Gd(III)-DTPA] (Magnevist) and Gd(III)-N, N′, N″, N‴-tetracarboxymethyl-1,4,7,10-tetraazacyclododecane [Gd(III)-DOTA] increase relaxation rates of surrounding protons, these agents have been widely used in MR contrast media [8, 9]. However, the rapid clearance of these LMW contrast agents from the vascular space limits their application in time-dependent imaging studies [10]. Moreover, their relatively low molar relaxivity properties [11] mandate the use of larger doses (0.1mM/kg), which has led to growing concern due to gadolinium toxicity as reported recently in the syndrome of nephrogenic systemic fibrosis (NSF) [12].
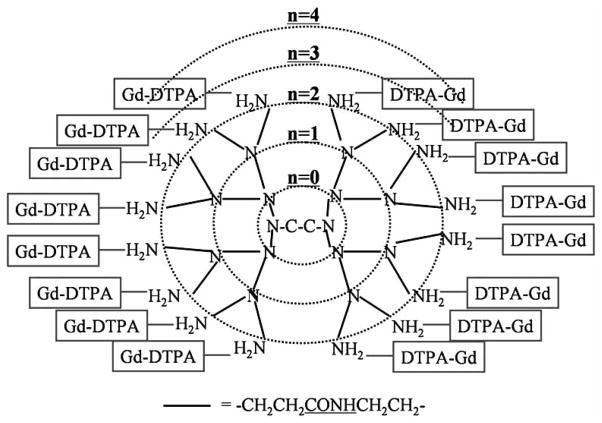
Macromolecular imaging agents were developed to overcome some of these limitations. Due to their multivalent surfaces, well-defined architectures, and nanoscale sizes, dendrimers are optimal nanoparticles to serve as a core platform to carry multiple copies of small molecules such as Gd(III)-chelates for MR contrast [1]. Such agents must be on the surface of the dendrimer in order to allow them to freely interact with water molecules. Derivatives of the two major classes of MR chelates, DTPA and DOTA, are used to sequester the metal ions at the surface of dendrimers [5, 11, 13] (Fig. 2). Although there are subtle differences between chemical properties of DTPA and DOTA, when chelated with Gd(III), the differences in R1 relaxivity are neglible (4.7mM−1s−1 at 20MHz and 25 °C) [5]. In addition, physical and pharmacokinetic properties of dendrimers conjugated with Gd(III) DOTA chelates and dendrimer-Gd(III)-1B4M-DTPA chelates are comparable. One great advantage of macromolecular contrast agents is their increased relaxivity owing to their slower tumbling rate in solution. Dendrimer-Gd(III)-1B4M-DTPA using larger than generation-5 dendrimer showed 6–8 fold relaxivity gains (30–40 mM−1s−1 at 20–64 MHz and 25 °C) compared with small molecular agents [14, 15]. In addition to the favorable pharmacokinetics, high relaxivity can minimize the injected dose of gadolinium ions, which could decrease the concern of metal ion toxicity.
Fig. (2).
Schema of the MRI contrast agent made based on EDA core - PAMAM interior - Amide surface dendrimer.
Lauterbur and colleagues were the first to demonstrate an in vivo application of dendrimer-based MR contrast agents in the early 1990s. Due to the blood pool properties demonstrated early on, their usefulness as vascular imaging was quickly recognized. In addition, it was demonstrated that relaxivity values correlated positively with dendrimer generation such that relaxivity of MR contrast agents based on PAMAM-Gd(III) chelates increased with each dendrimer generation number [1].
Research in dendrimer-based MR contrast agents has revealed that modification of dendrimer properties such as size, cores and interiors, and exterior shell chemistry permit application-specific customization [16]. Among these tunable properties, size has been shown to have a tremendous effect on in vivo behavior, even when comparing molecules with similar chemical properties [5]. Small changes in diameter of MR contrast agents can greatly impact their pharmacokinetics, permeability across the vascular wall, excretion route, and recognition by the reticulo-endothelial system [5]. To further explore these effects, Kobayashi et al. synthesized a library of novel dendrimer-based macromolecular MRI-contrast agents with molecular weights ranging from 29–3850 kD and molecular sizes ranging from 3 to 15 nm with variations in properties that influence blood retention, tissue perfusion, and excretion rates [5].
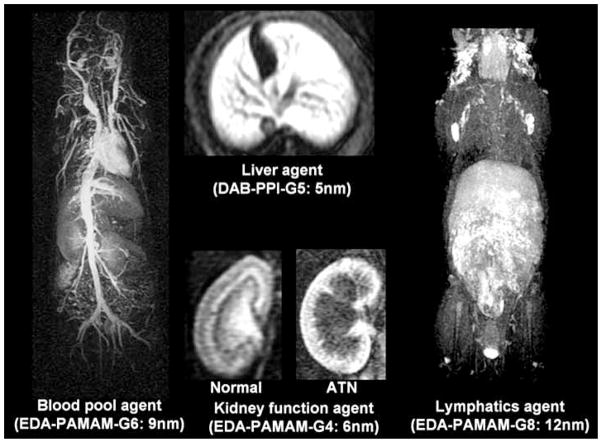
The versatility of dendrimer nanoparticle behavior has led to the development of several interesting dendrimer-based MR contrast agent applications. As mentioned above, macromolecular MR contrast agents are useful for vascular imaging, specifically microvascular imaging and determining functional capillary density in tumor tissues [5]. Among the dendrimer-based agents, generation (G)7 and G8 agents permit visualization of the blood vessels both within and outside tumors most clearly and PEG conjugation further improves image quality as well as excretion profiles [5]. Dendrimers with hydrophobic properties have proven useful in hepatic imaging. Utilizing the predisposition of slightly less hydrophilic macromolecules to accumulate in the liver, a G5 DAB/PPI dendrimer with a hydrophobic 64 surface amine core unit (DAB-Am64-(1B4M-Gd)64, DABG5, was shown to homogenously enhance liver parenchyma [5]. Furthermore, DABG5 applied to dynamic micro-MRIs enabled serial visualization of growing metastatic colon cancer foci as small as 0.3-mm in diameter within the livers of living mice [5].
The utility of dendrimer-based MR contrast agents has also been demonstrated in the functional assessment of organs such as the kidney. Although small dendrimer based agents are quickly excreted from the kidneys, Kobayashi et al. found that certain agents are retained in the blood vessels or urinary tract with minimal perfusion into the extravascular tissue and that after glomerular filtration, they are concentrated at the proximal tubule, forming a high intensity band [5]. The absence or delayed formation of this high intensity band correlated well with renal tubular function disturbances associated with acute tubular necrosis (ATN) in a mouse model and the enhancement pattern was predictive of the pathogenesis of acute and/or chronic renal failure (e.g. heavy metal exposure, ischemia, obstruction, and sepsis) [17, 18]. Of the dendrimer-based MR contrast agents, it was shown that a G2 DAB agent was the most effective for evaluation of renal function [5] (Fig. 3).
Fig. (3).
MR images of several different organ systems obtained using the dendrimer-based contrast agent optimal for visualization of the given organ.
Research currently focuses on development of novel synthetic chemistry to improve efficiency and yields of dendrimer-based MR agents. Standard conjugates of bifunctional chelates to amino-terminated dendrimers use the isothiocyanate form of 1B4M-DTPA in an aqueous phase reaction. Such methods derived from preparation of monoclonal antibody conjugates with bifunctional chelates and involve lengthy procedure times. In addition, in order to counteract the competing decomposition and disproportion reactions of the reactive isothiocyanate group, a large excess of bifunctional chelator must be used. Xu et al. recently introduced a new synthetic approach for preparation of two G4 PAMAM MR agents, wherein tert-butyl-protected forms of 2-(4-isothicyantobenzyl)-6-methyldiethylenetiramine pentaacetic acid (1B4M-DTPA), bearing either an isothiocyanate or a succinimidyl ester moiety, respectively, were conjugated to the primary amine groups of the dendrimer outer shell [16]. This new technique yielded similar relaxivity values and had comparable MR imaging properties to the G4 agents prepared by the typical aqueous chemistry route, however had much higher yields and was obtained with greater efficiency [16].
Another research direction to improve dendrimer-based MRI contrast agents is to make the agent biodegradable [19, 20]. Lu ZR et al. introduced disulfide groups close to the gadolinium chelate in the interior of the dendrimer to catabolize and release the chelated gadolinium ions as small molecules through renal excretion which may be a clever method of avoiding heavy metal toxicity associated with free gadolinium ions.
CT
Iodinated contrast media has evolved over the past century to its current state where it is used routinely in patients undergoing contrast enhanced computed tomography (CT) scans [13]. However, the current approved CT contrast agents belong to the class of LMW compounds, similar to the case of approved MR agents. Such LMW agents quickly equilibrate between intravascular and extracellular fluid and are rapidly excreted from the body [11]. Thus, macromolecular contrast agents, which possess different in vivo properties such as prolonged intravascular half-life and initial volumes of distribution that approximate plasma are better suited for quantitative characterization of tissues, blood vessels, and pathologic alterations associated with cancer, ischemic injury, and angiogenesis [21–24].
The ideal macromolecular CT contrast agent would have an intravascular distribution, relatively high iodine concentrations or other radiodense atoms, high stability, near or complete monodispersity, low osmolality, high water solubility, limited viscosity, positive biocompatibility and complete elimination from the body [6]. With this in mind, a PEG core dendrimer-based iodinated contrast agent was developed. PEG possesses several advantageous attributes for such applications. In aqueous solution, PEG physically repulses other surrounding macromolecules, thus improving solubility. In addition, attachment of PEG to biomacromolecules increases blood half-life while dramatically reducing immunogenicity. Due to its extremely narrow distribution it is possible to synthesize nearly monodisperse PEG conjugates.
However, PEG possesses only two termini available for conjugation, therefore a method for amplification of termini was needed to enable it to be used as a dendrimer core. Fu et al. achieved this using dendritic polylysines as terminal amplifiers conjugated to a PEG core via carbamate linkages [13]. Triiodophthalamide moieties were conjugated to the free amino terminal of the paired, symmetrical dendritic polylysines extending from the PEG macro core to create an iodinated contrast agent compatible with CT imaging [13]. This agent is currently intended for intravascular use and represents a new development in dendrimer-based contrast agents with potential applications for quantitative microvascular characterization of disease states such ischemic injury, inflammation, and cancer. In addition, modification of this technology with targeting moieties will increase its applications in molecular imaging [13].
In general, X-ray CT is much less sensitive than MRI so higher concentrations of iodine are needed, which makes the chemical synthesis of macromolecular contrast agents challenging. As seen with Gadolinium-dendrimers, prolonged retention of iodinated dendrimer agents may prove problematic. The main side-effects of iodinated agents are anaphlactoid reactions, and damage to the kidneys causing acute renal failure. For this reason iodinated agents are contra-indicated in patients with existing renal insufficiency, or those patients at significant risk for renal disease (e.g. patients with diabetes mellitus, multiple myeloma); patients on dialysis are exempt, as dialysis can be used to remove the agent. However, due to the increased size of macromolecular agents, dialysis may not be as successful as it is for the removal of LMW contrast agents, possibly leading to additional risk for in vivo use.
DUAL-MODALITY AGENTS
By taking advantage of the numerous attachment sites available on a single dendrimer molecule, new synthetic chemistry techniques have led to the development of dual-modality magnetic resonance and fluorescence imaging agents for molecular imaging. By reducing a unique disulfide bond in the core of the Gd(III)-1B4M-DTPA chelated to the G2 PAMAM dendrimer and then attaching a maleimide-functionalized biotin, a novel approach has emerged for the preparation of biotinylated dendrimer-based MRI agent [6]. To create dual-use functionality, the agent is optimized for optical imaging by immobilizing four copies of the agent to fluorescently labeled avidin, thus, creating a supramolecular avidin-biotin-dendrimer-Gd(III) complex. This system has been shown to effectively target ovarian tumors, delivering chelated Gd(III) and fluorophores such as Rhodamine green to permit dual modality MR and fluorescence molecular imaging of disseminated peritoneal ovarian cancer [6].
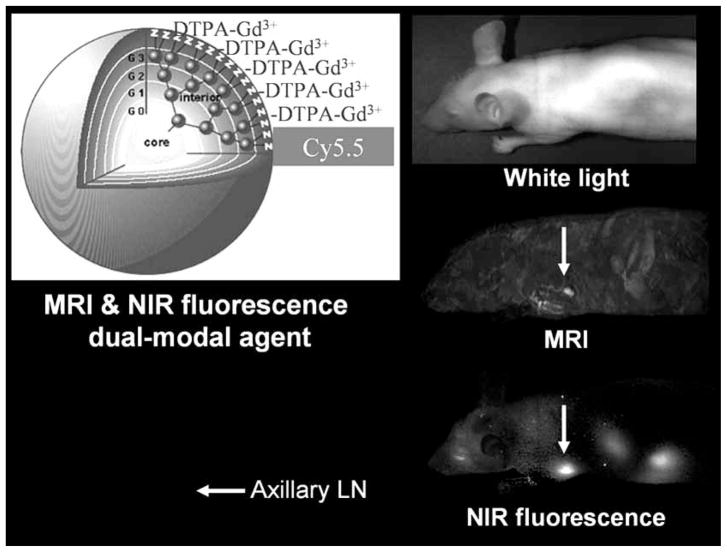
Recently another dual MR and optical fluorescence imaging agent was introduced for the localization of sentinel lymph nodes (SLNs) in mice [25]. Using a G6 PAMAM dendrimer, labeled with Cy5.5 near infrared (NIR) fluorophore coupled with a Gd, a macromolecular MR/NIR optical contrast agent was synthesized [25]. By injecting the agent into the mammary glands of healthy mice and examining the lymphatic drainage from the breast, investigators were able to identify SLNs using both MR and optical imaging, although MR permitted better and more specific resolution near the injection site [25] (Fig. 4).
Fig. (4).
MRI and near infrared fluorescence imaging obtained with a dendrimer-based dual-modal contrast agent.
Another promising application of dendrimer chemistry is the development of combined molecular imaging/therapeutic agents. Recently, Pan et al. developed a PAMAM dendrimer with a modified magnetic nanoparticle (MNP) conjugated with antisense oligonucleotides (asODN). It was shown that asODN-dendrimer-MNP composites effectively entered various human cancer cell lines, causing considerable down-regulation of the targeted gene product, and inhibiting cell growth [26]. In addition, as MNPs are widely used MR imaging contrast agents, there is the potential dual application of this agent as a combined gene therapy-diagnostic molecular imaging agent [26].
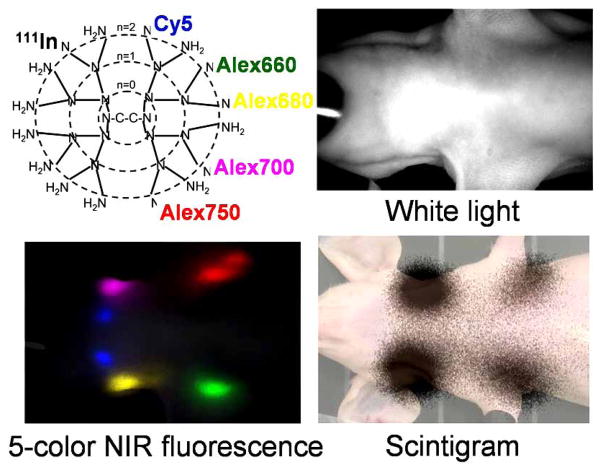
Another dual modality, dendrimer-based imaging agent is a radioisotope- and 5-color NIR fluorophore-labeled G6-PAMAM dendrimer which has been synthesized and used for lymphatic imaging [27]. The similar sensitivity of radionuclide and fluorescence imaging allowed the injected dose of the agent to be decreased to 1/100 compared with the comparable MRI and optical agent for visualizing the lymphatic system. The radionuclide-optical combination also better duplicates the agents in use clinically for sentinel node imaging (Fig. 5).
Fig. (5).
Scintigraphy and 5-color near infrared fluorescence imaging obtained with a series of dendrimer-based dual-modal multiple color contrast agents.
Multi-modality contrast agents have clear benefits. The ability of dendrimers to provide an excellent platform for the incorporation of multiple imaging beacons on each carrier molecule, confers a great advantage for their use as multimodality imaging agents.
TOXICITY
Physical properties of dendrimer-based contrast agents not only affect imaging applications but also influence agent-related toxicity. For example, the prolonged blood pool retention times of dendrimer-based MR agents have limited their clinical use secondary to the theorized increased time for release of toxic Gd(III) ions from unstable Gd(III) chelation [3]. Until this is definitively demonstrated not to be a problem, drug developers may be reluctant to develop MR-based dendrimer compounds. The clinical application of imaging agents, therefore, must take into consideration both imaging properties and toxicity profiles.
In a comparison of the pharmacokinetics of small-dendrimer based macromolecular MR contrast agents by Kobayashi et al., diaminobutane (DAB) dendrimer-based agents cleared more rapidly from the body than polyamidoamine (PAMAM) dendrimer-based agents with the same number of branches. In addition, smaller dendrimer conjugates were more rapidly secreted than larger ones. PAMAM-G2, DAB-G3, and DAB-G2 dendrimer-based contrast agents showed the fastest clearance. Therefore, it has been proposed that these agents are most suitable for clinical use due to a theoretical lower risk of toxicity secondary to prolonged retention compared to similar agents [3].
CONCLUSION
Given their ability to deliver an imaging payload with target specificity, dendrimer-based agents are desirable components of molecular imaging contrast media. The versatility of in vivo behavior achieved by nanoscale changes in dendrimer size highlights the ability of dendrimer-based contrast agents to be customized for application-specific needs literally by modifying the dendrimer on an atom-by-atom scale. In addition, continued work with dendrimer libraries will further elucidate the relationships between size and chemical properties, and in vivo behavior.
Improvement of efficiency in dendrimer-based contrast agent synthesis will increase their availability for clinical applications. Towards this, it is crucial that the field continue to develop novel synthetic preparation techniques specific to dendrimer-based contrast agent chemistry rather than rely on modification of pre-existing techniques for other types of contrast agents.
Perhaps the most exciting new horizon is the increasing number of dual modality agents. The development of agents with multi-modality imaging properties permits generation of synergistic data using the best imaging characteristics from each single modality. Finally, the ability of dendrimers to not only image, but also deliver therapeutic payloads represents a clear direction for future research and perhaps clinical benefit.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Tomalia DA, Reyna LA, Svenson S. Dendrimers as multipurpose nanodevices for oncology drug delivery and diagnostic imaging. Biochem Soc Trans. 2007;35:61–67. doi: 10.1042/BST0350061. [DOI] [PubMed] [Google Scholar]
- 2.Svenson S, Tomalia DA. Dendrimers in biomedical applications--reflections on the field. Adv Drug Deliv Rev. 2005;57:2106–2129. doi: 10.1016/j.addr.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 3.Kobayashi H, Kawamoto S, Jo SK, Bryant HL, Jr, Brechbiel MW, Star RA. Macromolecular MRI contrast agents with small dendrimers: pharmacokinetic differences between sizes cores. Bioconjug Chem. 2003;14:388–394. doi: 10.1021/bc025633c. [DOI] [PubMed] [Google Scholar]
- 4.Malik N, Wiwattanapatapee R, Klopsch R, Lorenz K, Frey H, Weener JW, Meijer EW, Paulus W, Duncan R. Dendrimers: relationship between structure and biocompatibilityin vitro and preliminary studies on the biodistribution of 125I-labelled polyamidoamine dendrimers in vivo. J Control Release. 2000;65:133–148. doi: 10.1016/s0168-3659(99)00246-1. [DOI] [PubMed] [Google Scholar]
- 5.Kobayashi H, Brechbiel MW. Nano-sized MRI contrast agents with dendrimer cores. Adv Drug Deliv Rev. 2005;57:2271–2286. doi: 10.1016/j.addr.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 6.Xu H, Regino CA, Koyama Y, Hama Y, Gunn AJ, Bernardo M, Kobayashi H, Choyke PL, Brechbiel MW. Preparation and preliminary evaluation of a biotin-targeted, lectin-targeted dendrimer-based probe for dual-modality magnetic resonance and fluorescence imaging. Bioconjug Chem. 2007;18:1474–1482. doi: 10.1021/bc0701085. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman JM, Gambhir SS. Molecular imaging: the vision and opportunity for radiology in the future. Radiology. 2007;244:39–47. doi: 10.1148/radiol.2441060773. [DOI] [PubMed] [Google Scholar]
- 8.Lauffer RB. Paramagnetic metal complex as water proton relaxation agents for NMR imaging: Theory and design. Chem Rev. 1987;87:901–927. [Google Scholar]
- 9.Caravan P, Ellison JJ, McMurry TJ, Lauffer RB. Gadolinium (III) chelates as MRI contrast agents: Structure, dynamics, and applications. Chem Rev. 1999;99:2293–2352. doi: 10.1021/cr980440x. [DOI] [PubMed] [Google Scholar]
- 10.Comblin VGD, Hermann M, Humblet V, Jacques V, Mesbashi M, Sauvage C, Desreux JF. Designing new MRI contrast agents: A coordination chemistry challenge. Coord Chem Rev. 1999;186:451–470. [Google Scholar]
- 11.Raymond KN, Pierre VC. Next generation, high relaxivity gadolinium MRI agents. Bioconjug Chem. 2005;16:3–8. doi: 10.1021/bc049817y. [DOI] [PubMed] [Google Scholar]
- 12.Thomsen HS, Marckmann P, Logager VB. Nephrogenic systemic fibrosis (NSF): A late adverse reaction to some of the gadolinium based contrast agents. Cancer Imaging. 2007;7:130–137. doi: 10.1102/1470-7330.2007.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fu Y, Nitecki DE, Maltby D, Simon GH, Berejnoi K, Raatschen HJ, Yeh BM, Shames DM, Brasch RC. Dendritic iodinated contrast agents with PEG-cores for CT imaging: Synthesis and preliminary characterization. Bioconjug Chem. 2006;17:1043–1056. doi: 10.1021/bc060019c. [DOI] [PubMed] [Google Scholar]
- 14.Bryant LH, Jr, Brechbiel MW, Wu C, Bulte JW, Herynek V, Frank JA. Synthesis relaxometry of high-generation (G = 5, 7, 9 and 10) PAMAM dendrimer-DOTA-gadolinium chelates. J Magn Reson Imaging. 1999;9:348–352. doi: 10.1002/(sici)1522-2586(199902)9:2<348::aid-jmri30>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 15.Kobayashi H, Brechbiel MW. Dendrimer-based nanosized MRI contrast agents. Curr Pharm Biotechnol. 2004;5:539–549. doi: 10.2174/1389201043376571. [DOI] [PubMed] [Google Scholar]
- 16.Xu H, Regino CA, Bernardo M, Koyama Y, Kobayashi H, Choyke PL, Brechbiel MW. Toward improved syntheses of dendrimer-based magnetic resonance imaging contrast agents: new bifunctional diethylenetriaminepentaacetic acid ligands and nonaqueous conjugation chemistry. J Med Chem. 2007;50:3185–3193. doi: 10.1021/jm061324m. [DOI] [PubMed] [Google Scholar]
- 17.Dear JW, Kobayashi H, Jo SK, Holly MK, Hu X, Yuen PS, Brechbiel MW, Star RA. Dendrimer-enhanced MRI as a diagnostic and prognostic biomarker of sepsis-induced acute renal failure in aged mice. Kidney Int. 2005;67:2159–2167. doi: 10.1111/j.1523-1755.2005.00321.x. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi H, Kawamoto S, Brechbiel MW, Jo SK, Hu X, Yang T, Diwan BA, Waldmann TA, Schnermann J, Choyke PL, Star RA. Micro-MRI methods to detect renal cysts in mice. Kidney Int. 2004;65:1511–1516. doi: 10.1111/j.1523-1755.2004.00532.x. [DOI] [PubMed] [Google Scholar]
- 19.Lu ZR, Mohs AM, Zong Y, Feng Y. Polydisulfide Gd(III) chelates as biodegradable macromolecular magnetic resonance imaging contrast agents. Int J Nanomed. 2006;1:31–40. doi: 10.2147/nano.2006.1.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu R, Wang Y, Wang X, Jeong EK, Parker DL, Lu ZR. In vivo evaluation of a PAMAM-cystamine-(Gd-DO3A) conjugate as a biodegradable macromolecular MRI contrast agent. Exp Biol Med (Maywood) 2007;232:1081–1089. doi: 10.3181/0702-RM-33. [DOI] [PubMed] [Google Scholar]
- 21.Su MY, Najafi AA, Nalcioglu O. Regional comparison of tumor vascularity and permeability parameters measured by albumin-Gd-DTPA and Gd-DTPA. Magn Reson Med. 1995;34:402–411. doi: 10.1002/mrm.1910340318. [DOI] [PubMed] [Google Scholar]
- 22.Brasch RC. Rationale and applications for macromolecular Gd-based contrast agents. Magn Reson Med. 1991;22:282–287. doi: 10.1002/mrm.1910220225. discussion 300–283. [DOI] [PubMed] [Google Scholar]
- 23.Daldrup-Link HE, Brasch RC. Macromolecular contrast agents for MR mammography: Current status. Eur Radiol. 2003;13:354–365. doi: 10.1007/s00330-002-1719-1. [DOI] [PubMed] [Google Scholar]
- 24.Schwitter J, Saeed M, Wendland MF, Derugin N, Canet E, Brasch RC, Higgins CB. Influence of severity of myocardial injury on distribution of macromolecules: Extravascular versus intravascular gadolinium-based magnetic resonance contrast agents. J Am Coll Cardiol. 1997;30:1086–1094. doi: 10.1016/s0735-1097(97)00245-3. [DOI] [PubMed] [Google Scholar]
- 25.Koyama Y, Talanov VS, Bernardo M, Hama Y, Regino CA, Brechbiel MW, Choyke PL, Kobayashi H. A dendrimer-based nanosized contrast agent dual-labeled for magnetic resonance and optical fluorescence imaging to localize the sentinel lymph node in mice. J Magn Reson Imaging. 2007;25:866–871. doi: 10.1002/jmri.20852. [DOI] [PubMed] [Google Scholar]
- 26.Pan B, Cui D, Sheng Y, Ozkan C, Gao F, He R, Li Q, Xu P, Huang T. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res. 2007;67:8156–8163. doi: 10.1158/0008-5472.CAN-06-4762. [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi H, Koyama Y, Barrett T, Hama Y, Regino CAS, Shin SI, Jang BS, Le N, Palk CH, Choyke PL, Urano Y. Multomodal nanoprobes for radionuclide and five-color near-infrared optical lymphatic imaging. ACS Nano. 2007;1:258–264. doi: 10.1021/nn700062z. [DOI] [PMC free article] [PubMed] [Google Scholar]