Abstract
Chronic stress is a risk factor for depression, and chronic stress can induce cognitive impairments associated with prefrontal cortical dysfunction, which are also major components of depression. We have previously shown that 5-weeks of chronic intermittent cold (CIC) stress induced a reversal learning deficit in rats, associated with reduced serotonergic transmission in the orbitofrontal cortex (OFC), that was restored by chronic treatment with a selective serotonin reuptake inhibitor (SSRI). However, the mechanisms underlying the beneficial cognitive effects of chronic SSRI treatment are currently unknown. Thus, the purpose of the present study was to investigate the potential modulatory influence specifically of 5-HT2A-receptors in the OFC on reversal learning, and their potential contribution to the beneficial cognitive effects of chronic SSRI treatment. Bilateral microinjections of the selective 5-HT2A-receptor antagonist, MDL 100,907 into OFC (0.02–2.0 nmoles) had a dose-dependent detrimental effect on a reversal learning task, suggesting a facilitatory influence of 5-HT2A-receptors in the OFC. In the next experiment, rats were exposed to 5-weeks of CIC stress, which compromised reversal learning, and treated chronically with the SSRI, citalopram (20 mg/kg/day) during the final 3 weeks of chronic stress. Chronic CIT treatment improved reversal learning in the CIC-stressed rats, and bilateral microinjection of MDL 100,907 (0.20 nmoles, the optimal dose from the preceding experiment) into OFC once again had a detrimental effect on reversal learning, opposing the beneficial effect of citalopram. We conclude that 5-HT2A-receptors in the OFC facilitate reversal learning, and potentially contribute to the beneficial cognitive effects of chronic SSRI treatment.
Keywords: antidepressant, chronic stress, orbitofrontal cortex, reversal learning, serotonin-2A receptor subtype
Introduction
Chronic stress is a risk factor in depression (Anisman and Zacharko, 1982; Caspi et al., 2003; Kendler et al., 1999; Kessler, 1997). Moreover, altered regulation of serotonergic neurotransmission interacts with stress to increase risk for depression (Caspi et al., 2003). However, little is known about the mechanisms underlying this interaction, nor how long-term regulatory changes in serotonergic function induced by chronic stress might contribute to the symptoms of depression. Likewise, although selective serotonin reuptake inhibitors (SSRIs) are the most widely used class of antidepressant drugs, little is known about how long-term drug-induced alterations in serotonergic function might contribute to the beneficial effects of SSRIs on mood and cognition in depression.
The prefrontal cortex (PFC) is involved in executive functions that are disrupted in depression, including cognitive flexibility, the ability to modify previously learned associations and behavioral patterns in response to a changing environment (see Kehagia et al., 2010). Cognitive dysfunction, specifically deficits of cognitive flexibility, may contribute to the perseverative emotional biases that are important in the development and maintenance of depression (Beck, 1976; Coles and Heimberg, 2002; Mathews and Mackintosh, 1998). Depressed patients exhibit cognitive biases for emotionally salient material, particularly related to stressful life events (Beck, 1976), abnormal responses to performance feedback, a narrowing of attentional focus to depression-relevant thoughts, and difficulty shifting attentional set from one affective dimension to another, consistent with perseverative attention to themes of loss and worthlessness, and the persistent ruminations prevalent in depression (Austin et al., 2001; Fossati et al., 1999; Merriam et al., 1999; Murphy et al., 1999). In addition to cognitive impairments, there is evidence of decreased cortical volume and functional dysregulation of the PFC in depression, including regions of both hypo- and hyper-metabolic activity (Mayberg, 2003; Rogers et al., 2004; Sheline, 2003; see Price and Drevets, 2010).
In previous studies, we have used a behavioral assay for cognitive flexibility, the Attentional Set-shifting Test (AST)(Birrell and Brown, 2000), to address mechanisms by which monoaminergic neurotransmission in PFC of rats can modulate cognitive flexibility, and how chronic stress can induce deficits of cognitive flexibility that are sensitive to antidepressantdrug treatment (Bondi et al., 2010; Bondi et al., 2008; Lapiz et al., 2007; Lapiz-Bluhm and Morilak, 2010). We have shown that 2 weeks of chronic intermittent cold stress (CIC) produced a selective reversal learning deficit (Lapiz-Bluhm et al., 2009), that was subsequently reversed by 3 weeks of chronic SSRI treatment, administered while the stress continued (Lapiz-Bluhm and Morilak, 2010). Similarly selective reversal learning deficits have been observed following lesions or 5-HT depletion specifically in orbitofrontal cortex (OFC) (Boulougouris et al., 2007; Clarke et al., 2007; McAlonan and Brown, 2003), and the reversal learning deficit in rats exposed to CIC stress was accompanied by reduced 5-HT release in OFC during the AST (Lapiz-Bluhm et al., 2009).
Thus, 5-HT has been implicated in reversal learning and cognitive flexibility in the OFC, but the mechanisms by which these effects are mediated remain unknown. Systemic administration of a 5-HT2A-receptor antagonist increased perseverative errors in an operant 2-choice serial reversal task, whereas systemic blockade of 5-HT2C-receptors had the opposite effect, improving performance (Boulougouris et al., 2008), as did direct administration of a 5-HT2C-receptor antagonist into OFC (Boulougouris and Robbins, 2010). Thus, 5-HT2C-receptors may compromise, and 5-HT2A-receptors facilitate reversal learning, but it is unknown if either of these has a role in the beneficial effects of SSRI treatment.
We showed recently that the beneficial effects of selective norepinephrine reuptake blockade on cognitive set-shifting, another form of cognitive flexibility, are mediated by α1-adrenergic receptors in the medial PFC (Bondi et al., 2010; Lapiz and Morilak, 2006). 5-HT2A-receptors and α1-receptors have similar distributions in PFC, and exert similar effects on the electrical activity of PFC pyramidal cells (Marek and Aghajanian, 1999). Thus, in the present study, we hypothesized that 5-HT2A-receptors in the OFC may likewise facilitate reversal learning, and contribute to the beneficial effects of chronic SSRI treatment in restoring cognitive flexibility compromised by prior exposure to CIC stress. First, bilateral microinjections of the selective 5-HT2A-receptor antagonist, MDL 100,907, were made directly into OFC prior to a reversal learning task. Then, the role of 5-HT2A-receptors in the beneficial effect of chronic SSRI treatment was investigated by microinjecting MDL 100,907 into the OFC of rats whose reversal learning capability had been compromised by prior exposure to CIC stress and restored by chronic SSRI treatment. Portions of this work have been presented in abstract form (Furr et al., 2010).
Methods
Animals
A total of 158 adult male Sprague Dawley rats were used in these experiments. Rats weighed 220–240 g upon arrival, and were individually housed under a 12-hr light cycle (lights on from 0700 to 1900 hr) and given food and water ad libitum. Experiments were conducted during the light phase of the cycle. All procedures were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio, and were consistent with NIH guidelines for the care and use of laboratory animals. All efforts were made to minimize pain, distress, and the number of animals used.
Chronic intermittent cold (CIC) stress
CIC stress was applied as described previously (Lapiz-Bluhm and Morilak, 2010). During the light phase of the cycle, rats in the CIC stress condition were weighed and transported in their home cages, with food, water, and bedding, into a cold room maintained at 4 °C for 6 hr, then returned to the housing room. This was repeated daily for 5 weeks. Control rats remained in the housing room for a comparable time, and were handled regularly when they were weighed.
Attentional set-shifting test (AST)
An abbreviated AST was conducted according to published procedures (Lapiz-Bluhm and Morilak, 2010), but only through completion of the first reversal task (R1), which was shown previously to be compromised selectively by both serotonin depletion and CIC stress (Lapiz-Bluhm et al., 2009). Seven days prior to testing, rats were placed on a restricted diet of 14 g of food per day, with water freely available. The testing apparatus was a custom-built rectangular white wooden arena (45 × 71 × 22 cm) with a removable white divider separating one-third of the length of the arena, forming a start box as well as a holding area following each trial. To begin each trial, the rat was placed in the start box and given access to the rest of the arena by lifting the divider. A white Plexiglas panel divided the far third of the arena into two sections. During testing, a terracotta pot (internal rim diameter 7 cm; depth 6 cm) was placed in each section. Each pot was defined by a pair of cues along two stimulus dimensions; the texture of the digging medium with which it was filled, and a distinct odor applied to the inner rim. One-fourth of a Honey Nut Cheerio (General Mills Cereals, Minneapolis, MN) was buried 2 cm below the surface of the digging medium in the “positive” pot. In all discrimination trials, a small quantity of powdered Cheerio was sprinkled onto the medium in both pots to ensure that the rat learned the discrimination and was not making choices by smelling the reward. Digging was defined as vigorous displacement of the medium to retrieve the reward buried in the pot. Simply investigating the rim of the pot or the surface of the digging medium with paws or snout without displacing material was not scored as a ‘dig’. Therefore, the rats were able to access visual, tactile and olfactory cues associated with the pots to make their choices. The behavioral procedure was conducted over 3 days:
Day 1: Habituation
Two unscented pots were placed in the home cage and re-baited every 5 min, with the cheerio covered with increasing amounts of sawdust (3 trials with no sawdust, 3 with the pots one-third full, 3 with the pots half-full and 3 completely full). Once the rat had successfully retrieved the reward from the pots each time, it was transferred to the testing arena and given three consecutive trials to retrieve the reward from both sawdust-filled pots.
Day 2: Training
On the following day rats were trained to complete two simple discriminations, with 6 consecutive correct responses required to reach criterion in each. In the first discrimination, both pots were filled with the same medium (sawdust) and scented with different odors (lemon vs. rosewood), with only one odor associated with reward. After reaching criterion, two new unscented pots were introduced, each filled with a different medium (shredded paper vs. felt strips). All rats were trained using the same stimulus exemplars in the same order. The positive and negative cues for each rat were randomly determined and equally represented. These training stimuli were not used again during testing.
Day 3: Testing
On the following day, the rats were tested on a series of discriminations. To proceed to the next stage, they had to reach a criterion of 6 consecutive correct trials. The first task was a simple discrimination, similar to the training trials, involving only one stimulus dimension. Half the rats were required to discriminate between two odors, only one of which was associated with reward, with both pots filled with sawdust. For the other half, this first discrimination involved the digging media, and both pots were unscented (for clarity, the remainder of this description will refer only to the example with odor discrimination). The second stage was then a compound discrimination, in which the same discrimination was required (e.g., odor), but the second, irrelevant stimulus was introduced. Only one odor was associated with reward, and the two different digging media were paired randomly with the odors over successive trials. Once the rats reached criterion, the last stage (R1) was then a reversal of the previous compound discrimination, in which the same odors and media were used, and odor was still the relevant dimension, but the negative odor from the previous stage was now positive (i.e., associated with the reward), and the positive odor from the previous stage was now negative. The same was true for rats tested with medium as the discriminative dimension. The dependent measure on this test was Trials to Criterion (TTC), the number of trials required to reach the criterion of 6 consecutive correct responses on the R1 reversal task.
Drugs
MDL 100,907 ((R)-(+)- α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenyl)ethyl]-4-piperidinemethanol), is a selective 5-HT2A-receptor antagonist (Axon Medchem, Groningen, The Netherlands). Due to the low solubility of MDL 100,907 in aqueous solution, it was dissolved with sonication in a vehicle comprised of 20% 2-hydroxypropyl-β-cyclodextrin (HBC) in filtered saline For chronic delivery by osmotic minipump, the SSRI, citalopram HBr (CIT; Shanco International, Hazlet, NJ), was dissolved in a vehicle of 10% ethanol in filtered saline to a final concentration of approximately 150 mg/ml, calculated to deliver 20 mg/kg/day free-base.
Experiment 1: Test the suitability of 20% HBC as a vehicle for microinjections into OFC
Because MDL 100,907 had to be dissolved in 20% HBC, it was first necessary to conduct a control experiment to determine if microinjections of the HBC vehicle into the OFC would have any effect on reversal learning in the R1 task compared to microinjections of saline vehicle, which we have demonstrated previously to have no effect (Lapiz-Bluhm and Morilak, 2010).
Thirteen rats were given one week to acclimatize to the animal facility after arrival. They were anesthetized (ketamine 43mg/ml, acepromazine 1.4 mg/ml, xylazine 8.6mg/ml given in 1.0 ml/kg, i.m.) and placed in a stereotaxic apparatus for bilateral implantation of microinjection guide cannulae (22 ga stainless steel, 11 mm length), aimed so that their tips were located 1 mm above the OFC (coordinates from bregma: AP: +2.9 mm, ML: ±2.6 mm, DV: −4.2 mm, corresponding to plate 9 in (Paxinos and Watson, 1998)). After surgery, rats were given antibiotic (Penicillin G, 300 000 IU/ml/kg), and individually housed for 14 days. Beginning seven days prior to testing, food was restricted to 14 g/day, with water freely available. Rats then underwent habituation, training and behavioral testing on the AST over 3 days, as described above. On both the habituation and training days, they were also acclimated to handling and removal of the obdurators. On the test day, all rats were taken through the simple and compound discrimination tasks. After completion of the compound discrimination, the obdurators were removed and replaced with microinjectors (30 ga stainless steel tubing) connected by a fluid-filled line to a Hamilton syringe mounted on a syringe pump (Instech Labs, Plymouth Meeting, PA). The injectors extended 1 mm beyond the tips of the guide cannulae, placing them in the OFC. The rats were then given acute bilateral microinjections into the OFC (0.50 μl/side) of either saline vehicle or 20% HBC vehicle, delivered over 2 min at a rate of 0.25 μl/min (n=6–7/group). The microinjectors were left in place for an additional 2 min to allow for diffusion before withdrawing. After the completion of the microinjections, the rats were returned to their home cage for a 5-min recovery period before testing resumed with the R1 reversal task.
Experiment 2: Effects of the selective 5-HT2A-receptor antagonist, MDL 100,907 microinjected into the OFC on reversal learning
Having confirmed that the HBC vehicle alone had no effect on reversal learning when microinjected into OFC (see Results), 68 rats were used in experiment 2 to test the effects of direct microinjections of MDL 100,907 (n=7–12/group) into the OFC. The rats were given at least one week to acclimatize to the housing facility after arrival. They were then stereotaxically implanted with bilateral guide cannulae aimed at the OFC as above. Antibiotic was administered, and rats were individually housed for 14 days following surgery. Seven days prior to testing, food was restricted to 14 g/day. The rats were habituated, trained and tested over three days, as described above. After the habituation and training procedures, rats were briefly acclimatized on each day to handling, removal and replacement of the obdurators. On the test day, rats were taken through the simple and compound discrimination stages. After completion of the compound discrimination, bilateral microinjections were made into the OFC of either 20% HBC vehicle (0.50 μl/side) or MDL 100,907 (0.02, 0.10, 0.20, 1.00, or 2.00 nmoles/0.50 μl/side) at a rate of 0.25 μl/min over a 2-min period. The microinjectors were left in place for an additional 2 min to allow for diffusion before withdrawal. Rats were returned to their home cages for 5 min post-injection before testing resumed with the R1 reversal stage of the AST.
To test for anatomical specificity, two groups of rats (n=8/group) subsequently received bilateral microinjections of vehicle or an effective dose of MDL 100,907 (0.20 nmoles) using the same approach, but 2.2 mm dorsal to the OFC target, in the Fr1 region of frontal cortex.
Experiment 3: Role of 5-HT2A-receptors in the OFC in the beneficial effects of chronic SSRI treatment on reversal learning that has been compromised by chronic stress
Seventy-five rats were randomly assigned to 8 groups defined by the chronic stress treatment (control or CIC), chronic drug treatment (vehicle or CIT) and the drug microinjected acutely into OFC prior to R1 reversal testing (HBC-vehicle or MDL 100,907). Rats underwent 14 days of control or CIC stress treatment. On day 15, the rats were stereotaxically implanted with bilateral microinjection guide cannulae aimed at the OFC. An osmotic minipump was also implanted intraperitoneally (Alzet model 2ML4, Cupertino, CA), pre-filled with either vehicle (10% ethanol in saline) or CIT at a concentration calculated to deliver 20 mg/kg/day of the free base. This dose was determined in pilot studies to achieve steady-state plasma drug levels within the target therapeutic range (unpublished data). After antibiotic administration and 3 days of post-surgical recovery, CIC stress treatment or control conditions were resumed for an additional 3 weeks. As above, food was restricted to 14 g/day seven days prior to test day. Testing occurred 3 days after the termination of CIC or control treatments.
On the test day, all rats were taken through the simple and compound discriminations, after which they received acute bilateral microinjections into the OFC of either HBC vehicle (0.50 μl/side) or MDL 100,907 (0.20 nmoles/0.50 μl/side), given at a rate of 0.25 μl/min over a 2-min period, as described above. The microinjectors remained in place for an additional 2 min to allow for drug diffusion before withdrawal. Rats were returned to their home cages for 5 min post-injection before testing resumed with the R1 reversal stage of the AST.
Statistical Analyses
In all experiments, investigators conducting the behavioral test were blind to the experimental treatment condition of the rat being tested. Mean trials to criterion on the simple discrimination task on the training day were first compared by ANOVA, to ensure that acquisition and general performance capability were comparable between experimental groups. A two-way MANOVA was also performed on the tasks preceding the R1 reversal stage on the testing day, to ensure that the groups were comparable prior to the acute microinjections.
In all experiments, the dependent measure of interest was the number of trials required to reach criterion (TTC) on the R1 reversal stage. In experiment 1, the effect of microinjecting 20% HBC vehicle compared to saline vehicle prior to the R1 task was assessed using a t-test. For experiment 2, performance on the R1 reversal task following acute microinjections of different doses of MDL 100,907 were analyzed by one-way ANOVA. In experiment 3, results were first analyzed by a three-way ANOVA assessing the effects of CIC stress, chronic CIT treatment and acute microinjections of MDL 100,907. Given a significant main effect and drug interaction involving CIC stress, these data were subsequently analyzed post hoc by 2-way ANOVA for both the unstressed and CIC-stressed animals, to assess more specifically the differences in drug effects in these two groups. Subsequent pairwise comparisons were made using the Newman-Keuls test. Significance was determined at p<0.05 in all analyses.
After testing, rats were euthanized and brains were collected for histological analysis. Rats with one or both cannulae located outside the OFC target were excluded from analysis, as were any rats in which a microinjection failed due to cannulae blockage, or rats that failed to complete testing through the reversal stage for any reason. This resulted in elimination of 9 rats who did not complete training, 5 who did not complete testing, 3 due to misplacement of one or both cannulae, and 3 due to cannulae blockage. These rats were not included in the total number reported as being used in this study.
Results
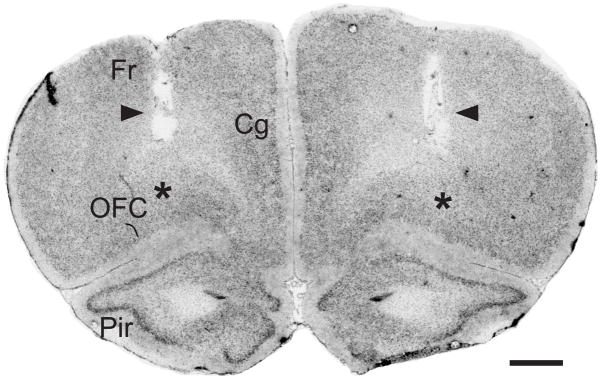
Figure 1 shows a representative example of the histological localization of bilateral guide cannulae tracks and microinjection sites in the OFC.
Figure 1.
Representative photomicrograph of a Cresyl Violet-stained coronal section, corresponding to plate 7 in the atlas of Paxinos and Watson (1998), showing the tracks of the guide cannulae implanted bilaterally above the OFC (arrowheads). Microinjection sites were 1 mm beyond the tip of the cannulae, in the OFC (asterisks). Abbreviations: Cg, cingulate cortex; Fr, frontal cortex; OFC, orbital frontal cortex; Pir, piriform cortex. Scale bar = 1 mm.
Experiment 1: Suitability of 20% HBC as a vehicle for microinjections into OFC
Performance on the reversal task was comparable after 20% HBC and saline vehicle microinjections into OFC. There were no significant differences on the number of trials to reach criterion (t=0.297, p=0.77, n=6–7/group), indicating that 20% HBC is a suitable vehicle for microinjections into the OFC prior to testing on the reversal learning task.
Experiment 2: Effects of 5-HT2A-receptor blockade in OFC on reversal learning
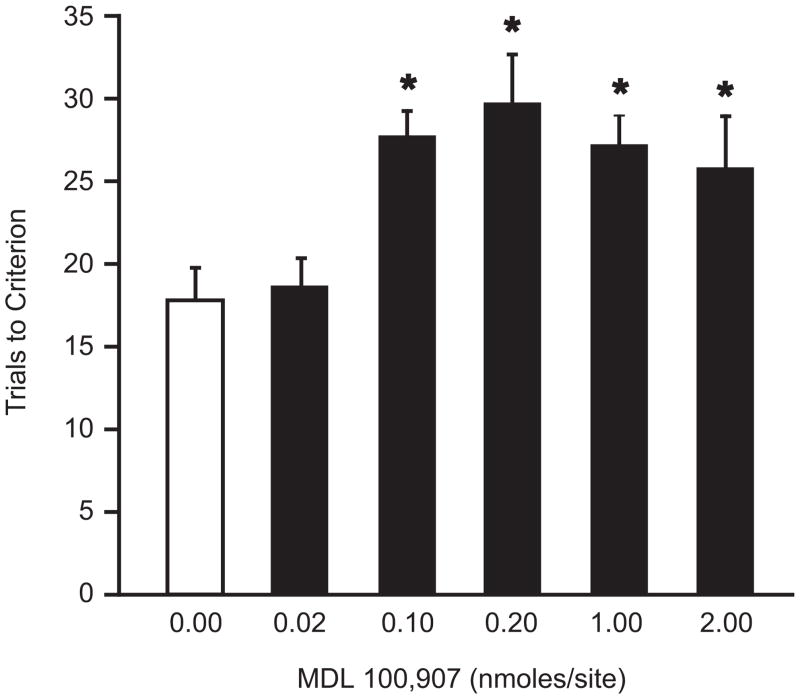
Analysis of trials to criterion on the two test stages prior to drug microinjections revealed no pre-test differences between the groups (F5,46=1.449, p>0.20, data not shown). Bilateral microinjections of MDL 100,907 had a significant, dose-related detrimental effect on reversal learning compared to HBC-vehicle control injections (F5,46=5.086, p<0.001, n=7–12/group; see Figure 2). Post-hoc comparisons revealed a significant increase in trials to criterion in rats that received MDL 100,907 at doses from 0.10–2.00 nmoles compared to HBC-vehicle controls (p<0.05). Based on this result, 0.20 nmoles was selected as the optimal dose of MDL 100,907 to be used in Experiment 3. This same dose was also used to test for anatomical specificity of the observed effect, and to control for potential diffusion of drug up the cannula tracks. Bilateral microinjection of MDL 100,907 (0.20 nmoles/side) into a site 2.2 mm dorsal to the OFC target had no effect on reversal learning compared to vehicle (t14 = 0.531, p= 0.60).
Figure 2.
Acute bilateral microinjections of the selective 5-HT2A-receptor antagonist, MDL 100,907, into the OFC immediately prior to testing on the reversal stage of the attentional set-shifting test impaired reversal learning compared to vehicle microinjections in control rats. Significant reversal deficits were observed in rats given MDL 100,907 at 0.10, 0.20, 1.00 and 2.00 nmoles/0.50 μl/side (*p<0.05 compared to the vehicle-injected control group by Newman-Keuls post hoc comparisons; mean ± S.E.M., n=7–12/group).
Experiment 3: Role of 5-HT2A-receptors in the OFC in the beneficial effects of chronic SSRI treatment on reversal learning that has been compromised by CIC stress
Control and cold-stressed rats learned the simple discrimination during the training session comparably, indicating no pre-existing difference between treatment groups in the ability to learn the contingency and perform the required tasks, and there were no group differences in performance on the two test stages prior to the R1 reversal task (all p>0.05, data not shown).
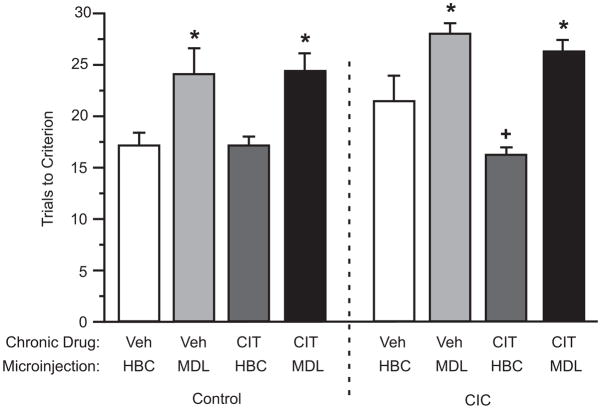
Figure 3 shows the effect of acute bilateral microinjections of 0.20 nmoles of MDL 100,907 into the OFC on the beneficial effects of chronic CIT treatment in CIC-stressed rats. Three-way ANOVA indicated a significant main effect for CIC stress (F1,67 = 6.71, p<0.01), replicating the detrimental effect on reversal learning reported previously (Lapiz-Bluhm et al., 2009). There were also significant main effects of chronic CIT treatment (F1,67 = 4.12, p<0.05) and MDL 100,907 microinjection (F1,67 = 34.69, p<0.001), and a significant CIC stress x CIT interaction (F1,67 = 4.69, p<0.05). Post hoc comparisons indicated that CIC stress significantly increased trials to criterion in vehicle treated animals, and that CIT treatment significantly reduced trials to criterion in CIC-stressed animals, also in replication of our previous results (Lapiz-Bluhm and Morilak, 2010). There was no interaction involving MDL 100,907, suggesting that it exerted similar effects in all treatment conditions. This was confirmed by subsequent post hoc two-way ANOVAs, indicating that chronic CIT treatment had no significant effect in control rats, but exerted a beneficial effect in CIC-stressed rats (F1,31 = 17.16, p<0.001), and that a significant main effect of MDL 100,907 was observed in both control (F1,36 = 13.52, p<0.001) and CIC-stressed rats (F1,31 = 32.38, p<0.001). A significant CIT x MDL interaction existed only in the CIC-stressed group (F1,31 = 7.57, p<0.01), with post hoc comparison by the Newman Keuls test confirming that MDL 100,907 exerted a detrimental effect on reversal learning in all treatment conditions, and reversed the cognitive improvement seen in CIC-stressed rats treated chronically with CIT (Figure 3).
Figure 3.
Effects of acute bilateral microinjections of MDL 100,907 into the OFC on reversal learning in CIC-stressed rats treated chronically with citalopram (CIT). CIC stress compromised reversal learning (main effect, p < 0.01), and chronic CIT treatment improved reversal learning in CIC-stressed rats but not in controls (+p<0.01, effect of CIT compared to the corresponding vehicle-treated group). The effect of CIT was reversed by microinjections of MDL 100,907 into OFC, which increased trials to criterion on the reversal task in all treatment conditions (*p<0.05, compared to corresponding HBC-vehicle control groups) (mean ± S.E.M., n=7–11/group).
Discussion
This study addressed the potential role of 5-HT2A-receptors in modulation of reversal learning in OFC, and in the beneficial cognitive effects of chronic treatment with the antidepressant SSRI, citalopram. Reversal learning is a form of cognitive flexibility that enables behavioral adaptation to changing internal states and environmental circumstances (see Rygula et al., 2010). The neural substrate of reversal learning has been shown to include the orbitofrontal cortex in rodents (Boulougouris et al., 2007; Clarke et al., 2007; McAlonan and Brown, 2003), as well as in humans (Cools et al., 2002; Fellows and Farah, 2003; Hampshire and Owen, 2006). Further, serotonin has been implicated in the modulation of reversal learning in the OFC (Clarke et al., 2007; Lapiz-Bluhm et al., 2009). We have shown previously that systemic treatment with the SSRI, citalopram, reversed the selective impairment in reversal learning induced in rats following CIC stress treatment, and this improvement was associated with an increase in 5-HT release in the OFC (Lapiz-Bluhm and Morilak, 2010). However, the mechanism by which 5-HT might exert its beneficial effects on reversal learning in the OFC is not known.
The primary observation in the present study is that local administration of the selective 5-HT2A-receptor antagonist, MDL 100,907 into the OFC prior to testing on the R1 task of the AST attenuated reversal learning. This is consistent with a previous study in which systemic administration of MDL 100,907 compromised serial reversal learning on a spatial 2-choice operant task (Boulougouris et al., 2008). In contrast with the present results, however, local infusion of MDL 100,907 into OFC by the same group had no effect on reversal learning, whereas the 5-HT2C receptor antagonist, SB 242084, enhanced reversal learning similarly after either systemic or local administration into OFC (Boulougouris et al., 2008; Boulougouris and Robbins, 2010). One possible explanation for the discrepant findings after local microinjections of MDL 100,907 into OFC may be in the nature of the learning involved in the two experiments. Lesion studies have demonstrated that the OFC is essential to reversal learning on the AST (McAlonan and Brown, 2003), which involves associating reward with a specific cue in one of two different sensory modalities (e.g., odor vs. texture), then switching contingencies between the cues within one of those modalities. By contrast, the 2-choice operant task used by Boulougouris et al. is a spatial learning task, wherein the lever associated with reward is defined by its spatial location, and reversal involves switching the spatial location associated with reward, rather than switching the valence of distinct sensory cues. This difference may render the OFC less essential in the spatial task, as other regions involved in both reversal learning and spatial learning (e.g., hippocampus or striatum) may maintain reversal learning capability in the face of compromised OFC function, and may also be sites at which facilitation by 5-HT2A receptors can be maintained despite blockade of these receptors in the OFC.
Secondly, we examined the potential contribution of 5-HT2A-receptors in OFC to the beneficial cognitive effects of chronic treatment with the SSRI antidepressant drug, citalopram. Replicating our previous result (Lapiz-Bluhm and Morilak, 2010), chronic treatment with citalopram improved reversal learning that had been impaired by prior exposure to CIC stress, but had no effect on cognitive capability in unstressed controls. This is consistent with the literature suggesting that beneficial effects of antidepressant drug treatment are evident only in affected subjects, both in human clinical studies (e.g., Gelfin et al., 1998), and in animal studies comparing antidepressant responses of controls to subjects that have been compromised prior to treatment, for instance by genetic predisposition or chronic stress, using both behavioral and molecular-cellular measures (e.g., see Balu et al., 2009; David et al., 2009; El Khoury et al., 2006; Murray et al., 2008; Piras et al., 2010; Willner, 1997). In the present study, bilateral microinjections of MDL 100,907 into the OFC reversed the citalopram-induced improvement in reversal learning after 5 weeks of CIC stress treatment, indicating that the beneficial effects of citalopram may be due, at least in part, to activation of 5-HT2A-receptors in OFC. However, a definitive conclusion regarding the degree to which the beneficial effects of SSRI treatment may require 5-HT2A-receptor activation cannot be determined from these data, because the local blockade of 5-HT2A-receptors in OFC had a detrimental effect on reversal learning in control as well as chronically stressed rats, treated either with citalopram or vehicle. Thus, it is possible that the effects of MDL 100,907 may be additive with those of citalopram, rather than strictly antagonistic. Nonetheless, the fact that 5-HT2A-receptor activation remains capable of facilitating reversal learning in animals compromised by chronic stress suggests that, even if these receptors are not primarily responsible for the beneficial cognitive effects of SSRI treatment, it may be possible to enhance these effects by promoting or maintaining 5-HT2A-receptor activity in the OFC during chronic antidepressant treatment.
The detrimental effect on reversal learning resulting from blockade of 5-HT2A-receptors in the OFC is reminiscent of the facilitatory influence exerted by α1-adrenergic receptors in the medial PFC on cognitive set-shifting (Lapiz and Morilak, 2006), and the role of α1-adrenergic receptors in the beneficial cognitive effects of antidepressant drugs that block norepinephrine reuptake (Bondi et al., 2010). The cellular actions of 5-HT2A and α1-adrenergic receptors are mediated by similar signal transduction pathways, involving activation of phospholipase C by Gαq/11 (Hannon and Hoyer, 2008; Wu et al., 1992). Moreover, 5-HT2A and α1-adrenergic receptors both facilitate glutamate release in PFC, and both enhance the excitatory responses of pyramidal cells to glutamate (Marek and Aghajanian, 1999). It is unlikely that the effects of MDL 100,907 were attributable to non-selective blockade of α1-receptors, as MDL 100,907 has >100–500-fold greater affinity and potency at 5-HT2A receptors compared to α1-receptors (Kehne et al., 1996). In addition, we have shown that the selective reversal learning deficit induced by CIC stress is sensitive to serotonergic modulation and chronic treatment with an SSRI, but not with the selective NE reuptake blocker, desipramine (Lapiz-Bluhm and Morilak, 2010; Lapiz-Bluhm et al., 2009). Thus, similarities in the modulatory influence of 5-HT2A and α1-adrenergic receptors in PFC may underly similar but specific facilitatory roles in the beneficial cognitive effects of antidepressant drugs that block the reuptake of 5-HT and norepinephrine, respectively.
Reversal learning is a complex process, requiring error detection, behavioral inhibition (i.e., suppression of the previously learned pre-eminent response), and overcoming the learned avoidance of a previously negative stimulus that is now positive (see Clarke et al., 2007). Depletion of serotonin in the OFC specifically impaired the inhibition of pre-eminent responses, resulting in perseverative behavior (Clarke et al., 2007). A subsequent study using a modified probabilistic reversal learning task showed that increasing serotonergic tone with SSRI treatment enhanced cognitive flexibility and decreased perseverative behavior by facilitating reward sensitivity and reducing negative feedback sensitivity, thus increasing the successful completion of reversal learning, whereas serotonin depletion had the opposite effect (Bari et al., 2010). This is perhaps consistent with the negative emotional biases and enhanced sensitivity to negative feedback seen in depressed patients (Beats et al., 1996; Murphy et al., 2003).
However, the role of serotonin, and specifically of 5-HT2A receptors in different aspects of cognitive flexibility is complex and sometimes contradictory. A lack of behavioral inhibition can also manifest as impulsivity, and serotonin depletion has been shown to increase impulsivity as measured by premature responding on the 5-choice serial reaction time test (Winstanley et al., 2004). However, by contrast with results indicating that blockade of 5-HT2A receptors with MDL 100,907 is detrimental to reversal learning (Boulougouris et al., 2008, and the present study), MDL 100,907 improved performance and reduced impulsivity on the 5-choice test (Winstanley et al., 2004). Interestingly, despite these disparate observations, the effects of 5-HT2C receptors were found in both cases to be opposite to those of 5-HT2A receptors.
Reduced 5-HT activity has been implicated in depression, and 5-HT is critical to the clinical efficacy of SSRI antidepressants (Delgado et al., 1999). However, elucidation of the involvement specifically of 5-HT2A-receptors either in depression or in the efficacy of antidepressant drug treatment has remained elusive and controversial (see Carr and Lucki, 2011; Celada et al., 2004). Consistent with a beneficial effect of 5-HT2A receptor antagonism on impulsivity, MDL 100,907 has been shown to exhibit antidepressant-like effects alone, and to enhance the effects of a low dose of fluoxetine on a test in which impulsivity is a component of the behavioral measure, namely, the differential reinforcement of a low rate 72-sec schedule of reinforcement (DRL 72-s, Marek et al., 2005). Such observations are further consistent with both clinical and preclinical studies suggesting that blockade of 5-HT2A-receptors contributes to or enhances antidepressant efficacy (e.g., see Pandey et al., 1995).
Effects of chronic stress on 5-HT2A receptor expression are similarly complex. Differential changes in 5-HT2A receptor expression have been reported in hippocampus, hypothalamus and frontal cortex following acute or repeated footshock associated with learned helplessness. Whereas reduced 5-HT2A receptor expression in hippocampus was associated with learned helplessness behavior after both acute and repeated shock, it was suggested that the elevated expression of 5-HT2A receptors in frontal cortex seen only after repeated shock may have been an adaptive response to reduced serotonergic transmission (Dwivedi et al., 2005). Chronic unpredictable stress also elevated cortical 5-HT2A receptor binding density in rats, and this was reversed by antidepressant treatment (Ossowska et al., 2002). By contrast, repeated restraint stress had no effect on cortical 5-HT2 receptor binding (Watanabe et al., 1993). Thus, in at least some contexts, chronic stress may up-regulate 5-HT2A-receptor expression, as in depression, and in both cases this may represent a compensatory response to altered serotonergic tone (e.g., see Shelton et al., 2009). In such contexts, the restoration of serotonergic activity at 5-HT2A-receptors in OFC by chronic SSRI treatment may improve cognitive flexibility. However, the apparently opposing effects of 5-HT2A-receptor blockade on cognitive flexibility on one hand, and on depressed mood and other indices of antidepressant efficacy on the other, may account for the disturbingly high incidence of partial antidepressant response, persistence of residual symptoms, loss of clinical efficacy over time, or even treatment resistance.
In sum, the primary observation in this study is that blockade of 5-HT2A-receptors in the OFC compromised reversal learning capability on the AST. Secondly, activation of this receptor subtype may contribute to the beneficial cognitive effects of chronic SSRI treatment in alleviating a selective deficit in reversal learning induced by exposing rats to chronic cold stress. These results add to an accumulating body of both clinical and preclinical evidence suggesting that deficits of cognitive flexibility may be central to the etiology and symptomatology of psychiatric disorders associated with chronic stress, in particular major depressive disorder. Such observations suggest that a more fine-grained assessment of the cognitive deficits exhibited by depressed patients might identify specific etiological substrates, and may eventually indicate more specific, or even individualized therapeutic approaches. More generally, elucidating the mechanisms by which antidepressant drugs can exert their therapeutic benefits may lead to the development of more effective or specific antidepressant strategies.
Acknowledgments
We thank Mr. Ankur Joshi and Ms. Kale Naegeli for technical assistance, and Ms. Ayomide Okanlawon for assistance with pilot experiments conducted during an undergraduate summer research internship. This work was supported by the National Institute of Mental Health Grants MH083669 and MH072672.
Footnotes
Statement of Interest
The authors have no conflicts of interest to report, nor any involvement to disclose, financial or otherwise, that may bias the conduct, interpretation or presentation of this work.
References
- Anisman H, Zacharko RM. Depression: The predisposing influence of stress. Behavioural Brain Science. 1982;5:89–137. [Google Scholar]
- Austin M-P, Mitchell P, Goodwin GM. Cognitive deficits in depression: Possible implications for functional neuropathology. British Journal of Psychiatry. 2001;178:200–206. doi: 10.1192/bjp.178.3.200. [DOI] [PubMed] [Google Scholar]
- Balu DT, Hodes GE, Anderson BT, Lucki I. Enhanced sensitivity of the MRL/MpJ mouse to the neuroplastic and behavioral effects of chronic antidepressant treatments. Neuropsychopharmacology. 2009;34:1764–1773. doi: 10.1038/npp.2008.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, et al. Serotonin modulates sensitivity to reward and negative feedback in a probabilistic reversal learning task in rats. Neuropsychopharmacology. 2010;35:1290–1301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychological Medicine. 1996;26:591–604. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- Beck AT. Cognitive therapy and the emotional disorders. New York: Int. Univ. Press; 1976. [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. Journal of Neuroscience. 2000;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Jett JD, Morilak DA. Beneficial effects of desipramine on cognitive function of chronically stressed rats are mediated by alpha1-adrenergic receptors in medial prefrontal cortex. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:913–923. doi: 10.1016/j.pnpbp.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi CO, Rodriguez G, Gould GG, Frazer A, et al. Chronic unpredictable stress induces a cognitive deficit and anxiety-like behavior in rats that is prevented by chronic antidepressant drug treatment. Neuropsychopharmacology. 2008;33:320–331. doi: 10.1038/sj.npp.1301410. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural Brain Research. 2007;179:219–228. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Glennon JC, Robbins TW. Dissociable effects of selective 5-HT2A and 5-HT2C receptor antagonists on serial spatial reversal learning in rats. Neuropsychopharmacology. 2008;33:2007–2019. doi: 10.1038/sj.npp.1301584. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Robbins TW. Enhancement of spatial reversal learning by 5-HT2C receptor antagonism is neuroanatomically specific. Journal of Neuroscience. 2010;30:930–938. doi: 10.1523/JNEUROSCI.4312-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr GV, Lucki I. The role of serotonin receptor subtypes in treating depression: A review of animal studies. Psychopharmacology. 2011;213:265–287. doi: 10.1007/s00213-010-2097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, et al. Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Celada P, Puig MV, Amargós-Bosch M, Adell A, et al. The therapeutic role of 5-HT1A and 5-HT2A receptors in depression. Journal of Psychiatry and Neuroscience. 2004;29:252–265. [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Walker SC, Dalley JW, Robbins TW, et al. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cerebral Cortex. 2007;17:18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- Coles ME, Heimberg RG. Memory biases in the anxiety disorders: Current status. Clinical Psychology Review. 2002;22:587–627. doi: 10.1016/s0272-7358(01)00113-1. [DOI] [PubMed] [Google Scholar]
- Cools R, Clark L, Owen AM, Robbins TW. Defining the neural mechanisms of probabilistic reversal learning using event-related functional magnetic resonance imaging. Journal of Neuroscience. 2002;22:4563–4567. doi: 10.1523/JNEUROSCI.22-11-04563.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David DJ, Samuels BA, Rainer Q, Wang J-W, et al. Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression. Neuron. 2009;62:479–493. doi: 10.1016/j.neuron.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado PL, Miller HL, Salomon RM, Licinio J, et al. Tryptophan-depletion challenge in depressed patients treated with desipramine or fluoxetine: Implications for the role of serotonin in the mechanism of antidepressant action. Biological Psychiatry. 1999;46:212–220. doi: 10.1016/s0006-3223(99)00014-1. [DOI] [PubMed] [Google Scholar]
- Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology. 2005;48:204–214. doi: 10.1016/j.neuropharm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- El Khoury A, Gruber SHM, Mørk A, Mathé AA. Adult life behavioral consequences of early maternal separation are alleviated by escitalopram treatment in a rat model of depression. Prog Neuro-Psychopharmacol Biological Psychiatry. 2006;30:535–540. doi: 10.1016/j.pnpbp.2005.11.011. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: Evidence from reversal learning paradigm. Brain. 2003;126:1830–1837. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Fossati P, Amar G, Raoux N, Ergis AM, et al. Executive functioning and verbal memory in young patients with unipolar depression and schizophrenia. Psychiatry Research. 1999;89:171–187. doi: 10.1016/s0165-1781(99)00110-9. [DOI] [PubMed] [Google Scholar]
- Furr A, Lapiz-Bluhm MD, Morilak DA. Society for Neuroscience Abstracts 36. 2010. Role of 5-HT2A receptors in orbitofrontal cortex in the beneficial effect of chronic SSRI treatment against a chronic cold stress-induced cognitive deficit. Online Program no., 697.10. [Google Scholar]
- Gelfin Y, Gorfine M, Lerer B. Effect of clinical doses of fluoxetine on psychological variables in healthy volunteers. American Journal of Psychiatry. 1998;155:290–292. doi: 10.1176/ajp.155.2.290. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Owen AM. Fractionating attentional control using event-related fMRI. Cerebral Cortex. 2006;16:1679–1689. doi: 10.1093/cercor/bhj116. [DOI] [PubMed] [Google Scholar]
- Hannon J, Hoyer D. Molecular biology of 5-HT receptors. Behavioural Brain Research. 2008;195:198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Current Opinions in Neurobiology. 2010;20:199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Baron BM, Carr AA, Chaney SF, et al. Preclinical characterization of the potential of the putative atypical antipsychotic MDL100,907 as a potent 5-HT2A antagonist with a favorable CNS safety profile. Journal of Pharmacology and Experimental Therapeutics. 1996;277:968–981. [PubMed] [Google Scholar]
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. American Journal of Psychiatry. 1999;156:837–841. doi: 10.1176/ajp.156.6.837. [DOI] [PubMed] [Google Scholar]
- Kessler RC. The effects of stressful life events on depression. Annual Review of Psychology. 1997;48:191–214. doi: 10.1146/annurev.psych.48.1.191. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Bondi CO, Morilak DA. Chronic treatment with desipramine improves cognitive performance of rats in an attentional set shifting test. Neuropsychopharmacology. 2007;32:1000–1010. doi: 10.1038/sj.npp.1301235. [DOI] [PubMed] [Google Scholar]
- Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137:1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Lapiz-Bluhm MDS, Morilak DA. A cognitive deficit induced in rats by chronic intermittent cold stress is reversed by chronic antidepressant treatment. International Journal of Neuropsychopharmacology. 2010;13:997–1009. doi: 10.1017/S1461145710000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapiz-Bluhm MDS, Soto-Piña AE, Hensler JG, Morilak DA. Chronic intermittent cold stress and serotonin depletion induce deficits of reversal learning in an attentional set-shifting test in rats. Psychopharmacology. 2009;202:329–341. doi: 10.1007/s00213-008-1224-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek GJ, Aghajanian GK. 5-HT2A receptor or α1-adrenoceptor activation induces excitatory postsynaptic currents in layer V pyramidal cells of the medial prefrontal cortex. European Journal of Pharmacology. 1999;367:197–206. doi: 10.1016/s0014-2999(98)00945-5. [DOI] [PubMed] [Google Scholar]
- Marek GJ, Martin-Ruiz R, Abo A, Artigas F. The selective 5-HT2A receptor antagonist M100907 enhances antidepressant-like behavioral effects of the SSRI fluoxetine. Neuropsychopharmacology. 2005;30:2205–2215. doi: 10.1038/sj.npp.1300762. [DOI] [PubMed] [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22:539–560. [Google Scholar]
- Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. British Medical Bulletin. 2003;65:193–207. doi: 10.1093/bmb/65.1.193. [DOI] [PubMed] [Google Scholar]
- McAlonan K, Brown VJ. Orbital prefrontal cortex mediates reversal learning and not attentional set shifting in the rat. Behavioural Brain Research. 2003;146:97–103. doi: 10.1016/j.bbr.2003.09.019. [DOI] [PubMed] [Google Scholar]
- Merriam EP, Thase ME, Haas GL, Keshavan MS, et al. Prefrontal cortical dysfunction in depression determined by Wisconsin Card Sorting Test performance. American Journal of Psychiatry. 1999;156:780–782. doi: 10.1176/ajp.156.5.780. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Michael A, Robbins TW, Sahakian BJ. Neuropsychological impairments in patients with major depressive disorder: The effects of feedback on task performance. Psychological Medicine. 2003;33:455–467. doi: 10.1017/s0033291702007018. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, et al. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Murray F, Smith DW, Hutson PH. Chronic low dose corticosterone exposure decreased hippocampal cell proliferation, volume and induced anxiety and depression like behaviours in mice. European Journal of Pharmacology. 2008;583:115–127. doi: 10.1016/j.ejphar.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Ossowska G, Nowak G, Klenk-Majewska B, Danilczuk Z, et al. Effect of imipramine on brain D-1 and 5-HT-2A receptors in a chronic unpredictable stress model in rats. Polish Journal of Pharmacology. 2002;54:89–93. [PubMed] [Google Scholar]
- Pandey SC, Davis JM, Pandey GN. Phosphoinositide system-linked serotonin receptor subtypes and their pharmacological properties and clinical correlates. Journal of Psychiatry and Neuroscience. 1995;20:215–225. [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- Piras G, Giorgi O, Corda MG. Effects of antidepressants on the performance in the forced swim test of two psychogenetically selected lines of rats that differ in coping strategies to aversive conditions. Psychopharmacology. 2010;211:403–414. doi: 10.1007/s00213-010-1904-x. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers MA, Kasai K, Koji M, Fukuda R, et al. Executive and prefrontal dysfunction in unipolar depression: A review of neuropsychological and imaging evidence. Neuroscience Research. 2004;50:1–11. doi: 10.1016/j.neures.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Rygula R, Walker S, Clarke H, Robbins TW, et al. Differential contributions of the primate ventrolateral prefrontal and orbitofrontal cortex to serial reversal learning. Journal of Neuroscience. 2010;30:14552–14559. doi: 10.1523/JNEUROSCI.2631-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI. Neuroimaging studies of mood disorder effects on the brain. Biological Psychiatry. 2003;54:338–352. doi: 10.1016/s0006-3223(03)00347-0. [DOI] [PubMed] [Google Scholar]
- Shelton RC, Sanders-Bush E, Manier DH, Lewis DA. Elevated 5-HT 2A receptors in psotmortem prefrontal cortex in major depression is associated with reduced activity of protein kinase A. Neuroscience. 2009;158:1406–1415. doi: 10.1016/j.neuroscience.2008.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Sakai RR, McEwen BS, Mendelson S. Stress and antidepressant effects on hippocampal and cortical 5-HT1A and 5-HT2 receptors and transport sites for serotonin. Brain Research. 1993;615:87–94. doi: 10.1016/0006-8993(93)91117-b. [DOI] [PubMed] [Google Scholar]
- Willner P. Validity, reliability and utility of the chronic mild stress model of depression: A 10-year review and evaluation. Psychopharmacology. 1997;134:319–329. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DEH, Dalley JW, Glennon JC, et al. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: Interactions with global 5-HT depletion. Psychopharmacology. 2004;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Wu D, Katz A, Lee C-H, Simon MI. Activation of phospholipase C by α1-adrenergic receptors is mediated by the α subunits of Gq family. Journal of Biological Chemistry. 1992;267:25798–25802. [PubMed] [Google Scholar]