Abstract
It is not known whether or not muscle spasm of the back muscles presented in patients with sciatic scoliosis caused by lumbar disc herniation produces muscle pain and/or tenderness. Pressure pain thresholds (PPTs) of the lower back and low-back pain were examined in 52 patients (13 of 52 presenting sciatic scoliosis) with lumbar disc herniation who complained of radicular pain and in 15 normal subjects. PPTs were measured at five points bilaterally using an electronic pressure algometer. Low-back pain was evaluated using visual analogue scale (VAS) ratings. All patients complained of radicular leg pain and were divided into the following three groups according to the presence of and the region of low-back pain: no low-back pain group, low-back pain with no laterality group, and low-back pain dominantly on the herniation side group; the VAS rating on the side ipsilateral to the herniation side was higher than that on the contralateral side. In the normal subjects, there were no statistically significant differences between sides in mean PPTs at all sites examined. PPTs were not lower in the spasmodic side (concave side) than the convex side in patients with sciatic scoliosis. PPTs on the herniation side were significantly lower than those on the contralateral side in patients with low-back pain dominantly on the herniation side. Furthermore, the areas of low PPTs were beyond the innervation area of dorsal ramus of L5 and S1 nerve root. It was considered that not only the peripheral mechanisms but also the hyper excitability of the central nervous system might contribute in lowering PPTs of the lower back on the herniation side.
Keywords: Pressure pain threshold, Muscle spasm, Low-back pain, Lumbar disc herniation, Sciatic scoliosis
Introduction
In patients with musculoskeletal disorders, increased muscle tonus around the painful site is sometimes noticed [26]. Concerning low-back pain patients, Roland pointed out in a review article that although the precise pathological diagnosis of low-back pain is difficult to make, muscle spasm of the back is not a rare clinical feature [34].
Spasm of the lower back muscle has been considered to elicit secondary low-back pain and tenderness of the lower back [34]. However, the lack of convincing evidence to support muscle hyperactivity, which is thought to cause pain, in various musculoskeletal disorders has been pointed out in recent reviews [26]. Therefore, the commonly accepted classical concept of a ‘muscle pain-spasm-muscle pain vicious cycle’, with pain and hyperactivity of the muscle reinforcing one another, has been open to question [26, 35]. Furthermore, it was reported that animal experiments of the low-back muscle pain model also did not produce hyper tonus of the back muscles [22]. Taken together, these findings put forth the question of whether or not secondary low-back pain is truly produced by spasm of the back muscles.
Regarding the neural mechanisms in the development of hypersensitivity of the tissues, such as muscle tenderness, attention has recently focused on central neural mechanisms in addition to peripheral mechanisms. A barrage of nociceptive input results in changes in the response properties of dorsal horn and brain stem neurons, such as wide-dynamic range neurons (WDR) and nociceptive-specific neurons [40, 42]. This neuroplasticity alters the normal processing of nociceptive and non-nociceptive information, and, consequently, pain thresholds may be lowered. Several clinical reports also suggest the involvement of central mechanisms in the lowering of pressure pain thresholds (PPTs) in musculoskeletal disorders [23]. In addition to the neural mechanism mentioned above, muscle exertion [31, 32], psychologic stress [28], ovarian cycle [10] and many other factors have been reported to influence PPTs.
However, the influence of the central mechanisms on pain threshold in low-back disorders has not been well understood. One of the reasons is that the etiology of low-back pain varies and the diagnosis is difficult. Furthermore, it is difficult to eliminate the influence of muscle spasm of the back muscle, which is sometimes presented in low-back disorders.
To elucidate the relationship between muscle spasm, pain and tenderness of the back muscles, we consider sciatic scoliosis due to lumbar disc herniation to be a good model. Since spasm of the low-back muscle is generally unilateral and usually occurs on the side contralateral to the herniation [27, 37], we could, therefore, separately observe the effect of low-back pain and muscle spasm on the pressure pain threshold of the lower back on the respective side.
In the present study, we examined firstly the side differences of PPTs of the lower back in the normal subjects. Secondly, in order to elucidate the influence of muscle spasm on PPTs, PPTs of each side of the lower back in patients with sciatic scoliosis caused by lumbar disc herniation were investigated. Finally, we studied the relationship between the low-back pain intensity and the PPTs of the lower back.
Materials and methods
A group of 52 patients (18 females, 34 males; mean age 41±14 years) and 15 control subjects (one female, 14 males; mean age 27±3 years) participated in the study. All patients were diagnosed as single-level unilateral lumbar disc herniation (L4/5; 25 and L5/S; 27) by MRI. The duration from the onset of sciatic pain to the examination ranged from 1 to 360 days, averaging 55±77 days. Sciatic scoliosis was defined as a Cobb angle of more than 5 degrees between L1 and L5 in anteroposterior lumbar radiographs in the standing position and a lack of a history of idiopathic scoliosis. From this definition, 12 patients (six females, six males; mean age 33±8 years) were diagnosed as sciatic scoliosis. As normal subjects, healthy volunteers with no history of low-back pain were recruited.
An electronic pressure algometer (Somedic, Farsta, Sweden) was used to measure PPTs. This algometer consisted of a gun-shaped handle with a pressure-sensitive strain gauge at the tip. The probe of the tip was covered with 1.0-cm2 neoplane rubber, to avoid adverse skin pain stimuli. The display showed pressure (kPa) and a scale indicating the rate of pressure force increase. The scale enabled the examiner to keep any desired rate of pressure increase. In this study a rate of 50 kPa/s was chosen. The subject indicated pain threshold by pressing a button which froze the current pressure value on the digital display.
All measurements were made by the same investigator (J.H.), with the subjects in a relaxed prone position. The subjects were fully informed that the investigation aimed at determining the individual pain threshold, not pain tolerance. The algometer was demonstrated and the subjects were instructed to push the button exactly at the moment when the pressure sensation turned into a pain sensation. PPT was measured at the following five points bilaterally: Med.1, 3 and 5; 2 cm lateral to the L1, 3 and 5 spinous process, and Lat. 1 and 3; 5 cm lateral to the L1 and 3 spinous process (Fig. 1). Prior to PPT measurement, all subjects had tried pressure stimulation of a thoracic level. In this study, patients who perceived pain radiating to the buttock or lower extremity when the pressure was applied to the measuring point were excluded.
Fig. 1.
PPT measurement sites are indicated on the left side of the lower back. Definition of low-back pain area is indicated by the shaded area on the right side of lower back
Low-back pain was evaluated on the right and left sides separately using a visual analogue scale (VAS) rating (0–100 mm) at the time when they felt the strongest pain regardless of their position. Low-back pain was defined as pain in the area between the L1 spinous process level and the iliac crest (Fig. 1).
In study 1, mean PPTs at each point were compared between the right and left sides in the normal subjects.
In study 2, mean PPTs at each point were compared between the concave side (considered as the muscle spasm side) and the convex side in patients with sciatic scoliosis.
In study 3, patients were divided into the following three groups: no low-back pain, low-back pain with no laterality, and low-back pain dominantly on the herniation side; the VAS rating on the side ipsilateral to the herniation side was higher than that on the contralateral side.
Mean PPTs at each point were compared between the ipsilateral side and the side contralateral to the lumbar disc herniation.
Study 4: in order to determine the relationship between the VAS rating and PPT, the difference between the herniation and contralateral side VAS ratings and PPTs were calculated at Med. 5. To obtain positive values in both variables, calculation for VAS ratings and PPT were performed in opposite ways (i.e., ipsilateral-contralateral for VAS and contralateral-ipsilateral for PPT).
Additionally, to examine whether sciatic pain (nociceptive information derived from the ventral ramus) affects the PPTs of the low back or not, the relationship between leg pain VAS ratings and PPTs differences (PPTcontralateral–PPTipsilateral) was calculated at Med. 5, in the group without low-back pain but suffering from sciatic pain.
For each point, mean PPTs between the two sides were analyzed by Wilcoxon signed-ranks test. Data are presented as mean±SD. In study 1, Kruskal–Wallis test and Mann–Whitney’s U-test was used to analyze differences among the measured points in the control group. In study 4, a linear regression analysis was used. A P-value of less than 0.05 was considered to be statistically significant.
Results
Study 1
In the normal subjects, at Lat.1 and Lat.3, there was a tendency for a low PPT on the left side, but it was not statistically significant. At Med.1, Med.3 and Med.5, there were no statistically significant side differences in mean PPTs (Table 1). Therefore, to compare among the five sites, the data of each site from the right and left sides were averaged.
Table 1.
Comparison of PPT at the right and left side in normal subjects
| Right (kPa) | Left (kPa) | P value | |
|---|---|---|---|
| Med. 1 | 441.3±123.0 | 438.1±130.0 | 0.7764 |
| Med. 3 | 418.5±158.4 | 424.5±135.1 | 0.6832 |
| Med. 5 | 401.3±162.1 | 395.5±170.7 | 0.925 |
| Lat. 1 | 362.1±136.7 | 331.8±135.4 | 0.0535 |
| Lat. 3 | 335.3±126.4 | 314.0±121.4 | 0.0691 |
The mean PPTs differed significantly among the five sites examined (P=0.0033)(Fig. 2). More lateral sites showed lower PPTs (Lat.1; 347.0±134.6 kPa and Lat.3; 324.6±122.3 kPa) compared with medial sites (Med.1; 439.7±124.4 kPa, Med.3; 421.5±421.5 kPa and Med.5; 398.4±163.6 kPa) (Fig. 2). Mean PPTs on the caudal most site tended to show lower PPTs (Med. 5; 398.4±163.6 kPa) than the cranial most site (Med.1; 439.7±124.4 kPa) but this was not statistically significant (Fig. 2).
Fig. 2.
Mean (±SD) PPT at five sites in control subjects. Mean PPT differed significantly among the five sites examined. Lateral sites showed lower PPT (Lat.1 and Lat.3) compared with medial sites (Med.1, Med.3 and Med.5). *P<0.05; **P<0.01
Study 2
Thirteen patients presented sciatic with scoliosis. Eleven of 13 patients had a lumbar disc herniation at the convex side of scoliosis. Low-back pain intensity on the concave side (mean VAS: 17.0±20.4 mm) was lower than the convex side (mean VAS: 47.0±28.2 mm). There were no statistical differences between the concave (muscle spasm) and convex sides at Med. 1, Lat. 3 and Lat. 5 (Table 2). The difference in intensity at Med. 3 and Med. 5 was statistically significant. However, the concave (muscle spasm) side showed a higher PPT than the convex side (Table 2). These results indicated that the muscle spasm side is not more sensitive to pressure.
Table 2.
Comparison of PPT at the convex side and concave side in sciatic scoliosis
| Convex (kPa) | Concave (kPa) | P value | |
|---|---|---|---|
| Med. 1 | 444.5±208.9 | 428.4±155.7 | 0.8139 |
| Med. 3 | 328.3±140.4 | 406.4±161.7 | 0.0499* |
| Med. 5 | 285.9±107.8 | 346.3±120.8 | 0.0186* |
| Lat. 1 | 331.6±121.9 | 357.3±152.6 | 0.2393 |
| Lat. 3 | 304.3±161.0 | 336.5±124.3 | 0.2094 |
*P<0.05
Study 3
In the group with low-back pain dominantly on the herniation side (n=30), the mean VAS rating was 57.2±23.1 mm on the herniation side and 12.8±20.2 mm on the contralateral side. The mean PPT on the herniation side was significantly lower than that on the contralateral side at all five sites (Table 3).
Table 3.
Comparison of PPT at the herniation side and contralateral side in the group with low-back pain dominantly on the herniation side
| Herniation (kPa) | Contra. (kPa) | P value | |
|---|---|---|---|
| Med. 1 | 451.4±214.8 | 489.5±200.3 | 0.0196* |
| Med. 3 | 348.5±179.6 | 442.4±194.7 | <0.0001* |
| Med. 5 | 267.7±105.1 | 387.2±153.6 | <0.0001* |
| Lat. 1 | 325.6±133.0 | 361.6±143.8 | 0.0532 |
| Lat. 3 | 297.1±157.2 | 336.7±127.3 | 0.0201* |
*P<0.05
The mean PPTs in the group without low-back pain (n=16) did not differ between the herniation side and the contralateral side (Table 4). In the group with low-back pain with no laterality (n=6), the mean VAS rating was 45.7±25.0 mm. The mean PPTs at all five sites did not differ between the herniation side and the contralateral side (Table 5).
Table 4.
Comparison of PPT at the herniation side and contralateral side in the group without low-back pain
| Herniation (kPa) | Contra. (kPa) | P value | |
|---|---|---|---|
| Med. 1 | 493.6±219.6 | 443.5±260.2 | 0.0787 |
| Med. 3 | 402.6±211.2 | 413.5±195.8 | 0.5521 |
| Med. 5 | 362.4±214.2 | 376.1±220.1 | 0.2343 |
| Lat. 1 | 352.6±145.0 | 355.6±153.9 | 0.8361 |
| Lat. 3 | 355.5±159.8 | 347.1±138.2 | 0.9176 |
Table 5.
Comparison of PPT at the herniation side and contralateral side in the low-back pain with no laterality group
| Herniation (kPa) | Contra. (kPa) | P value | |
|---|---|---|---|
| Med. 1 | 431.0±194.3 | 453.7±198.3 | 0.2945 |
| Med. 3 | 405.3±134.5 | 370.0±143.0 | 0.0747 |
| Med. 5 | 411.3±160.5 | 410.5±185.8 | 0.9165 |
| Lat. 1 | 381.2±177.3 | 387.7±155.9 | 0.9165 |
| Lat. 3 | 354.7±170.1 | 342.2±166.9 | 0.7532 |
Study 4
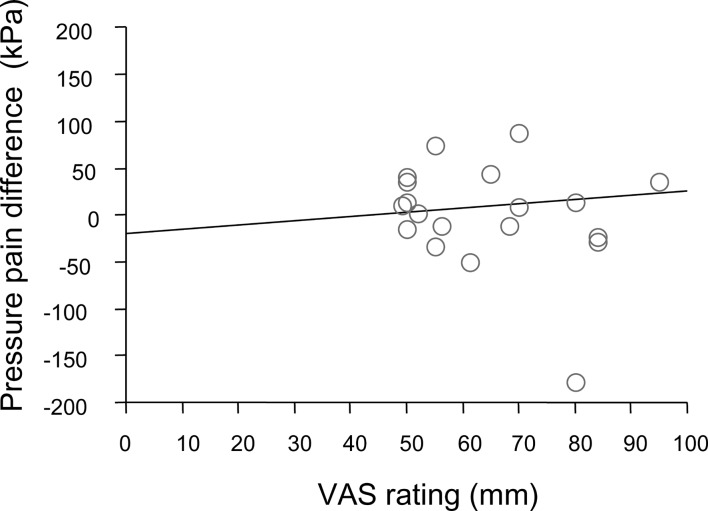
As shown in Fig. 3, a significant linear correlation was found between low-back pain VAS rating differences and PPT differences at Med. 5 (R=0.505, P=0.0001). There was no correlation between the leg pain VAS rating and PPT differences at Med. 5 (R=0.105, P=0.66; Fig. 4).
Fig. 3.
A significant linear correlation between low-back pain VAS rating differences (VAS ipsilateral-VAScontralateral) and PPT differences (PPTcontralateral-PPTipsilateral) at Med. 5 (correlation coefficient: R=0.55; P=0.2)
Fig. 4.
There was no correlation between the leg pain VAS rating and PPT differences (PPTcontralateral-PPTipsilateral) at Med. 5 in group without low-back pain (correlation coefficient: R=0.105; P=0.66)
Discussion
Manual palpation is the most widely used clinical method to assess muscle pain. However, this method is subjective and it is difficult to quantify or standardize. In contrast, the reliability and validity of PPTs have been reported in patients with a variety of musculoskeletal pain syndromes [8, 33], as well as in asymptomatic subjects [3, 9, 14, 18, 24].
According to the previous report, although the PPTs inter-observer reliability of short-term has been reported to be good [9, 18, 19], the second immediate consecutive measurements were slightly lower [18, 24], likely due to local irritation from the first session. Therefore, in the present study, PPTs were measured only once at each point.
It has been reported that there are differences in PPT between different tissues and anatomical sites. Considering the PPT differences between the right and left sides, Brennum et al. [6] reported that the PPT on the dominant side in fingers is higher than that on the non-dominant side. However, no difference has been reported between the right and left sides in PPT in the body trunk [2, 18, 24].
It was reported that muscle exertion [31, 32], psychologic stress [28], ovarian cycle [10] and many other factors influence PPTs. However, these factors may have a general influence on the bilateral body [31, 32].
In accordance with the previous report, here PPTs on the right and left sides of the lower back in the normal subjects showed no statistical differences. However, there were site differences; in particular, the more lateral sites (L1 and L3) of the lower back showed lower PPTs.
In the present study, PPTs of the back muscles of patients with sciatic scoliosis were examined to elucidate the influence of muscle spasm on the PPTs. Clinically, muscle spasm caused by painful disease is difficult to define, and further, the neural mechanisms are not well understood. Prolonged sustained contraction of the muscle is well known to be painful. Several authors have reported that hyperactivity of the muscles does not always exist in musculoskeletal pain conditions [26]. However, the demise of the hyperactivity–causality model does not mean that motor performance is unrelated in the presence of pain.
Lund et al. [26] suggested that the changes in motor performance in several chronic pain conditions are caused by protective adaptations explained by the action of pain on segmental interneurons. Johansson and Sojka [20] proposed that the increased muscle tone is due to the increase in stretch reflex activity caused by increased γ-motoneuron discharge. Hirayama et al. [17] reported that noxious stimulation increases prolonged stretch reflex activity caused by the central sensitization of the spinal neurons using sciatic scoliosis in an animal model. Although several neural mechanisms of muscle spasm have been reported, it remains to be proven whether or not muscle spasm causes muscle pain.
In the present study, it was revealed that the low-back pain intensity on the concave side (spasm side) was not higher than that on the convex side. Furthermore, PPTs on the concave side (spasm side) also were not lower than those on the convex side. These results can be interpreted as follows: (1) muscle spasm of the back muscles presented in sciatic scoliosis did not cause muscle pain, (2) muscle spasm produced muscle pain, but the influence of low-back pain produced by lumbar disc herniation masked the effect. In order to confirm this finding, further longitudinal study (i.e., between the periods during and after suffering from the symptoms) within the patient group and a comparative study using age, gender, etc. with matched controls will be necessary.
Regarding the low-back pain caused by lumbar disc herniation, little information is available in the literature about the nature and the area of the low-back pain [29, 36, 39], since it is less pronounced than sciatic pain and few studies have been undertaken. According to clinical and animal experiments, the possible sources of low-back pain caused by lumbar disc herniation are the dorsal ramus component of an injured nerve root [4], the lumbar disc itself [25, 36] and compressed dura [21, 25, 36]. Although it is probable that the dorsal ramus component of an injured nerve root may cause unilateral low-back pain, the lumbar disc and dura are innervated bilaterally [30], which may affect PPTs on both sides.
Therefore, here low-back pain was evaluated separately on the right and left sides. The group without low-back pain showed no side differences in the PPTs, and were considered to not be affected by any low-back pain source. The low-back pain with no laterality group also showed no side differences in PPTs.
In contrast, PPTs on the herniation side were significantly lower than those on the contralateral side in patients with low-back pain dominantly on the herniation side.
One possible explanation is hypersensitivity of the sensory neurons of an injured dorsal ramus caused by lumbar disc herniation. It has been reported that injured sensory neurons frequently become electrically hyperexcitable due to upregulation of voltage-dependent ion channels [1, 12, 13], which may partly contribute to the hyperalgesia in the animal model. The presence of a “tender point”, usually noticed around the sciatic nerve in patients with lumbar disc herniation, is well known [16, 38]. However, the area where decreased PPTs were found was not restricted only to the area innervated by the injured dorsal ramus of L5 or S1 nerve root. There are several deep structures, such as multifidus muscle and the facet joint, underlying the Med. 1, Med. 3 and Med. 5 sites. Multifidus muscle is known to be innervated unisegmentally [5, 7]. Recently, the multifidus muscle was revealed to be polysegmentally innervated, from EMG data obtained from neurotomy of the medial branches of the dorsal ramus; however, the medial branch of the dorsal ramus innervates several segmental levels caudally, but not rostrally [41]. Iliocostalis muscle underlies the sites at Lat.1 and Lat.3. However, it does not receive a nerve supply from the L5 spinal nerve, which has no lateral branch of the dorsal ramus. These facts indicate that the results that lowering of PPTs at Med. 1, Med. 3 and Lat.1 and Lat. 3 could not be simply explained by only peripheral mechanisms.
It has been reported that a barrage of nociceptive input results in changes to the response properties of dorsal horn neurons and amplifies other noxious or non-noxious inputs [40]. It was reported that experimentally induced muscle pain lowers pain threshold in an area distant to the pain induction in humans [15]. Furthermore, in animal studies, expansion of receptive fields of the deep structures, as well as receptive fields of the skin, has been found as a manifestation of central hyerexcitability [11, 42]. Such central processing may explain the lowering of PPTs detected beyond the innervation area of the L5 and S1 dorsal ramus in this study.
Conclusion
This study showed that there were no differences between sides in mean PPTs in the normal subjects. In patients with sciatic scoliosis, PPTs on the spasmodic side (concave side) were not lower than those on the convex side. PPTs on the herniation side were significantly lower than those on the contralateral side in patients with low-back pain dominantly on the herniation side. The site of lowed PPTs was beyond the innervation area of the L5 and S1 dorsal ramus, which indicates the involvement of central neuromechanisms.
References
- 1.Abe M, Kurihara T, Han W, Shinomiya K, Tanabe T. Changes in expression of voltage-dependent ion channel subunits in dorsal root ganglia of rats with radicular injury and pain. Discussion 1525. Spine. 2002;27:1517–1524. doi: 10.1097/00007632-200207150-00007. [DOI] [PubMed] [Google Scholar]
- 2.Antonaci F, Bovim G, Fasano ML, Bonamico L, Shen JM. Pain threshold in humans. A study with the pressure algometer. Funct Neurol. 1992;7:283–288. [PubMed] [Google Scholar]
- 3.Antonaci F, Sand T, Lucas GA. Pressure algometry in healthy subjects: inter-examiner variability. Scand J Rehabil Med. 1998;30:3–8. doi: 10.1080/003655098444255. [DOI] [PubMed] [Google Scholar]
- 4.Bogduk N. Lumbar dorsal ramus syndrome. Med J Aust. 1980;2:537–541. doi: 10.5694/j.1326-5377.1980.tb100759.x. [DOI] [PubMed] [Google Scholar]
- 5.Bogduk N, Wilson AS, Tynan W. The human lumbar dorsal rami. J Anat. 1982;134:383–397. [PMC free article] [PubMed] [Google Scholar]
- 6.Brennum J, Kjeldsen M, Jensen K, Jensen TS. Measurements of human pressure-pain thresholds on fingers and toes. Pain. 1989;38:211–217. doi: 10.1016/0304-3959(89)90240-6. [DOI] [PubMed] [Google Scholar]
- 7.Campbell WW, Vasconcelos O, Laine FJ. Focal atrophy of the multifidus muscle in lumbosacral radiculopathy. Muscle Nerve. 1998;21:1350–1353. doi: 10.1002/(SICI)1097-4598(199810)21:10<1350::AID-MUS21>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 8.Chung SC, Kim JH, Kim HS. Reliability and validity of the pressure pain thresholds (PPT) in the TMJ capsules by electronic algometer, Discussion 177. Cranio. 1993;11:171–176. doi: 10.1080/08869634.1993.11677961. [DOI] [PubMed] [Google Scholar]
- 9.Chung SC, Um BY, Kim HS. Evaluation of pressure pain threshold in head and neck muscles by electronic algometer: intrarater and interrater reliability. Cranio. 1992;10:28–34. doi: 10.1080/08869634.1992.11677888. [DOI] [PubMed] [Google Scholar]
- 10.Cimino R, Farella M, Michelotti A, Pugliese R, Martina R. Does the ovarian cycle influence the pressure-pain threshold of the masticatory muscles in symptom-free women. J Orofac Pain. 2000;14:105–111. [PubMed] [Google Scholar]
- 11.Cook AJ, Woolf CJ, Wall PD, McMahon SB. Dynamic receptive field plasticity in rat spinal cord dorsal horn following C-primary afferent input. Nature. 1987;325:151–153. doi: 10.1038/325151a0. [DOI] [PubMed] [Google Scholar]
- 12.Devor M. Neuropathic pain: what do we do with all these theories. Acta Anaesthesiol Scand. 2001;45:1121–1127. doi: 10.1034/j.1399-6576.2001.450912.x. [DOI] [PubMed] [Google Scholar]
- 13.Dib-Hajj SD, Fjell J, Cummins TR, Zheng Z, Fried K, LaMotte R, Black JA, Waxman SG. Plasticity of sodium channel expression in DRG neurons in the chronic constriction injury model of neuropathic pain. Pain. 1999;83:591–600. doi: 10.1016/S0304-3959(99)00169-4. [DOI] [PubMed] [Google Scholar]
- 14.Fischer AA. Pressure algometry over normal muscles. Standard values, validity and reproducibility of pressure threshold. Pain. 1987;30:115–126. doi: 10.1016/0304-3959(87)90089-3. [DOI] [PubMed] [Google Scholar]
- 15.Graven-Nielsen T, Arendt-Nielsen L, Svensson P, Jensen TS. Stimulus-response functions in areas with experimentally induced referred muscle pain—a psychophysical study. Brain Res. 1997;744:121–128. doi: 10.1016/S0006-8993(96)01077-3. [DOI] [PubMed] [Google Scholar]
- 16.Gunn CC, Milbrandt WE. Tenderness at motor points. A diagnostic and prognostic aid for low-back injury. J Bone Joint Surg Am. 1976;58:815–825. [PubMed] [Google Scholar]
- 17.Hirayama J, Takahashi Y, Nakajima Y, Takahashi K, Yamagata M, Moriya H. Effects of electrical stimulation of the sciatic nerve on background electromyography and static stretch reflex activity of the trunk muscles in rats: possible implications of neuronal mechanisms in the development of sciatic scoliosis. Spine. 2001;26:602–609. doi: 10.1097/00007632-200103150-00009. [DOI] [PubMed] [Google Scholar]
- 18.Hogeweg JA, Langereis MJ, Bernards AT, Faber JA, Helders PJ. Algometry. Measuring pain threshold, method and characteristics in healthy subjects. Scand J Rehabil Med. 1992;24:99–103. [PubMed] [Google Scholar]
- 19.Isselee H, De Laat A, Lesaffre E, Lysens R. Short-term reproducibility of pressure pain thresholds in masseter and temporalis muscles of symptom-free subjects. Eur J Oral Sci. 1997;105:583–587. doi: 10.1111/j.1600-0722.1997.tb00221.x. [DOI] [PubMed] [Google Scholar]
- 20.Johansson H, Sojka P. Pathophysiological mechanisms involved in genesis and spread of muscular tension in occupational muscle pain and in chronic musculoskeletal pain syndromes: a hypothesis. Med Hypotheses. 1991;35:196–203. doi: 10.1016/0306-9877(91)90233-O. [DOI] [PubMed] [Google Scholar]
- 21.Kallakuri S, Cavanaugh JM, Blagoev DC. An immunohistochemical study of innervation of lumbar spinal dura and longitudinal ligaments. Spine. 1998;23:403–411. doi: 10.1097/00007632-199802150-00001. [DOI] [PubMed] [Google Scholar]
- 22.Kang YM, Wheeler JD, Pickar JG. Stimulation of chemosensitive afferents from multifidus muscle does not sensitize multifidus muscle spindles to vertebral loads in the lumbar spine of the cat. Spine. 2001;26:1528–1536. doi: 10.1097/00007632-200107150-00005. [DOI] [PubMed] [Google Scholar]
- 23.Koelbaek Johansen M, Graven-Nielsen T, Schou Olesen A, Arendt-Nielsen L. Generalised muscular hyperalgesia in chronic whiplash syndrome. Pain. 1999;83:229–234. doi: 10.1016/S0304-3959(99)00106-2. [DOI] [PubMed] [Google Scholar]
- 24.Kosek E, Ekholm J, Nordemar R. A comparison of pressure pain thresholds in different tissues and body regions. Long-term reliability of pressure algometry in healthy volunteers. Scand J Rehabil Med. 1993;25:117–124. [PubMed] [Google Scholar]
- 25.Kuslich SD, Ulstrom CL, Michael CJ. The tissue origin of low back pain and sciatica: a report of pain response to tissue stimulation during operations on the lumbar spine using local anesthesia. Orthop Clin North Am. 1991;22:181–187. [PubMed] [Google Scholar]
- 26.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–694. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 27.Matsui H, Ohmori K, Kanamori M, Ishihara H, Tsuji H. Significance of sciatic scoliotic list in operated patients with lumbar disc herniation. Spine. 1998;23:338–342. doi: 10.1097/00007632-199802010-00010. [DOI] [PubMed] [Google Scholar]
- 28.Michelotti A, Farella M, Tedesco A, Cimino R, Martina R. Changes in pressure-pain thresholds of the jaw muscles during a natural stressful condition in a group of symptom-free subjects. J Orofac Pain. 2000;14:279–285. [PubMed] [Google Scholar]
- 29.Nakamura SI, Takahashi K, Takahashi Y, Yamagata M, Moriya H. The afferent pathways of discogenic low-back pain. Evaluation of L2 spinal nerve infiltration. J Bone Joint Surg Br. 1996;78:606–612. [PubMed] [Google Scholar]
- 30.Ohtori S, Takahashi Y, Takahashi K, Yamagata M, Chiba T, Tanaka K, Hirayama J, Moriya H. Sensory innervation of the dorsal portion of the lumbar intervertebral disc in rats. Spine. 1999;24:2295–2299. doi: 10.1097/00007632-199911150-00002. [DOI] [PubMed] [Google Scholar]
- 31.Persson AL, Hansson GA, Kalliomaki A, Moritz U, Sjolund BH. Pressure pain thresholds and electromyographically defined muscular fatigue induced by a muscular endurance test in normal women. Clin J Pain. 2000;16:155–163. doi: 10.1097/00002508-200006000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Persson AL, Hansson GA, Kalliomaki J, Sjolund BH. Increases in local pressure pain thresholds after muscle exertion in women with chronic shoulder pain. Arch Phys Med Rehabil. 2003;84:1515–1522. doi: 10.1016/S0003-9993(03)00273-9. [DOI] [PubMed] [Google Scholar]
- 33.Reid KI, Gracely RH, Dubner RA. The influence of time, facial side, and location on pain-pressure thresholds in chronic myogenous temporomandibular disorder. J Orofac Pain. 1994;8:258–265. [PubMed] [Google Scholar]
- 34.Roland M. A critical review of the evidence for pain-spasm-pain cycle in spinal disorders. Clin Biomech. 1986;1:102–109. doi: 10.1016/0268-0033(86)90085-9. [DOI] [PubMed] [Google Scholar]
- 35.Simons DG, Mense S. Understanding and measurement of muscle tone as related to clinical muscle pain. Pain. 1998;75:1–17. doi: 10.1016/S0304-3959(97)00102-4. [DOI] [PubMed] [Google Scholar]
- 36.Smyth MJ, Wright V. Sciatica and intervertebral disc; an experimental study. J Bone Joint Surg Am. 1958;40:1401–1418. [PubMed] [Google Scholar]
- 37.Suk KS, Lee HM, Moon SH, Kim NH. Lumbosacral scoliotic list by lumbar disc herniation. Spine. 2001;26:667–671. doi: 10.1097/00007632-200103150-00023. [DOI] [PubMed] [Google Scholar]
- 38.Takahashi Y, Hirayama J, Seiji O, Tauchi T, Kazuhisa T. Anatomical nature of radicular leg pain analyzed by clinical findings. Pain Res. 2000;15:87–96. [Google Scholar]
- 39.Vucetic Clin Orthop Rel Res. 1995;320:65. [PubMed] [Google Scholar]
- 40.Woolf CJ, Thompson SW, King AE. Prolonged primary afferent induced alterations in dorsal horn neurones, an intracellular analysis in vivo and in vitro. J Physiol (Paris) 1988;83:255–266. [PubMed] [Google Scholar]
- 41.Wu PB, Date ES, Kingery WS. The lumbar multifidus muscle in polysegmentally innervated. Electromyogr Clin Neurophysiol. 2000;40:483–485. [PubMed] [Google Scholar]
- 42.Yu XM, Sessle BJ, Hu JW. Differential effects of cutaneous and deep application of inflammatory irritant on mechanoreceptive field properties of trigeminal brain stem nociceptive neurons. J Neurophysiol. 1993;70:1704–1707. doi: 10.1152/jn.1993.70.4.1704. [DOI] [PubMed] [Google Scholar]