Abstract
Low back pain (LBP) is often accompanied by changes in gait, such as a decreased (preferred) walking velocity. Previous studies have shown that LBP diminishes the normal velocity-induced transverse counter-rotation between thorax and pelvis, and that it globally affects mean erector spinae (ES) activity. The exact nature and causation of these effects, however, are not well understood. The aim of the present study was to examine in detail the effect of walking velocity on global trunk coordination and ES activity as well as their variability to gain further insights into the effects of non-specific LBP on gait. The study included 19 individuals with non-specific LBP and 14 healthy controls. Gait kinematics and ES activity were recorded during treadmill walking at (1) a self-selected (comfortable) velocity, and (2) sequentially increased velocities from 1.4 up to maximally 7.0 km/h. Pain intensity, fear of movement and disability were measured before the experiment. The angular movements of thorax, lumbar and pelvis were recorded in three dimensions. ES activity was recorded with pairs of surface electrodes. Trunk–pelvis coordination and mean amplitude of ES activity were analyzed. In addition, invariant and variant properties of trunk kinematics and ES activity were studied using principal component analysis (PCA). Comfortable walking velocity was significantly lower in the LBP participants. In the transverse plane, the normal velocity-induced change in pelvis–thorax coordination from more in-phase to more antiphase was diminished in the LBP participants, while lumbar and pelvis rotations were more in-phase compared to the control group. In the frontal plane, intersegmental timing was more variable in the LBP than in the control participants, with additional irregular movements of the thorax. Rotational amplitudes were not significantly different between the LBP and control participants. In the LBP participants, the pattern of ES activity was affected in terms of increased (residual) variability, timing deficits, amplitude modifications and frequency changes. The gait of the LBP participants was characterized by a more rigid and less variable kinematic coordination in the transverse plane, and a less tight and more variable coordination in the frontal plane, accompanied by poorly coordinated activity of the lumbar ES. Pain intensity, fear of movement and disability were all unrelated to the observed changes in coordination, suggesting that the observed changes in trunk coordination and ES activity were a direct consequence of LBP per se. Clinically, the results imply that conservative therapy should consider gait training as well as exercises aimed at improving both intersegmental and muscle coordination.
Keywords: Chronic low back pain, Walking, Trunk coordination, Muscle coordination, Variability
Introduction
In individuals with low back pain (LBP), gait is often disordered. Although it appears to be a consistent finding that individuals with LBP walk more slowly than pain-free individuals [23, 24, 27, 47], it is at present not clear why LBP is accompanied by slower walking since the effects of LBP on gait are not well understood. It has been suggested that slower walking is a reflection of the presence of pain and/or fear-avoidance behavior associated with pain and may reflect an attempt to reduce pain by restricting movements of the spine [1, 57, 63]. Individuals with acute induced pain as well as those with chronic LBP show increased activity levels of the lumbar erector spinae (LES) during the swing phase of gait, when this muscle is normally hardly active [3]. These changes in muscle activity are often assumed to “guard” or “splint” the spine in individuals with LBP [3, 29, 59]. Nevertheless, individuals with LBP exhibit a normal range of movement during walking despite the presence of pain [27] and walking for about 10 min has been found to actually decrease the pain during acute LBP [50].
Apart from changes in mean muscle activity, LBP appears to be accompanied by various task-specific changes in muscular control which become manifest as altered patterns of muscle recruitment [16, 18, 20, 53]. In a previous study on the impact of induced acute pain in healthy individuals, we found that acute pain does not alter the trunk coordination during walking at different velocities [26]. However, acute pain affects muscular control in terms of increased (residual) variability, reflecting timing deficits and changes in the frequency content while leaving the global pattern of LES activity intact. In contrast, fear induced in healthy individuals has no effect at all on gait coordination.
These findings appear to contradict the assumption that individuals with LBP alter motor control by way of protective guarding or splinting [3, 32, 59]. Alternatively, one may hypothesize that individuals with LBP have difficulty in adequately controlling their movements, and hence in dealing with perturbations, and therefore adapt a slower walking velocity allowing more precise control. Under normal circumstances, walking is a highly flexible and adaptive activity that is continuously altered so as to meet both environmental and internal requirements. In normal walking, coordinated patterns of trunk and pelvis rotations and trunk muscle activity are important for the maintenance of dynamic equilibrium, to reduce the energy cost and to effectively deal with perturbations during locomotion [48, 52, 62, 64].
In unimpaired gait, transverse thorax–pelvis coordination evolves from more or less in-phase (synchronous pelvis and thorax rotation in the same direction) towards more antiphase coordination (synchronous counter-rotation) as walking velocity increases [25]. Previous studies on the effect of LBP on thorax–pelvis coordination during gait have shown that individuals with LBP are able to walk faster than their self-selected comfortable walking velocity but encounter problems in adjusting thorax–pelvis coordination with increasing walking velocity from more in-phase toward more antiphase coordination [27].
At present, it is not clear if changes in trunk coordination in chronic LBP are a direct effect of LBP as such or are mediated by pain, fear of movement or disability. Little is known about the relationship between changes in trunk coordination and changes in muscle activity during gait in LBP. The only study (as far as we know) that examined recruitment patterns of ES muscles during gait in LBP [59] showed an earlier onset of the LES in individuals with LBP, with identical profiles to those observed in the healthy control group. However, in this study, data were averaged over strides and subjects within each group yielding a mean picture of the EMG profiles for LBP and control participants. In this coarse-grained analysis, information about variability may have been lost.
Variability of movement patterns in LBP has received little attention, with only a few studies addressing the issue of variability [16, 20, 60], despite its potential as a window into motor control. Most studies on movement disorders inherently assume that motor variability (e.g., in kinematics, strides and muscle activity) is a purely random phenomenon, and therefore typically focus on mean values and ensemble averages, which may mask eventual structures in the variability of motor data. Within the field of motor control, however, it is by now well recognized that motor variability is not simply a reflection of random noise but often contains (hidden) features and regularities that may provide insight into both healthy and pathological motor control [8, 10, 11, 14, 17, 33, 43].
The aim of the present study was to examine in detail the consequences of LBP on trunk–pelvis coordination and ES muscle activity when walking at a self-selected velocity and at a wide range of prescribed velocities both lower and higher than the self-selected velocity. The analysis focused on coordination and variability of movement patterns, in addition to using more conventional measures such as amplitudes and stride length. Gaining more insight into how motor control is changed in LBP may be useful for diagnostic purposes and may help to quantify pathological movement patterns in assessing the efficacy of treatment strategies.
Materials and methods
Participants
Data were collected from 22 individuals with chronic non-specific LBP (13 women, 9 men) and 17 healthy individuals (8 women, 9 men). Due to storage failure, the EMG data of three of the LBP group (two women, one man) and three of the healthy group (all women) were lost, as a result of which the data had to be restricted to 33 individuals. LBP participants had a mean age of 38 years (range 21–52 years), a mean weight of 74.4 kg (range 49–97 kg), a mean height of 1.73 m (range 1.54–1.88 m), and the controls had a mean age of 31 years (range 20–46 years), a mean weight of 72.5 kg (range 52–105 kg) and a mean height of 1.80 m (range 1.58–1.98 m). Weight and height were not significantly different between the two groups. Age differed significantly between the two groups (t=2.0, P<0.02); however, no effect of age (or gender) was found on any of the dependent variables.
Individuals with LBP were recruited from cooperating exercise therapy practices. At the time of enrolment in the study, the treatment had not yet started. None of the individuals with low back pain received disability payment because of LBP, 14 participants with LBP had paid work, 3 were housewives and 2 were students. The average duration of the back pain was 1.2 years (range 3.5 months to 3 years).
The procedure was approved by the Ethics Committee of the Medical Centre of the Free University. All participants gave their written informed consent to participate in the study. The inclusion criteria for the LBP participants were: (1) medical diagnosis of non-specific LBP with pain and symptoms persisting for longer than 3 months for which medical treatment had been sought, (2) age between 18 and 65 years, and (3) ambulation without a walking aid. Participants were excluded if they had: (1) LBP of traumatic or structural origin, (2) LBP with neurological symptoms or pain radiation in the lower leg(s), (3) previous back surgery, (4) spinal tumors or infections, or (5) neurological and/or musculoskeletal disorders unrelated to LBP. All LBP participants underwent standard neurological and orthopedic physical examinations, including an assessment of range of movement of the trunk and lower extremities, of possible neurological symptoms and of Waddell’s non-organic signs [61]. An orthopedic surgeon performed the examination. Four LBP participants reported pain radiation in the buttock region and upper leg (three at the right side).
The healthy participants were recruited from employees of the university and of the Medical Centre of the Free University and had no history of LBP or any other musculoskeletal disorders.
Procedure
Prior to the experiment, the LBP participants completed the Tampa scale for kinesiophobia (TSK) to assess pain-related fear of movement [56] (scores <37 within normal limits), the Roland disability questionnaire (RDQ) [44] (0=no disabilities, 24=severe disabilities) and visual analogue scales (VAS) for actual pain intensity ratings and anticipated pain (0=no pain, 10=most severe pain). VAS for measuring actual pain intensity were also administered immediately after the experimental walking trial.
The experiment was performed on a treadmill. Before the recording began, participants walked for a few minutes on the treadmill at different velocities to become familiar with the treadmill and the experimental setup. Participants were instructed to walk as naturally as possible in the middle of the belt. First, recordings were performed at a self-selected (comfortable) walking velocity. Subsequently, treadmill velocity was increased sequentially with increments of 0.8 km/h from 1.4 km/h to a maximally attainable walking velocity of up to 7.0 km/h, allowing the participants to accustom themselves to each higher velocity level in a smooth and uniform manner. Participants indicated when the walking velocity was too high, at which point the experiment was stopped; the preceding velocity level was then designated as their “maximally attainable velocity”.
Recording
Angular rotations of the trunk segments were recorded using a 3D active marker movement registration system (Optotrak 3020, Northern Digital, Ontario, Canada). Clusters of three markers were fixed on a light plate mounted on rigid fixtures and attached to the trunk at the level of Th3, L2 (spinous processes), and the sacrum between posterior superior iliac spine using neoprene bands. The marker clusters defined the thoracic, lumbar and pelvic segments, respectively. The fixtures at the level of Th3 and L2 were designed to span the ES muscle and the spinous process. To be able to detect characteristic moments of the gait cycle, infrared light-emitting markers were placed on the heels and the fifth metatarsophalangeal joint.
In the area of electrode placement the skin was shaved and cleaned with alcohol. EMG activity from the ES muscle (mainly m. longissimus) left and right at the level of Th12, L2 and L4 process, was recorded with pairs of surface electrodes (Blue sensor N-00-S; Medicotest, Denmark; AG/AgCl discs, 1 cm diameter, 2 cm interelectrode distance). Electrodes were placed at a distance of 3 cm lateral from the vertebral column [55].
At each walking velocity, kinematic data were sampled at 100 Hz and EMG data at 1 kHz (Porti5; TMS-international, Enschede, The Netherlands), both for 30 s. The recordings of kinematic and EMG data were synchronized by means of a single trigger pulse starting both. All raw data were exported for analysis using Matlab 6.50 (Mathworks, Natic, Mass.). Before each condition a reference measurement was taken with the participant standing quietly upright (anatomical position).
Data processing
Kinematic data were analyzed in a global reference frame in the form of fixed xyz-Euclidian coordinates with the x-axis corresponding to the line of progression, the y-axis perpendicular to the x-axis and parallel to the ground and the z-axis pointing vertically upwards. The cluster markers represented the motion of the trunk segments. From each marker cluster a segment reference frame was defined and transformed to a new coordinate system oriented as in the global coordinate system. Based on the reference measurement, the segment axes were aligned with the global system of reference. Angular rotations of trunk segments were obtained from segment angles with respect to the axial in the transverse (axial rotation of the trunk and pelvis) and the frontal (trunk lateral flexion and pelvic list) plane of motion, and calculated as (four quadrant) arctangent, specified by the xyz-coordinates of the segment coordinate system. Heel strikes were estimated by means of the minimum in the vertical velocity of the toe marker, and toe-off at the maximum in the vertical velocity of the heel marker [40]. A stride cycle was defined as the distance between adjacent heel strikes of the same leg.
The EMG data were first rectified using the Hilbert transform [13]. Kinematic and EMG data were low-pass filtered using a fourth-order bidirectional Butterworth filter with cut-off frequencies of 10 and 20 Hz, respectively.
Using the stride intervals, kinematic and EMG data were split into sequential time series each containing a single stride cycle. Subsequently, each of these time series was time-normalized and resampled using a cubic spline interpolation to 0–100% of the stride cycle. For each kinematic or EMG recording per velocity at least 12 “individual” time series of equal length were generated, each containing a stride cycle (e.g., at 1.4 km/h the number of strides was seven).
Rotational amplitudes and trunk coordination
Rotational amplitudes of the thoracic (RAth), lumbar (RAlu) and pelvic (RApe) segments in the transverse and frontal plane, defined as the absolute angular difference from maximum to minimum rotation within a stride, were calculated from the respective time series and averaged per participant and velocity.
The coordination between trunk and pelvis segments in the transverse and frontal plane was expressed in terms of the continuous relative Fourier phase (RP). For this purpose, the Fourier phase was computed for each individual time series. To cope with time-dependent changes of the phases, we performed the Fourier transform within a finite frame size and shifted that frame in time (see Appendix). Phases for each signal were obtained at the fundamental movement frequency of the proximal segment by which the frame size was fixed at twice the corresponding period length [25]. Hence, the continuous RP between two signals x(t) and y(t) was calculated as RPxy=RPx(t)−RPy(t), with x, y representing the RP of thoracic and pelvic (RPthpe) or lumbar and pelvic (RPlupe) segment rotations in the transverse or frontal plane. For each RP, the corresponding SD was calculated providing a measure of the stability (in the sense of Lyapunov) of the coordination of interest. Continuous RPs were averaged for each participant and each velocity. In general, a phase difference of 180° indicates antiphase coordination and 0° in-phase coordination.
To evaluate the degree of coupling between thoracic and pelvic rotations as well as between lumbar and pelvic rotations in the transverse and frontal planes, the corresponding power spectra were correlated using weighted coherence (WC) statistics. WC summarizes the proportion of shared variances over a specified frequency band [41]. First, spectral densities were estimated using Welch’s periodogram method. The length of the chosen Hanning window was based on a 0.95% confidence interval with error bounds not exceeding 25% of the estimated spectral power. WC was calculated at the frequency band of the fundamental frequency ±0.2 Hz, indicating the strength of coupling normalized to values between 0 and 1.
Erector spinae activity
Double stance and swing phase ipsi- and contralateral to the right and left heel strikes were detected using the foot contact data. For each participant and each velocity, the rectified time- and amplitude-normalized EMG time series [65] were averaged over strides per muscle. Thereafter, the mean EMG activities of left and right Th12, L2 and L4 recordings of the ES muscles, during ipsi- and contralateral swing and between heel strike of one leg and toe-off of the other leg (double stance), were calculated.
Invariant and variant patterns of trunk coordination and muscle activity
Principal component analysis (PCA) was applied to detect similarities and deviations between LBP and control participants in trunk coordination and EMG activity patterns and to examine the relationship between LBP and variability. In general, this method allows detection of time-varying coherent patterns of activity (e.g., kinematic and EMG) vs more variant aspects within a data set. For a detailed description of this method we refer to a recent tutorial [10] and a clinical study in which it was applied [26]. To examine the consistency of patterns over stride cycles, kinematic and EMG recordings obtained at each walking velocity were split into sequential stride cycles. Every timeseries was rescaled to unit variance to eliminate overall amplitude effects. First, PCA was applied separately to the data of the LBP and control groups in order to evaluate eventual differences in the number of relevant principal modes (approximate “dimensionality”) between groups. Second, PCA was applied to the combined data set of LBP and control participants to examine deviations from normal pattern and, more interestingly, to analyze differences in variability.
In general, one distinguishes between global and residual patterns. Hence differences in variability can be quantified in terms of variations of those patterns (kinematic or EMG data for each walking velocity). To relate the variability of LBP participants to that of the control participants, we computed for each LBP participant the ratio of the variability of kinematic or EMG patterns divided by the respective ones of the entire control group. The individual ratios so obtained were then averaged over the LBP group. In other words, for the LBP group per velocity an averaged ratio was obtained indexing variability for segment rotations and ES patterns (see reference 26 for further details). Deviations in the global pattern of muscle activation between LBP participants and the control group were further examined by calculating the cross-correlation between the global patterns of muscles of LBP participants and that of the entire control group resulting in a maximal correlation at specific times.
Statistical analysis
Mean and SD of the RPs were calculated using circular statistics to account for phase wrapping [4]. The dependent variables were: transverse and frontal RAth, RAlu, RApe, RArlu, stride length, the mean and SD of RPthpe, RPlupe, WCthpe and WClupe, the averaged ES activity during ipsi- and contralateral swing and double stance of left and right Th12, L2 and L4, and the variance of the global and residual pattern of segment rotations and EMG activity.
Independent t-tests were performed on the dependent variables to compare the LBP and control groups at comfortable walking velocity. The effects of walking velocity, health status (LBP vs control) and their interaction on the dependent variables were tested using generalized estimating equations (GEE), which treat the measurements within participants as repeated measures. In contrast to other statistical methods for the analysis of repeated measures, GEE allows the analysis of unbalanced designs and designs with missing values. Standard errors (SE) and the corresponding confidence intervals (CI) are reported for significant effects of health status. The interaction effect was removed from the model if it proved not to be significant. Gender and age were entered as covariates. Paired t-tests were used to assess the differences in pain before and after walking in LBP participants. Spearman correlations (rs) were applied to examine the relationships between pain intensity, TSK and RDQ scores. Controlling for the maximally attained walking velocity, partial correlations were applied to assess the relationship between pain ratings, TSK and RDQ and the dependent variables. P<0.05 (two-sided) was taken as the level of significance.
Results
Averaged comfortable walking velocity was significantly lower (t=1.7, P<0.001) in the LBP group (mean 3.3 km/h, SD 1.1 km/h) than in the control group (mean 4.7 km/h, SD 0.7 km/h). All control participants were able to walk at all prescribed walking velocities. All 19 LBP participants could walk at velocities from 1.4 to 3.8 km/h, and 17 LBP participants walked up at velocities up to 4.6 km/h, 15 up to 5.4 km/h, 13 up to 6.2 km/h and 5 up to the maximum velocity of 7.0 km/h.
In the LBP group, pain intensity ratings did not differ significantly before and after walking (5.6±3 and 4.9±3, respectively). RDQ scores suggested moderate limitations in activities of daily living. The average TSK score indicated that pain-related fear-avoidance beliefs were present in the LBP group; 11 participants had a score higher than 37 with a mean of 44±5 (Table 1). Fear-avoidance belief correlated highly with the disability score. Pain levels correlated with TSK and RDQ scores, and RDQ also correlated with anticipated pain. Comfortable velocity and the maximally attained velocity were only significantly related to the RDQ (Table 1).
Table 1.
Mean scores for fear of movement (TSK), disability (RDQ), anticipated pain and actual pain and Spearman correlations (rs) in the LBP group
| TSK | RDQ | Anticipated pain | Actual pain | |
|---|---|---|---|---|
| Mean (SD) | 39 (6.8) | 10 (6) | 4.6 (3) | 5.6 (3) |
| Correlations (rs) | ||||
| TSK | ||||
| RDQ | 0.85** | |||
| Anticipated pain | 0.30 | 0.60** | ||
| Actual pain | 0.58** | 0.68** | 0.50* | |
| Comfortable velocity | −0.46 | −0.52* | −0.45 | −0.44 |
| Maximal velocity | −0.46 | −0.55* | −0.48 | −0.39 |
*P<0.05
**P<0.01
Rotational amplitudes and stride length
At all walking velocities, stride length was shorter in the LBP group than in the control group, and significantly shorter at velocities of 1.4, 2.2, 3.0 and 6.2 km/h (t=2.5, t=2.8, t=2.3 and t=2.9, respectively; P<0.05). Neither at the comfortable velocity, nor at the prescribed velocities, were significant differences between the LBP and control group found for RAth, RAlu and RApe in the transverse and frontal plane (Table 2).
Table 2.
Rotational amplitudes in the transverse and frontal plane of the thoracic (RAth), lumbar (RAlu) and pelvic (RApe) segments (in degrees) for the LBP and control groups at the comfortable and the prescribed walking velocities. Values are given as means with SDs in parentheses
| Velocity (km/h) | NLBPa | RAth | RAlu | RApe | |||
|---|---|---|---|---|---|---|---|
| LBP | Control | LBP | Control | LBP | Control | ||
| Transverse plane rotations | |||||||
| Comfortable | 19 | 9.4 (3.6) | 7.2 (2.9) | 8.7 (3.9) | 6.4 (2.1) | 5.5 (2.7) | 6.3 (2.3) |
| 1.4 | 19 | 12.1 (4.8) | 9 (2.4) | 12.9 (4.8) | 10.0 (3.2) | 10.5 (5.0) | 8.4 (2.6) |
| 2.2 | 19 | 11.8 (4.8) | 9.3 (3.4) | 12.0 (4.5) | 9.7 (3.5) | 9.5 (4.3) | 8.0 (3.4) |
| 3.0 | 19 | 10.5 (4.2) | 9.1 (3.5) | 9.9 (3.8) | 8.7 (3.6) | 8.3 (4.2) | 7.4 (3.5) |
| 3.8 | 19 | 8.9 (3.2) | 7.9 (2.9) | 8.0 (2.7) | 7.7 (3.2) | 7.7 (4.2) | 7.4 (3.3) |
| 4.6 | 17 | 7.2 (2.0) | 6.9 (2.3) | 7.0 (2.5) | 6.3 (2.6) | 9.1 (4.9) | 7.4 (2.3) |
| 5.4 | 15 | 6.5 (2.5) | 6.5 (2.4) | 7.5 (2.6) | 6.6 (2.5) | 11.0 (5.5) | 9.8 (3.0) |
| 6.2 | 13 | 6.8 (3.6) | 6.7 (3.0) | 8.8 (3.0) | 7.7 (2.9) | 12.6 (6.3) | 12.6 (4.3) |
| 7.0 | 5 | 5.5 (1.2) | 7 (3.4) | 10.0 (3.3) | 8.3 (3.2) | 17.5 (7.5) | 14.7 (5.3) |
| Frontal plane rotations | |||||||
| Comfortable | 19 | 3.8 (2.1) | 4.7 (1.6) | 3.3 (1.1) | 3.4 (1.2) | 4.6 (3.1) | 5.9 (2.8) |
| 1.4 | 19 | 3.3 (1.6) | 4.8 (3.5) | 3.3 (1.4) | 3.9 (1.6) | 4.0 (2.4) | 4.6 (2.2) |
| 2.2 | 19 | 3.7 (1.9) | 3.8 (1.6) | 3.0 (1.1) | 3.4 (1.0) | 4.5 (2.0) | 4.9 (2.5) |
| 3.0 | 19 | 3.7 (1.9) | 3.6 (1.3) | 3.2 (1.6) | 3.3 (1.2) | 5.2 (2.1) | 5.6 (1.9) |
| 3.8 | 19 | 4.0 (2.1) | 3.9 (1.6) | 3.4 (1.9) | 3.3 (1.3) | 6.1 (2.2) | 6.4 (1.8) |
| 4.6 | 17 | 4.1 (2.2) | 4.2 (1.3) | 3.6 (2.2) | 3.6 (1.2) | 7.0 (2.4) | 7.5 (2.2) |
| 5.4 | 15 | 4.1 (2.2) | 4.8 (1.5) | 3.8 (2.1) | 4.2 (1.2) | 8.2 (2.6) | 8.5 (3.0) |
| 6.2 | 13 | 5.0 (2.4) | 5.6 (2.2)) | 3.7 (1.3) | 4.7 (1.4) | 9.2 (2.5) | 9.1 (3.4) |
| 7.0 | 5 | 4.8 (1.1) | 6.3 (3.9) | 4.3 (1.7) | 5.2 (1.7) | 10.2 (3.0) | 9.9 (3.9) |
aNumber of LBP participants who could attain this velocity; for control participants N=14 at all velocities
Invariant and variant patterns of trunk coordination
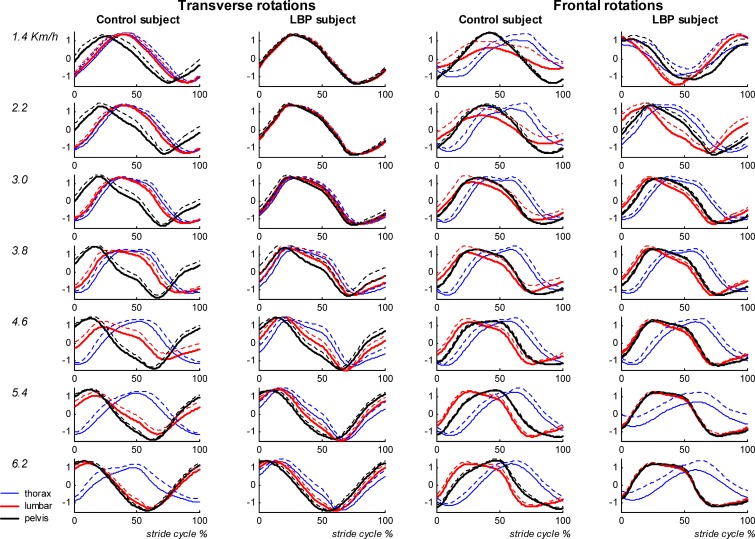
At the comfortable velocity and at all prescribed velocities, the first two principal modes covered about 84% of the spread of the data in the LBP group (52±6%+32±6%), the control group (50±6%+34±5%) and both groups together (51±6%+33±5%). The corresponding two patterns represented the coherent features of trunk and pelvis rotations in the transverse and frontal plane at all walking velocities, and captured the rotations that oscillated at the stride frequency. Analysis of the coefficients of the eigenvectors revealed an almost equal contribution of the LBP and control group to the two modes, suggesting that LBP had no effect on the global kinematic walking pattern. However, marked differences in the relative timing between the segment rotations were present. In particular, the transverse counter-rotation at higher walking velocities was less in the LBP group (see Fig. 1 for an example of one LBP and one control participant). To quantify differences in the timing between segments, the relative Fourier phase was calculated. The difference between the pattern of the first and second mode represented a phase shift of about 90°, and not so much between segments as between plane of rotation (i.e., transverse and frontal).
Fig. 1.
Average time series of the global pattern of segment rotations in the transverse (left two panels) and frontal (right two panels) planes are shown for one control and one LBP participant. The dotted lines represent the SD
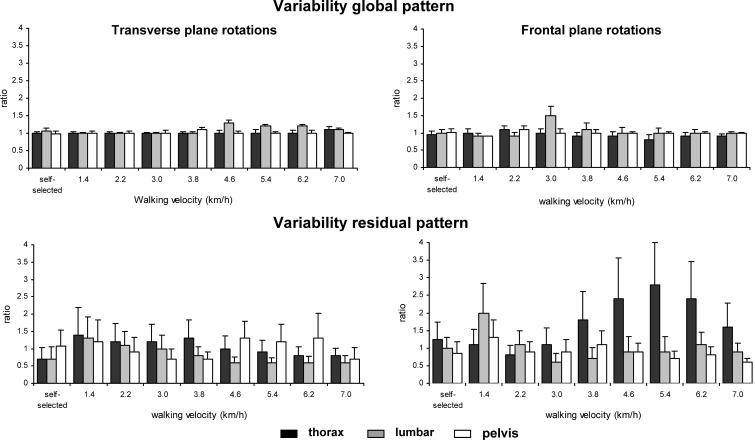
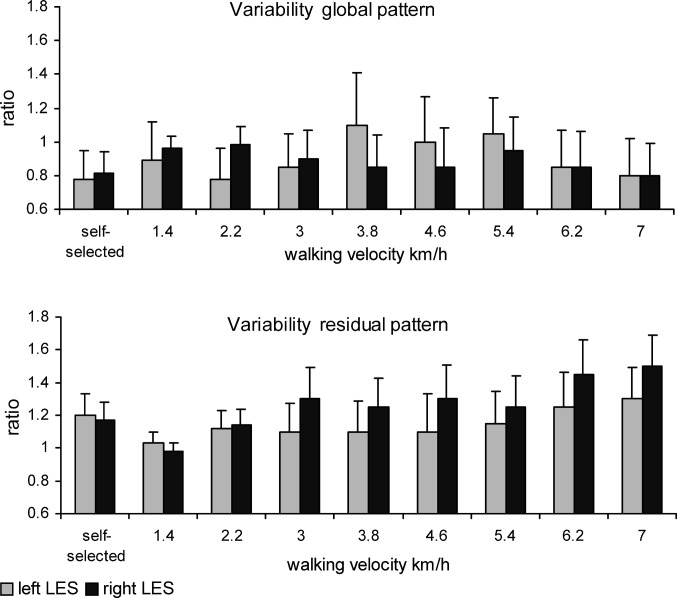
The global pattern of thoracic, lumbar and pelvic rotations was highly consistent across all participants and velocities. The variability of the global patterns of the thoracic, lumbar and pelvic rotations in the transverse and frontal planes was not significantly affected by health status, while the mean ratios of the global patterns were almost 1 at all walking velocities (Fig. 2, upper panel).
Fig. 2.
The variability of the global (upper panels) and residual (lower panels) patterns of segment rotations in the transverse and frontal plane quantified as the ratio between the average variance over stride cycles of each segment for each LBP participant and that of the control group (ratio). Error bars indicate SD
At the comfortable velocity, the variability of the residual patterns of transverse thoracic and lumbar rotations was significantly smaller in the LBP participants than in the healthy controls (t=2.12, P=0.04 and t=2.35, P=0.03, for the thoracic and lumbar segments, respectively).
At the prescribed walking velocities, the variability of transverse lumbar rotations was significantly smaller in the LBP participants than in the healthy controls (P=0.04; SE=0.025, CI=0.020–0.033), particularly at velocities higher than 4.6 km/h. The most pronounced effect of LBP was the larger variability of the residual pattern of frontal thoracic rotations (P<0.01; SE=0.03, CI=0.024–0.039) for walking velocities of 3.8 km/h and higher. This was also evidenced by the concomitant ratio, which, at some of the higher velocities, was even larger than 2 (see Fig. 2, lower panel).
Relative timing between segment rotations
Comfortable walking velocity
At the comfortable velocity, mean RPthpe in the transverse plane was significantly smaller in the LBP than in the control group (71° vs 111°, t=2.5, P=0.02), whereas in the frontal plane mean RPthpe and RPlupe did not differ significantly between the LBP group and thecontrol group. As regards the variability of relative timing, the SD of RPthpe in the frontal plane was significantly larger in the LBP group than in the control group (t=2.6, P=0.01; see Table 3). In accordance, the coupling between frontal thoracic and pelvic rotations was significantly weaker in the LBP group than in the control group (t=3.2, P<0.01, means 0.7±0.2 and 0.9±0.1, respectively).
Table 3.
Standard deviations of the relative phases between thoracic–pelvic (SD RPthpe) and lumbar–pelvic (SD RPlupe) frontal and transverse plane rotations for the LBP group and the control group. Values given are means with SDs in parentheses
| Velocity (km/h) | NLBPa | SD RPthpe | SD RPlupe | ||
|---|---|---|---|---|---|
| LBP | Control | LBP | Control | ||
| Transverse plane rotations | |||||
| Comfortable | 19 | 18 (13) | 19 (9) | 15 (13) | 16 (11) |
| 1.4 | 19 | 7 (8) | 8 (5) | 6 (8) | 7 (5) |
| 2.2 | 19 | 11 (11) | 14 (15) | 8 (10) | 12 (6) |
| 3.0 | 19 | 15 (8) | 15 (8) | 11(11) | 13 (8) |
| 3.8 | 19 | 14 (6) | 18 (9) | 11 (8) | 17 (8) |
| 4.6 | 17 | 15 (6) | 16 (5) | 10 (5) | 18 (9) |
| 5.4 | 15 | 15 (4) | 15 (4) | 7 (4) | 13 (5) |
| 6.2 | 13 | 16 (8) | 17 (9) | 6 (3) | 10 (5) |
| 7.0 | 5 | 12 (2) | 18 (16) | 4 (2) | 9 (6) |
| Frontal plane rotations | |||||
| Comfortable | 19 | 22 (15) | 11 (5) | 12 (9) | 12 (9) |
| 1.4 | 19 | 31 (19) | 28 (18) | 19 (16) | 20 (18) |
| 2.2 | 19 | 29 (17) | 23 (12) | 18 (14) | 16 (10) |
| 3.0 | 19 | 25 (13) | 19 (13) | 22 (21) | 19 (19) |
| 3.8 | 19 | 22 (15) | 16 (11) | 22 (20) | 15 (12) |
| 4.6 | 17 | 20 (14) | 11 (4) | 14 (10) | 10 (6) |
| 5.4 | 15 | 20 (12) | 11(7) | 12 (10) | 8 (4) |
| 6.2 | 13 | 14 (13) | 10 (7) | 10 (6) | 8 (5) |
| 7.0 | 5 | 19 (7) | 9 (3) | 6 (4) | 7 (6) |
aNumber of LBP participants who could attain this velocity; for control participants N=14 at all velocities
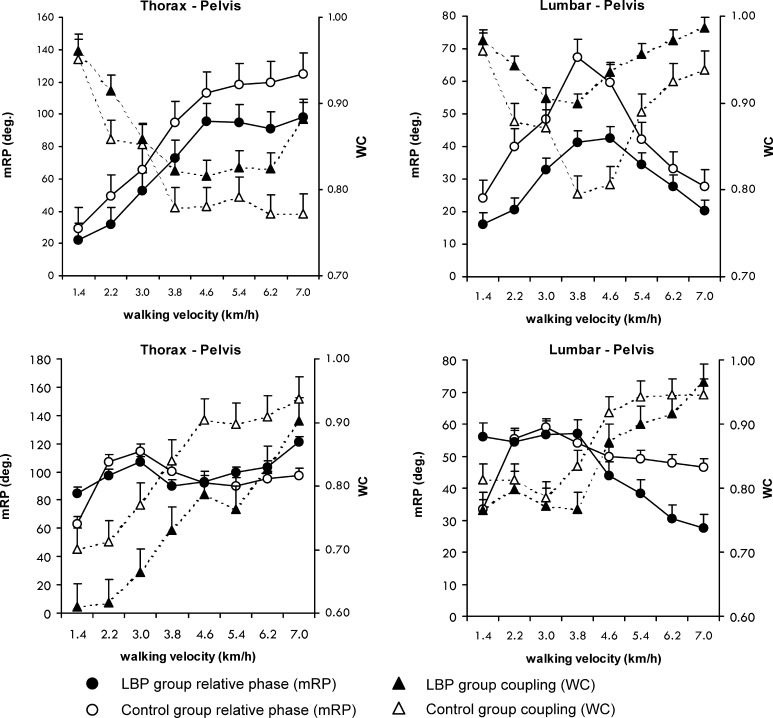
Effect of walking velocity
Figure 3 shows the effect of walking velocity on the mean relative phases and the coupling between the segment pairs. The SDs of the RPs are presented in Table 3. As can be appreciated from Fig. 3, mean RPthpe increased significantly with increasing velocity (P<0.01), whereas mean RPlupe first increased from 1.4 to 3.8 km/h and then decreased again in both groups. These velocity-induced changes in relative timing were accompanied by qualitatively similar changes in intersegmental coupling. Faster walking led to a decrease in WCthpe and WClupe up to a velocity of 3.8 km/h, after which WCthpe remained roughly constant and WClupe increased (both P<0.01; Fig. 3, upper panel). No significant effect of velocity was found on the variability of the relative phases in the transverse plane (Table 3).
Fig. 3.
Mean relative Fourier phase (mRP) and the coupling (WC) between thoracic–pelvic and lumbar–pelvic rotations in the transverse plane (upper panels) and frontal plane (lower panels). The left y-axis represents values of the mRP, and the right y-axis values of the coupling (WC) between the segments
In contrast to the relative phases in the transverse plane, mean RPthpe in the frontal plane hovered around 90° in both LBP and control participants, and was not affected significantly by velocity. With increasing velocity, however, the rotations in the frontal plane appeared to be more tightly coordinated, as was evidenced by a significant decrease in the SD of RPthpe and RPlupe (Table 3), as well as a significant increase in coupling strength with walking velocity in both groups of participants (both P<0.01; Fig. 3, lower panel).
Effect of health status (LBP)
In the transverse plane, mean RPthpe and RPlupe were significantly smaller in the LBP group than in the control group (P=0.02; SE=8.1, CI=6.53–10.66 and P=0.04; SE=6.7, CI=5.40–8.82, respectively). This effect was again reflected in the degree of intersegmental coupling. WClupe was significantly larger in the LBP group than in the control group (P=0.02; SE=0.025, CI=0.020–0.033, Fig. 3, upper panels). In addition, SD of RPlupe in the LBP group was smaller than in the control group (P=0.04; SE=1.2, CI=0.97–1.57; Table 3). In contrast to the intersegmental coordination in the transverse plane, the mean RPthpe of frontal plane rotations was not affected significantly by health status. Mean RPlupe was around 60° up to a velocity of 4.6 km/h, but then dropped to 30° in the LBP group. Consequently, the interaction between velocity and health status was significant (P=0.04), whereas the effect of health status as such was not (Fig. 3, lower panels). SDs of RPthpe and RPlupe in the frontal plane were significantly larger in the LBP group than in the control group (P<0.05; SE=3.1, CI=2.50–4.08 and P<0.01; SE=2.1, CI=1.69–2.76, respectively; Table 3). Furthermore, whereas intersegmental coupling increased with walking velocity in both groups of participants, it remained weaker in the LBP group. Specifically, WCthpe ranged from 0.6 to 0.9 in the LBP group and from 0.7 to 0.95 in the control group (P=0.03; SE=0.031, CI=0.023–0.051; Fig. 3, lower panels). Interactions between health status and velocity were not significant for either the SDs or the WCs.
Mean EMG amplitude
Comfortable walking velocity
At the comfortable velocity, averaged lumbar ES (LES) amplitudes increased significantly in the LBP group during the ipsilateral swing phase in left and right L2 (t=2.2, P=0.03; t=3.6, P<0.01) and L4 (t=2.9; P<0.01; t=3.0, P<0.01). In the contralateral swing phase, mean LES activity was significantly higher for left L4 (t=2.5, P=0.02), right L4 (t=2.8, P<0.01) and right L2 (t=2.3, P<0.03), whereas left L2 approached significance (P=0.07). Expressed as percentage increase in mean amplitude with respect to the control group [(LBP−control)/control)], LES activity was on average 51% elevated in the ipsilateral and 68% in the contralateral swing phases. Mean thoracic ES (TES) activity was increased (by 48%) only in the ipsilateral swing phase (t=2.6, P=0.02 and t=2.56, P=0.01 for left and right thorax, respectively).
Effect of walking velocity and health status (LBP)
As walking velocity increased, mean LES amplitude decreased during the swing phase up to a velocity of 4.6 km/h and then increased (P<0.01) in both groups of participants. Moreover, amplitudes of both L2 and L4 ES were significantly larger during the swing phase in the LBP than in the control group (P<0.05). On average, mean LES activity increased by 8, 21, 25, 39, 45, 32 and 48% for velocities from 1.4 to 7.0 km/h, respectively. The velocity by health status interactions were not significant. During double stance, no significant effect of health status or velocity was found on ES activity. Since neither health status nor velocity significantly affected mean TES activity during the swing phase, the TES muscles were excluded from the PCA analysis (see below).
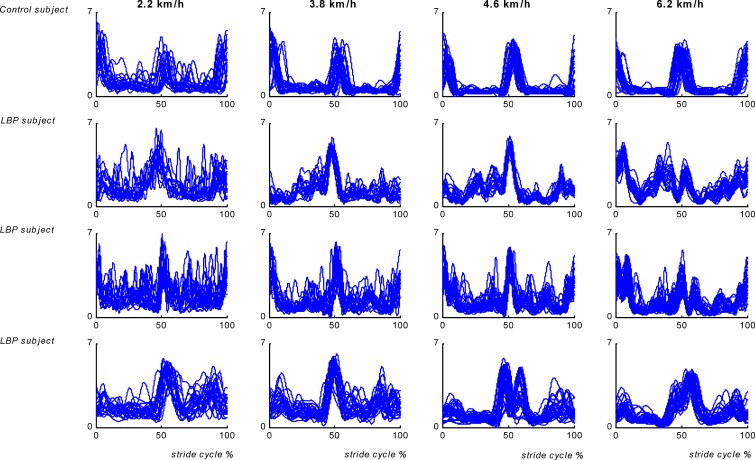
Visual inspection of the EMG time series revealed clear changes in LES activity patterns in the LBP participants over consecutive strides. Muscle activity appeared to be more variable both within and between strides in a variety of ways including larger phase shifts, additional frequencies and prolonged activity around heel strike. Within the LBP group marked individual differences were present (see Fig. 4).
Fig. 4.
Individual time series of superimposed stride cycles of the left ES recorded at the level of L4 for one control and three LBP participants at walking velocities of 2.2, 3.8, 4.6 and 6.2 km/h
Invariant and variant patterns of muscle activity
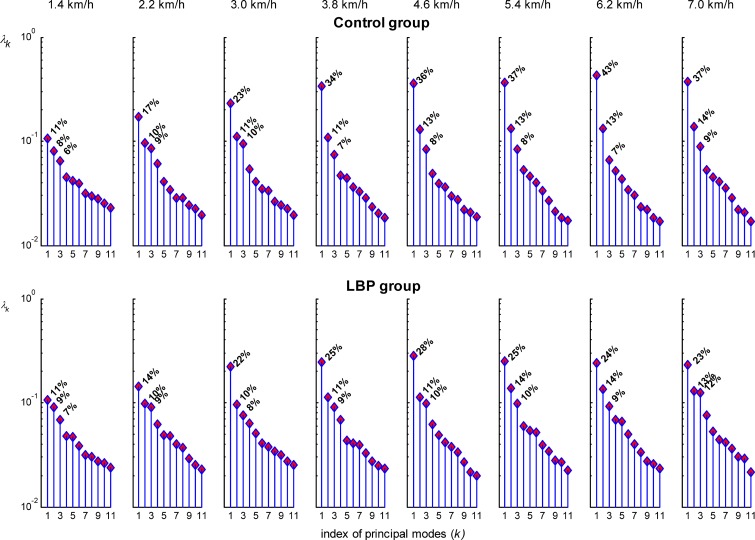
In both the LBP and control group, PCA revealed that, at the walking velocities of 1.4 and 2.2 km/h, the eigenvalue spectra did not show a clear gap between successive principal modes, but rather an exponential decrease in the spread. In other words, at these low walking velocities the activation pattern of the LES muscle was quite erratic, lacking consistent features. For velocities higher than 2.2 km/h, the first three principal modes, averaged over velocities, covered about 46% of the spread (25±3%+12±2%+10±2%) in the LBP group and 56% (35±6%+12±1%+8±1%) in the control group. As can be appreciated from Fig. 5, the first mode was smaller in the LBP group than in the control group and the gap between successive modes became successively smaller with increasing velocities, implying larger modifications of the normal basic EMG pattern in the LBP group.
Fig. 5.
Eigenvalue spectra (λκ) of the LBP group and control group plotted on a logarithmic scale. At walking velocities of 3.8 km/h and higher, the amount of variance explained by the first principal component is smaller in the LBP group than in the control group. Note that at velocities of 1.4 and 2.2 km/h no clear gap between successive modes is present in either group
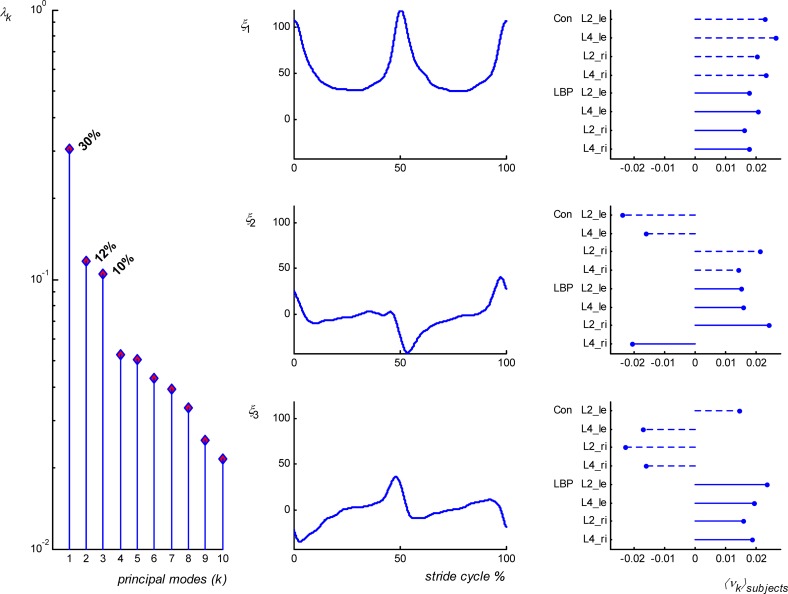
The number of relevant principal modes remained the same when PCA was applied to the data of both groups of participants collapsed together. At the comfortable velocity, and for the prescribed velocities in the range 3.8–7 km/h, the first three principal modes, again averaged over velocities, covered about 50% (29±4%+11±2+9±1%) of the spread. As an example, Fig. 6 shows the results of the PCA of EMG data of both groups combined for the walking velocity of 5.4 km/h. The distinctively biphasic activity pattern of LES muscles during the stride cycle at walking velocities higher than 2.2 km/h was captured by the first mode. The second mode represented modulations superimposed on the step frequency, that is, just after heel strike and around the double support phase, homolateral LES (with respect to toe off) increased and contralateral activity decreased. Adding a third mode to the first two shifted the peak activity to the left in the LBP group, implying that peak activation occurred earlier than in the control group. Similar patterns were observed for all velocities from 3.0 km/h and above.
Fig. 6.
PCA of EMG data obtained at a walking velocity of 5.4 km/h. The first column shows the eigenvalue spectra (λk); note the decrease in eigenvalues after mode 3. The second column shows the time series (ξ1..ξ3) corresponding to the first three modes, which together formed the global pattern of LES activity. The eigenvector coefficients (νk) are averaged over strides of LBP and control (Con) participants and represent the contribution of each muscle per group to the pattern (column 3)
Systematic differences were found for left and right LES activity but not for L2 and L4 recordings. Therefore, the results of each side were collapsed. Although the global pattern was present in the LBP group as a whole, the eigenvector coefficients indicated that for walking velocities of 3.0 km/h and higher their contribution to the first mode was smaller than in the control group. Hence, the variability of the global pattern was smaller in the LBP group than in the control group at the comfortable velocity (left LES t=3.9, P<0.01, and right LES t=3.3, P<0.01) as well as at the prescribed velocities (left and right LES, P=0.04; SE=0.034, CI=0.027–0.045 and SE=0.029, CI=0.023–0.029, respectively; Fig. 7, upper panel). In addition, the residual variability of left and right ES was larger in the LBP group than in the control group, both at the comfortable velocity (both t=4.2, P<0.01) and at the prescribed velocities (both Ps=0.03; SE=0.037, CI=0.030–0.049 and SE=0.038, CI=0.031–0.051, respectively; Fig. 7, lower panel). In the LBP participants, the residual pattern consisted of rather irregular deviations of the global pattern (phase shifts, amplitude modifications and additional bursts). The contribution of the LBP participants to the global pattern was not only smaller than that of the control participants, but their patterns also showed marked deviations from the normal LES activity pattern. This is also reflected in the maximal cross-correlation values and their corresponding time lags, as given in Table 4.
Fig. 7.
Variability of the global (upper panel) and residual pattern (lower panel) of left and right LES activity quantified by calculating the average variance over stride cycles for LBP participants over that of the control group (ratio). Error bars indicate SD
Table 4.
Cross-correlation values (Cr) and timelags (Lag) between the averaged global pattern of the control group and LBP participants of left and right ES activity. The values given are means with SDs in parentheses. Lags are expressed as percentage of stride cycle and negative signs (lower panel) indicate later peak activation
| Velocity (km/h) | left ES | Right ES | ||||
|---|---|---|---|---|---|---|
| Na | Cr | Lag % | Na | Cr | Lag % | |
| Comfortable | 11 | 0.81 (0.20) | +2.3 (2.9) | 11 | 0.55 (0.25) | +2.5 (2.6) |
| 3.0 | 14 | 0.66 (0.29) | +3.1 (2.9) | 13 | 0.66 (0.30) | +1.6 (2.8) |
| 3.8 | 15 | 0.76 (0.23) | +2.5 (2.6) | 15 | 0.57 (0.34) | +3.3 (4.0) |
| 4.6 | 14 | 0.80 (0.21) | +1.8 (2.2) | 13 | 0.56 (0.35) | +3.2 (4.7) |
| 5.4 | 9 | 0.69 (0.23) | +3.5 (2.0) | 9 | 0.71 (0.24) | +1.0 (1.8) |
| 6.2 | 9 | 0.62 (0.34) | +4.3 (5.5) | 9 | 0.67 (0.33) | +2.8 (5.0) |
| 7.0 | 4 | 0.74 (0.19) | +1.2 (1.2) | 3 | 0.68 (0.31) | +2.4 (4.9) |
| Averageb | 0.71 | +2.7 | 0.64 | +2.4 | ||
| Comfortable | 8 | 0.76 (0.10) | −4.3 (4.2) | 8 | 0.72 (0.17) | −5.3 (4.8) |
| 3.0 | 5 | 0.72 (0.32) | −2.7 (3.2) | 6 | 0.39 (0.36) | −3.7 (3.5) |
| 3.8 | 4 | 0.56 (0.32) | −4.0 (3.2) | 4 | 0.51 (0.36) | −2.3 (2.2) |
| 4.6 | 3 | 0.54 (0.39) | −5.3 (4.3) | 5 | 0.73 (0.15) | −1.5 (0.9) |
| 5.4 | 6 | 0.75 (0.29) | −4.6 (4.3) | 6 | 0.54 (0.23) | −5.7 (2.9) |
| 6.2 | 4 | 0.48 (0.33) | −5.6 (5.1) | 4 | 0.43 (0.27) | −7.2 (3.9) |
| 7.0 | 1 | 0.56 (0.50) | −7.0 (8.3) | 2 | 0.49 (0.29) | −4.9 (6.0) |
| Averageb | 0.60 | −4.9 | 0.52 | −4.2 | ||
aNumber of LBP participants with positive or negative time delay
bAveraged over all prescribed walking velocities
Pain intensity, fear of movement and disability
At the comfortable velocity, pain levels were correlated negatively with RPthpe (rs=0.46; P=0.048) and SD RPthpe (rs=0.45, P=0.05). At the prescribed velocities, when corrected for the maximally attained velocity, there were no significant correlations between the VAS, TSK or QDR scores with any of the dependent variables.
Discussion
The aim of the present study was to deepen current insights into the consequences of LBP for walking. We performed detailed analyses of the effect of walking velocity on both kinematics and ES activity, focusing on (1) the global coordination of the timing between trunk and pelvis segments in the frontal and transverse plane and the average amplitude of ES activity during different phases of the stride cycle, and (2) invariant and variant properties of trunk kinematics and muscle activity.
Walking velocity was found to affect the relative timing between segment rotations in both LBP and control participants. In line with previous studies [25, 26, 54], thoracic–pelvic rotations in the transverse plane evolved from more or less in-phase in the direction of antiphase coordination with increasing walking velocity. In contrast, the intersegmental coordination in the frontal plane did not change with walking velocity, although the segment pairs became more tightly coordinated with increasing walking velocity, as was evidenced by smaller standard deviations of the relative phases and higher coupling strengths.
As regards the kinematics, mainly the intersegmental coordination was disturbed in the LBP participants. Rotational amplitudes were not different between LBP and control participants. However, stride length was shorter, although not significantly so, at all velocities. The normal velocity-induced transverse counter-rotation between thorax and pelvis at higher walking velocities was less pronounced in the LBP participants. In addition, the LBP participants tended to move the lumbar and pelvic segments more synchronously and rigidly in the same direction. Both phase relations, i.e., thoracic–pelvic and lumbar–pelvic coordination, were characterized by a higher degree of coupling in the LBP group. These results are consistent with those of earlier studies on transverse plane coordination during gait in LBP [27, 46]. In contrast to the transverse plane, the intersegmental coordination in the frontal plane was more variable and less tightly coupled in the LBP than in the control participants. In addition, PCA revealed that after subtracting the common invariant features of the kinematic time series, in the LBP participants, the variability of the residual pattern was reduced in the transverse plane for lumbar rotations and was largely confined to thoracic rotations in the frontal plane, particularly at walking velocities higher than the average comfortable velocity. These additional frontal plane movements may be a strategy to compensate for the more rigid coordination in the transverse plane.
An overall effect of walking velocity was also found on the mean amplitude of LES during the swing phases. The average level of EMG activity decreased with walking velocity up to 4.6 km/h, which corresponds to the comfortable walking velocity of healthy individuals. The averaged LES activity during the swing phases was significantly greater in the LBP participants than in the control participants, whereas health status had no effect on lumbar or thoracic ES activity during doublestance. Besides overall changes in mean LES amplitudes, the results of the PCA indicated that muscular control in LBP was affected in terms of both alterations of the global pattern and a marked increase in variability of the residual pattern, both at the comfortable walking velocity and at the prescribed walking velocities. Compared to the control group, the first principal mode in the LBP group covered a smaller amount of the data’s spread, reducing its ‘distance’ relative to subsequent modes, especially at higher walking velocities. Hence, variability was spread out over subsequent modes, implying modifications of the normal biphasic LES pattern in the form of the appearance of phase shifts, (seemingly random) amplitude modifications and additional bursts.
When applied to the combined data set, PCA confirmed that the modifications of LES activity in the LBP participants affected the overall LES activity, i.e., LES activity was coordinated less than in healthy persons. Note that the observed increase in mean LES activity during the swing phase may in fact have resulted from these modifications, rather than being purely an amplitude effect. Our findings contradict those of Vogt et al. [59], who reported identical ES curves in LBP and healthy individuals. This discrepancy may be explained by the fact that we performed a more fine-grained analysis by examining the consistency of activity patterns over a finite bout of walking. Furthermore, in our analysis, the global pattern was based on the dominant principal modes, so that the resulting filter characteristics depended on the data themselves [10].
At very slow walking velocities (1.4 and 2.2 km/h), no consistent pattern of LES activity was observed in control and LBP participants alike. At these very low walking velocities, the duration of the double stance phase is relatively long, and the swing phase relatively short. Thus, presumably, the more erratic LES patterns at low velocities reflect ‘randomness’ due to balance problems in both groups of participants.
Actual pain intensity, anticipated pain, disability and fear-avoidance were all uncorrelated with the observed changes in the dependent kinematic and EMG variables. Only the level of disability was related significantly to walking velocity. When invited to walk at a comfortable velocity, individuals with LBP selected lower walking velocities than healthy individuals. Nevertheless, all LBP participants were able to walk faster than their preferred velocity, which raises the question: Why did the individuals with LBP walk with a lower velocity? In order to answer this question, it is important to recognize that spinal instability due to dysfunction of spinal structures or impaired control over trunk muscles is a major source of complaints in individuals with LBP [37, 38]. In principle, spinal stability may be regained through changes in muscle activity, but such changes may also lead to microtrauma and subsequent chronic pain and changes in sensory input, amplifying the spinal instability and reducing the ability to effectively deal with perturbations. When healthy individuals anticipate a perturbation, they contract trunk muscles to prevent balance loss. In individuals with LBP, however, such anticipatory behavior is diminished. They also have slower reaction times and less forceful corrections of ES activity to experimental perturbations than healthy individuals [19, 31, 42]. It is therefore conceivable that individuals with LBP attempt to enhance the control over their movements by walking slowly and more carefully, thus creating a greater margin of safety to deal with upcoming perturbations [50]. Similarly, it is conceivable that the observed changes in LES activity reflect an attempt to stabilize the spine by increasing its stiffness and to prevent the occurrence of unexpected perturbations to which the patient cannot adequately respond. In particular, the increased coupling between lumbar and pelvic rotations points to an increased stiffness of the spine. Accordingly, the avoidance of antiphase thorax–pelvis coordination by choosing a slow preferred walking velocity that does not require counter-rotation and/or increasing the intersegmental coupling strength can be interpreted as a strategy to minimize the probability of rotational perturbations of the spine that the individual with low back pain cannot adequately handle.
Besides changes in muscle recruitment, proprioception is also often affected in chronic low back pain, as evidenced by the fact that individuals with chronic LBP have reduced lumbar position sense [5, 15] and poor balance while standing [2, 30, 35]. In general, modulation and fine tuning of neuromuscular control on the basis of peripheral feedback play an important role in the control of walking. When this feedback is affected, the precise timing of trunk and pelvis rotations during walking may be hampered. Impaired motor control may promote chronicity or recurrent LBP and even be a risk factor for LBP [37, 38]. From this perspective, it has been suggested that people with mild deficits of the central nervous system [21], such as an imbalance in recruitment of agonist and antagonist trunk muscles [28, 36], slow reaction times [49] or poor proprioception [39], are at risk of developing musculoskeletal disorders.
The motor functioning of individuals with LBP is complicated further by the fact that, whereas walking more quickly with impaired coordination may enhance the mechanical instability of the spine, walking more slowly may have considerable disadvantages. Callaghan et al. [6] have shown that walking slowly without motion in the transverse plane increases static loading of the spine, whereas faster walking promotes more cyclic loading patterns. From the perspective of activities of daily living, normal walking in every day situations requires continuous adaptations to changes in circumstances and to potentially destabilizing factors in the environment—e.g., the ability to respond to sudden slips and trips.
In closing, some limitations of the present study should be discussed. First of all, the number of subjects that participated in the experiment was relatively small. In spite of the small sample size, however, numerous significant differences between the LBP and control group were found. The effect sizes for the main effects of group on the kinematic data (relative phase and variability) ranged from 0.86 to 0.93, with an associated estimated power ranging from 66 to 79%. The lowest power was obtained for the measures of variability of the global and filtered patterns of the EMG data, which ranged from 59 to 68%. In all likelihood, these lower values were due to the fact that there was considerable variability in LES activity among individuals with low back pain (see Fig. 4). These differences between participants reflect the non-homogeneity of the LBP population and show that a variety of neuromuscular strategies may be used to cope with the consequences of LBP. Pinpointing these strategies in a statistical sense, however, would require the assessment of a much larger number of LBP participants than included in the present study. To obtain a power of 80% with about the same effect sizes as found in the present study would require a sample size in the order of 28 subjects in each group. To identify different subgroups between the LBP patients, however, would require a group of about 140 LBP subjects in order to detect effect sizes between 0.3 and 0.5 [7].
Second, to be able to control walking velocity, the present experiment was performed on a treadmill. Treadmill walking, however, may be quite different from normal walking. Some researchers have indicated reduced amplitudes of thorax and pelvis rotations during treadmill walking as compared to level walking [58], whereas others have reported similar kinematics of the lumbar spine and pelvis [45]. Third, LBP participants were on average 7 years older than the controls and there were more females in the LBP group than in the control group. In principle, this could have affected the results, although post-hoc analysis did not reveal any significant effects of age or gender on the dependent measures. Note that age-dependency of gait parameters has been reported for much older adults than the participants in the present study [22, 34], while pelvis–thorax coordination appears to be unaffected by gender [9, 27]. Finally, in order to make the task demands severe, we used a wide range of walking velocities from 1.2 up to 7.0 km/h, a velocity just below the transition to running. Consequently, not all LBP participants were able to walk at all velocities. At velocities up to 6.2 km/h, the group sizes only differed marginally, but only five LBP participants could walk at 7.0 km/h.
Clinical implications
Although the study of variability may provide important insights into changes in motor control, changes in variability are often not easily measurable in a clinical setting. Indeed, the global patterns of coordination as being described by the relative phase between two moving segments is closely related to the level at which clinicians evaluate movement patterns. Studies on gait in LBP usually focus on measures such as walking velocity, stride length and amplitudes. The present study suggests that measures of intersegmental trunk coordination may be more informative about changes in gait and thus the motor functioning of individuals with LBP.
Nowadays, gait training or ‘speed’ walking in LBP is often part of rehabilitation programs and tests [12, 51, 66]. The results of the present study suggest that some caution should be applied in this respect. All LBP participants could walk at velocities higher than their comfortable velocity, but at these velocities they showed clear adaptations in movement coordination during which the spine may have been subjected to non-optimal loading. This implies that conservative therapy should include methods aimed at improving intersegmental and muscle coordination rather than walking velocity as such.
Conclusion
Collectively, the results of the kinematic analysis revealed that, in LBP participants, the coordination between transverse thoracic and pelvic rotations and lumbar and pelvic rotations was more rigid and less variable than in healthy controls, whereas the coordination between the segments in the frontal plane was less tight and more variable, particularly at walking velocities higher than the comfortable velocity. These changes in coordination were not accompanied by changes in rotational amplitudes.
Statistical analysis of the invariant and variant properties of kinematics and EMG revealed that the global pattern of segment rotations remained unaffected in the LPB participants, whereas the variability of the thoracic rotations in the frontal plane exhibited marked irregularities. The global pattern of LES activity deviated markedly from the normal pattern and the variability of the LES was significantly elevated, indicating poor coordination in the activation of the LES muscles. The slower walking of the LBP participants appeared to be a functional adaptation to altered motor control, which could not be related to actual pain, fear of movement/(re)injury or level of disability. The assessment of gait patterns in terms of coordination may help clinicians to quantify (the consequences of) movement impairments and may provide suggestions for interventions aimed at facilitating the emergence of desired coordination patterns.
Acknowledgements
This study was supported by grants from the Dutch Organization for Scientific Research # 904-65-090 (MW-NWO), the Dutch Association for Exercise Therapy Mensendieck (NVOM) and the Mensendieck Development Foundation (SOM). The authors thank Caroline van der Stok for her assistance with the data recording.
Appendix
For each time series, the Fourier phase was computed. To cope with time-dependent changes of the phases we performed the Fourier transform within a finite frame size ɛ, and shifted that frame in time. Phases were obtained at the fundamental movement frequency ω0 of the proximal segment by which the frame size was fixed at twice the corresponding period length ɛ=2T0=4π/ω0. In detail, we computed for a time series x(t), as follows:
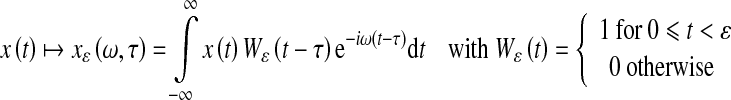 |
1 |
and defined the phase as
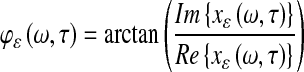 |
2 |
References
- 1.Ahern DK, Follick MJ, Council JR, Laser-Wolson N, Litchman H. Comparison of chronic low back pain patients and non-patient controls. Pain. 1988;34:153–160. doi: 10.1016/0304-3959(88)90160-1. [DOI] [PubMed] [Google Scholar]
- 2.Alexander KM, LaPier TL. Differences in static balance and weight distribution between normal subjects and subjects with chronic unilateral low back pain. J Orthop Sports Phys Ther. 1998;28:378–383. doi: 10.2519/jospt.1998.28.6.378. [DOI] [PubMed] [Google Scholar]
- 3.Arendt-Nielsen L, Graven-Nielsen T, Svarrer H, Svensson P. The influence of low back pain on muscle activity and coordination during gait: a clinical and experimental study. Pain. 1996;64:231–240. doi: 10.1016/0304-3959(95)00115-8. [DOI] [PubMed] [Google Scholar]
- 4.Batschelet E. Circular statistics in biology. London: Academic; 1981. [Google Scholar]
- 5.Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25:989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- 6.Callaghan JP, Patla AE, McGill SM. Low back three-dimensional joint forces, kinematics, and kinetics during walking. Clin Biomech. 1999;14:203–216. doi: 10.1016/S0268-0033(98)00069-2. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- 8.Collins JJ, De Luca CJ. Random walking during quiet standing. Phys Rev Lett. 1994;73:764–767. doi: 10.1103/PhysRevLett.73.764. [DOI] [PubMed] [Google Scholar]
- 9.Crosbie J, Vachalathiti R, Smith R. Age, gender and speed effects on spinal kinematics during walking. Gait Posture. 1997;5:13–20. doi: 10.1016/S0966-6362(96)01068-5. [DOI] [Google Scholar]
- 10.Daffertshofer A, Lamoth CJC, Meijer OG, Beek PJ. PCA in studying coordination and variability: a tutorial. Clin Biomech. 2004;19:415–428. doi: 10.1016/j.clinbiomech.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Dingwell JB, Ulbrecht JS, Boch J, Becker MB, O’Gorman JT, Cavanagh PR. Neuropathic gait shows only trends towards increased variability of sagittal plane kinematics during treadmill locomotion. Gait Posture. 1999;10:21–29. doi: 10.1016/S0966-6362(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 12.Fordyce WE. Behavioral methods in chronic pain and illness. St Louis: Mosby; 1976. [Google Scholar]
- 13.Gabor D. Theory of communication. IEE J Commun Eng. 1946;93:429–457. [Google Scholar]
- 14.Giakas G, Baltzopoulos V. Time and frequency domain analysis of ground reaction forces during walking: an investigation of variability and symmetry. Gait Posture. 1997;5:189–197. doi: 10.1016/S0966-6362(96)01083-1. [DOI] [Google Scholar]
- 15.Gill KP, Callaghan MJ. The measurement of lumbar proprioception in individuals with and without low back pain. Spine. 1998;23:371–377. doi: 10.1097/00007632-199802010-00017. [DOI] [PubMed] [Google Scholar]
- 16.Grabiner MD, Koh TJ, el Ghazawi A. Decoupling of bilateral paraspinal excitation in subjects with low back pain. Spine. 1992;17:1219–1223. doi: 10.1097/00007632-199210000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Hausdorff JM, Mitchell SL, Firtion R, Peng CK, Cudkowicz ME, Wei JY, Goldberger AL. Altered fractal dynamics of gait: reduced stride-interval correlations with aging and Huntington’s disease. J Appl Physiol. 1997;82:262–269. doi: 10.1152/jappl.1997.82.1.262. [DOI] [PubMed] [Google Scholar]
- 18.Hodges PW. Changes in motor planning of feedforward postural responses of the trunk muscles in low back pain. Exp Brain Res. 2001;141:261–266. doi: 10.1007/s002210100873. [DOI] [PubMed] [Google Scholar]
- 19.Hodges PW, Richardson CA. Altered trunk muscle recruitment in people with low back pain with upper limb movement at different speeds. Arch Phys Med Rehabil. 1999;80:1005–1012. doi: 10.1016/S0003-9993(99)90052-7. [DOI] [PubMed] [Google Scholar]
- 20.Hubley-Kozey CL, Vezina MJ. Differentiating temporal electromyographic waveforms between those with chronic low back pain and healthy controls. Clin Biomech. 2002;17:621–629. doi: 10.1016/S0268-0033(02)00103-1. [DOI] [PubMed] [Google Scholar]
- 21.Janda V. Muscles, central nervous motor regulation and back problems. In: Korr IM, editor. The neurobiologic mechanisms in manipulative therapy. New York: Plenum; 1978. pp. 27–41. [Google Scholar]
- 22.Judge JO, Ounpuu S, Davis RB. Effects of age on the biomechanics and physiology of gait. Clin Geriatr Med. 1996;12:659–678. [PubMed] [Google Scholar]
- 23.Keefe FJ, Hill RW. An objective approach to quantifying pain behavior and gait patterns in low back pain patients. Pain. 1985;12:153–161. doi: 10.1016/0304-3959(85)90285-4. [DOI] [PubMed] [Google Scholar]
- 24.Khodadadeh S, Eisenstein SM, Summers B, Patrick J. Gait asymmetry in patients with chronic low back pain. Neuroorthopedics. 1988;6:24–27. [Google Scholar]
- 25.Lamoth CJC, Beek PJ, Meijer OG. Pelvis-thorax coordination in the transverse plane during gait. Gait Posture. 2002;16:101–114. doi: 10.1016/S0966-6362(01)00146-1. [DOI] [PubMed] [Google Scholar]
- 26.Lamoth CJC, Daffertshofer A, Meijer OG, Moseley GL, Wuisman PIJM, Beek PJ. Effects of experimentally induced pain and fear of pain on trunk coordination and back muscle activity during walking. Clin Biomech. 2004;19:551–563. doi: 10.1016/j.clinbiomech.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Lamoth CJC, Meijer OG, Wuisman PIJM, Dieën J, Levin MF, Beek PJ. Pelvis–thorax coordination in the transverse plane during walking in persons with nonspecific low back pain. Spine. 2002;27:E92–E99. doi: 10.1097/00007632-200202150-00016. [DOI] [PubMed] [Google Scholar]
- 28.Lee J, Hoshino Y, Nakamura k, Kariya Y, Saita K, Ito K. Trunk muscle weakness as a risk factor for low back pain. Spine. 1999;24:54–57. doi: 10.1097/00007632-199901010-00013. [DOI] [PubMed] [Google Scholar]
- 29.Lund JP, Donga R, Widmer CG, Stohler CS. The pain-adaptation model: a discussion of the relationship between chronic musculoskeletal pain and motor activity. Can J Physiol Pharmacol. 1991;69:683–694. doi: 10.1139/y91-102. [DOI] [PubMed] [Google Scholar]
- 30.Luoto S, Aalto H, Taimela S, Hurri H, Pyykko I, Alaranta H. One-footed and externally disturbed two-footed postural control in patients with chronic low back pain and healthy control subjects. A controlled study with follow-up. Spine. 1998;23:2081–2090. doi: 10.1097/00007632-199810010-00008. [DOI] [PubMed] [Google Scholar]
- 31.Magnusson ML, Aleksiev AR, Wilder DG, Pope MH, Spratt KF, Lee SH, Goel VK, Weinstein JN. Unexpected load and asymmetric posture as etiologic factors in low back pain. Eur Spine J. 1996;5:23–25. doi: 10.1007/BF00307824. [DOI] [PubMed] [Google Scholar]
- 32.Main CJ, Watson PJ. Guarded movements: development of chronicity. J Musculoskel Pain. 1996;4:163–170. doi: 10.1300/J094v04n04_16. [DOI] [Google Scholar]
- 33.Maki BE. Gait changes in older adults: predictors of falls or indicators of fear. J Am Geriatr Soc. 1997;45:313–320. doi: 10.1111/j.1532-5415.1997.tb00946.x. [DOI] [PubMed] [Google Scholar]
- 34.McGibbon CA, Krebs DE. Age-related changes in lower trunk coordination and energy transfer during gait. J Neurophysiol. 2001;85:1923–1931. doi: 10.1152/jn.2001.85.5.1923. [DOI] [PubMed] [Google Scholar]
- 35.Mientjes MI, Frank JS. Balance in chronic low back pain patients compared to healthy people under various conditions in upright standing. Clin Biomech. 1999;14:710–716. doi: 10.1016/S0268-0033(99)00025-X. [DOI] [PubMed] [Google Scholar]
- 36.O’Sullivan P, Twomey L, Allison G. Altered abdominal muscle recruitment in low back pain patients following specific exercise intervention. J Orthop Sports Phys Ther. 1998;27:114–124. doi: 10.2519/jospt.1998.27.2.114. [DOI] [PubMed] [Google Scholar]
- 37.Panjabi MM. The stabilizing system of the spine. Part I: Function, dysfunction and enhancement. J Spinal Disord. 1992;5:383–389. doi: 10.1097/00002517-199212000-00001. [DOI] [PubMed] [Google Scholar]
- 38.Panjabi MM. The stabilizing system of the spine. Part I: Function, dysfunction and enhancement. J Spinal Disord. 1992;5:390–397. doi: 10.1097/00002517-199212000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Parkhurst TM, Burnett CN. Injury and proprioception in the lower back. J Orthop Sports Phys Ther. 1994;19:282–295. doi: 10.2519/jospt.1994.19.5.282. [DOI] [PubMed] [Google Scholar]
- 40.Pijnappels M, Bobbert MF, Dieën JH. Changes in walking pattern caused by the possibility of a tripping reaction. Gait Posture. 2001;14:11–18. doi: 10.1016/S0966-6362(01)00110-2. [DOI] [PubMed] [Google Scholar]
- 41.Porges SW, Bohrer RE, Cheung MN, Drasgow F, McCabe PM, Keren G. New time-series statistic for detecting rhythmic co-occurrence in the frequency domain: the weighted coherence and its application to psychophysiological research. Psychol Bull. 1980;88:580–587. doi: 10.1037//0033-2909.88.3.580. [DOI] [PubMed] [Google Scholar]
- 42.Radebold A, Cholewicki J, Panjabi MM, Patel TC. Muscle response pattern to sudden trunk loading in healthy individuals and in patients with chronic low back pain. Spine. 2000;25:947–954. doi: 10.1097/00007632-200004150-00009. [DOI] [PubMed] [Google Scholar]
- 43.Riley MA, Turvey MT. Variability of determinism in motor behavior. J Mot Behav. 2002;34:99–125. doi: 10.1080/00222890209601934. [DOI] [PubMed] [Google Scholar]
- 44.Roland M, Morris R. A study of the natural history of back pain. Part I: Development of a reliable and sensitive measure of disability in low back pain. Spine. 1983;8:141–144. doi: 10.1097/00007632-198303000-00004. [DOI] [PubMed] [Google Scholar]
- 45.Schache AG, Blanch PD, Rath DA, Wrigley TV, Starr R, Bennell KL. A comparison of overground and treadmill running for measuring the three-dimensional kinematics of the lumbo-pelvic-hip complex. Clin Biomech. 2001;16:667–680. doi: 10.1016/S0268-0033(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 46.Selles RW, Wagenaar RC, Smit TH, Wuisman PIJM. Disorders in trunk rotation during walking in patients with low back pain: a dynamical systems approach. Clin Biomech. 2001;16:175–181. doi: 10.1016/S0268-0033(00)00080-2. [DOI] [PubMed] [Google Scholar]
- 47.Spenkelink CD, Hutten MM, Hermens HJ, Greitemann BO. Assessment of activities of daily living with an ambulatory monitoring system: a comparative study in patients with chronic low back pain and nonsymptomatic controls. Clin Rehabil. 2002;16:16–26. doi: 10.1191/0269215502cr463oa. [DOI] [PubMed] [Google Scholar]
- 48.Stokes VP, Andersson CA, Forssberg H. Rotational and translational movement features of the pelvis and thorax during adult human locomotion. J Biomech. 1989;22:43–50. doi: 10.1016/0021-9290(89)90183-8. [DOI] [PubMed] [Google Scholar]
- 49.Taimela S, Kujala UM. Reaction times with reference to musculoskeletal complaints in adolescence. Percept Mot Skills. 1992;75:1075–1082. doi: 10.2466/pms.1992.75.3f.1075. [DOI] [PubMed] [Google Scholar]
- 50.Taylor NF, Evans OM, Goldie PA. The effect of walking faster on people with acute low back pain. Eur Spine J. 2003;12:166–172. doi: 10.1007/s00586-002-0498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Thomas LK, Hislop HJ, Waters RL. Physiological work performance in chronic low back disability: effects of a progressive activity program. Phys Ther. 1980;60:407–411. doi: 10.1093/ptj/60.4.407. [DOI] [PubMed] [Google Scholar]
- 52.Thorstensson A, Roberthson H. Adaptations to changing speed in human locomotion: speed of transition between walking and running. Acta Physiol Scand. 1987;131:211–214. doi: 10.1111/j.1748-1716.1987.tb08228.x. [DOI] [PubMed] [Google Scholar]
- 53.Dieën JH, Selen LP, Cholewicki J. Trunk muscle activation in low-back pain patients, an analysis of the literature. J Electromyogr Kinesiol. 2003;13:333–351. doi: 10.1016/S1050-6411(03)00041-5. [DOI] [PubMed] [Google Scholar]
- 54.Emmerik REA, Wagenaar RC. Effects of walking velocity on relative phase dynamics in the trunk in human walking. J Biomech. 1996;29:1175–1184. doi: 10.1016/0021-9290(95)00128-X. [DOI] [PubMed] [Google Scholar]
- 55.Vink P, Daanen HAM, Verbout AJ. Specificity of surface-EMG on the intrinsic lumbar back muscles. Hum Mov Sci. 1989;8:67–78. doi: 10.1016/0167-9457(89)90024-9. [DOI] [Google Scholar]
- 56.Vlaeyen JWS, Kole-Snijders AMJ, Boeren RGB, Goenman NH, Eek van H. Fear of movement/(re)injury in chronic low back pain and its relation to behavioral performance. Pain. 1995;62:363–372. doi: 10.1016/0304-3959(94)00279-N. [DOI] [PubMed] [Google Scholar]
- 57.Vlaeyen JWS, Linton SJ. Fear-avoidance and its consequences in chronic musculoskeletal pain: a state of the art. Pain. 2000;85:317–332. doi: 10.1016/S0304-3959(99)00242-0. [DOI] [PubMed] [Google Scholar]
- 58.Vogt L, Pfeifer K, Banzer W. Comparison of angular lumbar spine and pelvis kinematics during treadmill and overground locomotion. Clin Biomech. 2002;17:162–165. doi: 10.1016/S0268-0033(01)00111-5. [DOI] [PubMed] [Google Scholar]
- 59.Vogt L, Pfeifer K, Banzer W. Neuromuscular control of walking with chronic low-back pain. Man Ther. 2003;8:21–28. doi: 10.1054/math.2002.0476. [DOI] [PubMed] [Google Scholar]
- 60.Vogt L, Pfeifer K, Portscher M, Banzer W. Influences of nonspecific low back pain on three-dimensional lumbar spine kinematics in locomotion. Spine. 2001;26:1910–1919. doi: 10.1097/00007632-200109010-00019. [DOI] [PubMed] [Google Scholar]
- 61.Waddell G. A new clinical model for the treatment of low back pain. Spine. 1987;12:632–644. doi: 10.1097/00007632-198709000-00002. [DOI] [PubMed] [Google Scholar]
- 62.Waters RL, Morris JM. Electrical activity of muscles of the trunk during walking. J Anat. 1972;111:191–199. [PMC free article] [PubMed] [Google Scholar]
- 63.Watson P, Booker C, Main CJ. Evidence for the role of psychological factors in abnormal paraspinal activity in patients with chronic low back pain (CLBP) J Musculoskel Med. 1997;5:41–56. [Google Scholar]
- 64.White SG, McNair PJ. Abdominal and erector spinae muscle activity during gait: the use of cluster analysis to identify patterns of activity. Clin Biomech. 2002;17:177–184. doi: 10.1016/S0268-0033(02)00007-4. [DOI] [PubMed] [Google Scholar]
- 65.Winter DA, Yack HJ. EMG profiles during normal walking: stride-to-stride and inter-subject variability. Electroencephalogr Clin Neurophysiol. 1987;67:402–411. doi: 10.1016/0013-4694(87)90003-4. [DOI] [PubMed] [Google Scholar]
- 66.Wittink H, Rogers W, Gascon C, Sukiennik A, Cynn D, Carr DB. Relative contribution of mental health and exercise-related pain increment to treadmill test intolerance in patients with chronic low back pain. Spine. 2001;26:2368–2374. doi: 10.1097/00007632-200111010-00014. [DOI] [PubMed] [Google Scholar]