Abstract
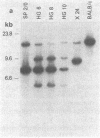
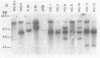
A series of three IgM, kappa monoclonal antibodies arising from a fusion of BALB/c spleen cells from mice immunized with beta-(1,6)-galactan-containing antigens have been analyzed. These three lines were found (i) to have homologous protein sequences in the heavy chain D region and at the sites of recombination between the heavy chain variable and D segment (VH-D) and the D and joining segment (D-JH), although amino acid substitutions were observed in both the heavy and light chain variable regions; (ii) to use identical heavy and light chain joining segments; and (iii) to demonstrate two identical (productive and nonproductive) kappa-chain rearrangements. A likely explanation for these observations is that the three lines are clonally related (arise from a common precursor) and that the observed heavy and light chain variable segment substitutions represent somatic point mutations. Because these antibodies are all of the IgM class, the results indicate that a somatic mutational mechanism is activated early in B-cell ontogeny and operates at both the heavy and light chain loci. Furthermore, the somatic mutation process appears to continue during the development of a given cell line, but is independent of class switching.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentley D. L., Rabbitts T. H. Evolution of immunoglobulin V genes: evidence indicating that recently duplicated human V kappa sequences have diverged by gene conversion. Cell. 1983 Jan;32(1):181–189. doi: 10.1016/0092-8674(83)90508-1. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Heavy chain variable region contribution to the NPb family of antibodies: somatic mutation evident in a gamma 2a variable region. Cell. 1981 Jun;24(3):625–637. doi: 10.1016/0092-8674(81)90089-1. [DOI] [PubMed] [Google Scholar]
- Cesari I. M., Weigert M. Mouse lambda-chain sequences. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2112–2116. doi: 10.1073/pnas.70.7.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S. P., Brown M., Rittenberg M. B. Immunologic memory to phosphorylcholine III. IgM includes a fine specificity population distinct from TEPC 15. J Immunol. 1982 Oct;129(4):1559–1562. [PubMed] [Google Scholar]
- Clarke S. H., Claflin J. L., Potter M., Rudikoff S. Polymorphism in anti-phosphocholine antibodies reflecting evolution of immunoglobulin families. J Exp Med. 1983 Jan 1;157(1):98–113. doi: 10.1084/jem.157.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S. H., Claflin J. L., Rudikoff S. Polymorphism in immunoglobulin heavy chains suggesting gene conversion. Proc Natl Acad Sci U S A. 1982 May;79(10):3280–3284. doi: 10.1073/pnas.79.10.3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Rudikoff S., Giusti A. M., Scharff M. D. Somatic mutation in a cultured mouse myeloma cell affects antigen binding. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1240–1244. doi: 10.1073/pnas.79.4.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory S., Tyler B. M., Adams J. M. Sets of immunoglobulin V kappa genes homologous to ten cloned V kappa sequences: implications for the number of germline V kappa genes. J Mol Appl Genet. 1981;1(2):103–116. [PubMed] [Google Scholar]
- Crews S., Griffin J., Huang H., Calame K., Hood L. A single VH gene segment encodes the immune response to phosphorylcholine: somatic mutation is correlated with the class of the antibody. Cell. 1981 Jul;25(1):59–66. doi: 10.1016/0092-8674(81)90231-2. [DOI] [PubMed] [Google Scholar]
- Dildrop R., Brüggemann M., Radbruch A., Rajewsky K., Beyreuther K. Immunoglobulin V region variants in hybridoma cells. II. Recombination between V genes. EMBO J. 1982;1(5):635–640. doi: 10.1002/j.1460-2075.1982.tb01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Feldmann R. J., Potter M., Glaudemans C. P. A hypothetical space-filling model of the V-regions of the galactan-binding myeloma immunoglobulin J539. Mol Immunol. 1981 Aug;18(8):683–698. doi: 10.1016/0161-5890(81)90060-2. [DOI] [PubMed] [Google Scholar]
- Gearhart P. J., Bogenhagen D. F. Clusters of point mutations are found exclusively around rearranged antibody variable genes. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3439–3443. doi: 10.1073/pnas.80.11.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhart P. J., Johnson N. D., Douglas R., Hood L. IgG antibodies to phosphorylcholine exhibit more diversity than their IgM counterparts. Nature. 1981 May 7;291(5810):29–34. doi: 10.1038/291029a0. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Bernard O. Sequences of the joining region genes for immunoglobulin heavy chains and their role in generation of antibody diversity. Proc Natl Acad Sci U S A. 1981 Jan;78(1):509–513. doi: 10.1073/pnas.78.1.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Loh E., Hubert J., Barstad P., Eaton B., Early P., Fuhrman J., Johnson N., Kronenberg M., Schilling J. The structure and genetics of mouse immunoglobulins: an analysis of NZB myeloma proteins and sets of BALB/c myeloma proteins binding particular haptens. Cold Spring Harb Symp Quant Biol. 1977;41(Pt 2):817–836. doi: 10.1101/sqb.1977.041.01.092. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Glaudemans C. P., Rudikoff S., Potter M. Structural requirements for the binding of derivatives of D-galactose to two homogeneous murine immunoglobulins. Biochemistry. 1974 Jul 16;13(15):3179–3184. doi: 10.1021/bi00712a028. [DOI] [PubMed] [Google Scholar]
- Jolley M. E., Rudikoff S., Potter M., Glaudemans C. P. Spectral changes on binding of oligosaccharides to murine immunoglobulin A myeloma proteins. Biochemistry. 1973 Jul 31;12(16):3039–3044. doi: 10.1021/bi00740a015. [DOI] [PubMed] [Google Scholar]
- Kim S., Davis M., Sinn E., Patten P., Hood L. Antibody diversity: somatic hypermutation of rearranged VH genes. Cell. 1981 Dec;27(3 Pt 2):573–581. doi: 10.1016/0092-8674(81)90399-8. [DOI] [PubMed] [Google Scholar]
- Kocher H. P., Berek C., Jaton J. C. The immune response of BALB/c mice to phosphorylcholine is restricted to a limited number of VH- and VL-isotypes. Mol Immunol. 1981 Dec;18(12):1027–1033. doi: 10.1016/0161-5890(81)90018-3. [DOI] [PubMed] [Google Scholar]
- Kurosawa Y., Tonegawa S. Organization, structure, and assembly of immunoglobulin heavy chain diversity DNA segments. J Exp Med. 1982 Jan 1;155(1):201–218. doi: 10.1084/jem.155.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushinski E. B., Potter M. Idiotypes on galactan binding myeloma proteins and anti-galactan antibodies in mice. J Immunol. 1977 Dec;119(6):1888–1893. [PubMed] [Google Scholar]
- Navia M. A., Segal D. M., Padlan E. A., Davies D. R., Rao N., Rudikoff S., Potter M. Crystal structure of galactan-binding mouse immunoglobulin J539 Fab at 4.5-A resolution. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4071–4074. doi: 10.1073/pnas.76.8.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollo R., Auffray C., Sikorav J. L., Rougeon F. Mouse heavy chain variable regions: nucleotide sequence of a germ-line VH gene segment. Nucleic Acids Res. 1981 Aug 25;9(16):4099–4109. doi: 10.1093/nar/9.16.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlita M., Potter M., Rudikoff S. kappa-Chain restriction in anti-galactan antibodies. J Immunol. 1982 Aug;129(2):615–618. [PubMed] [Google Scholar]
- Potter M. Antigen-binding myeloma proteins of mice. Adv Immunol. 1977;25:141–211. [PubMed] [Google Scholar]
- Radbruch A., Liesegang B., Rajewsky K. Isolation of variants of mouse myeloma X63 that express changed immunoglobulin class. Proc Natl Acad Sci U S A. 1980 May;77(5):2909–2913. doi: 10.1073/pnas.77.5.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao D. N., Rudikoff S., Krutzsch H., Potter M. Structural evidence for independent joining region gene in immunoglobulin heavy chains from anti-galactan myeloma proteins and its potential role in generating diversity in complementarity-determining regions. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2890–2894. doi: 10.1073/pnas.76.6.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudikoff S., Giusti A. M., Cook W. D., Scharff M. D. Single amino acid substitution altering antigen-binding specificity. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1979–1983. doi: 10.1073/pnas.79.6.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudikoff S. Immunoglobulin structure--function correlates: antigen binding and idiotypes. Contemp Top Mol Immunol. 1983;9:169–209. doi: 10.1007/978-1-4684-4517-6_6. [DOI] [PubMed] [Google Scholar]
- Rudikoff S., Pawlita M., Pumphrey J., Mushinski E., Potter M. Galactan-binding antibodies. Diversity and structure of idiotypes. J Exp Med. 1983 Nov 1;158(5):1385–1400. doi: 10.1084/jem.158.5.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudikoff S., Rao D. N., Glaudemans C. P., Potter M. kappa Chain joining segments and structural diversity of antibody combining sites. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4270–4274. doi: 10.1073/pnas.77.7.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Sakano H., Maki R., Kurosawa Y., Roeder W., Tonegawa S. Two types of somatic recombination are necessary for the generation of complete immunoglobulin heavy-chain genes. Nature. 1980 Aug 14;286(5774):676–683. doi: 10.1038/286676a0. [DOI] [PubMed] [Google Scholar]
- Seidman J. G., Leder A., Nau M., Norman B., Leder P. Antibody diversity. Science. 1978 Oct 6;202(4363):11–17. doi: 10.1126/science.99815. [DOI] [PubMed] [Google Scholar]
- Selsing E., Storb U. Somatic mutation of immunoglobulin light-chain variable-region genes. Cell. 1981 Jul;25(1):47–58. doi: 10.1016/0092-8674(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Weigert M., Gatmaitan L., Loh E., Schilling J., Hood L. Rearrangement of genetic information may produce immunoglobulin diversity. Nature. 1978 Dec 21;276(5690):785–790. doi: 10.1038/276785a0. [DOI] [PubMed] [Google Scholar]
- Weigert M., Perry R., Kelley D., Hunkapiller T., Schilling J., Hood L. The joining of V and J gene segments creates antibody diversity. Nature. 1980 Jan 31;283(5746):497–499. doi: 10.1038/283497a0. [DOI] [PubMed] [Google Scholar]