Abstract
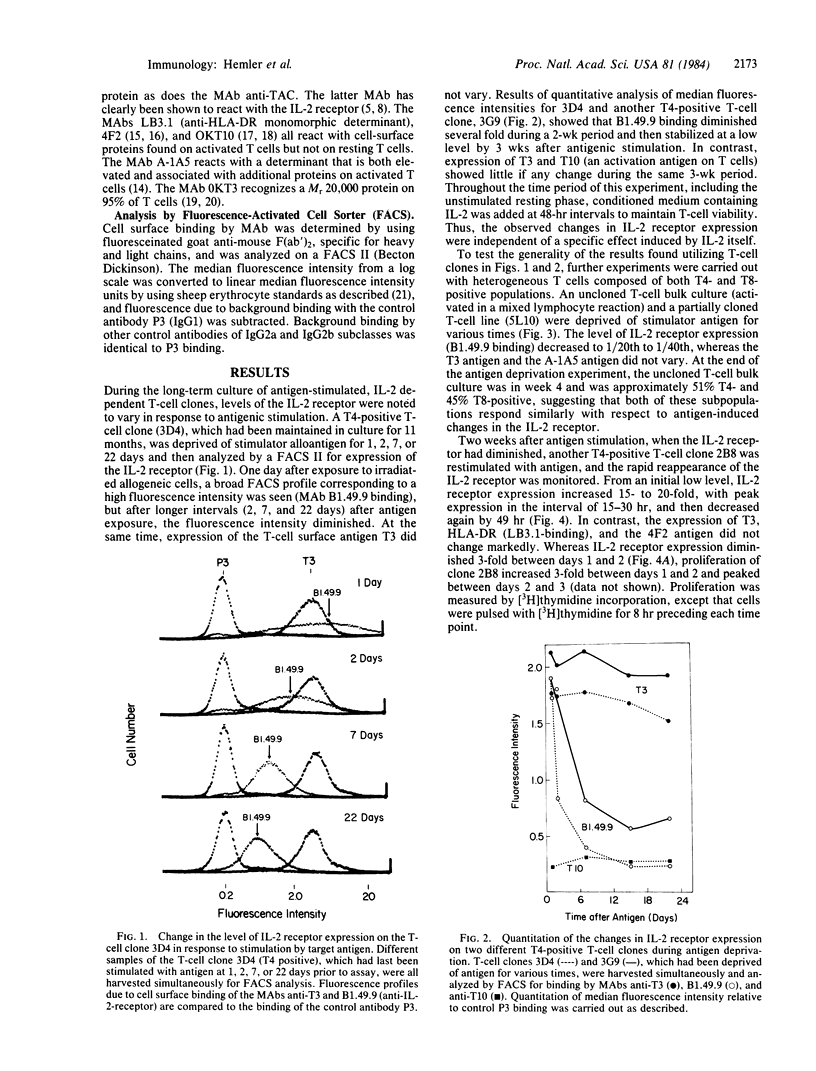
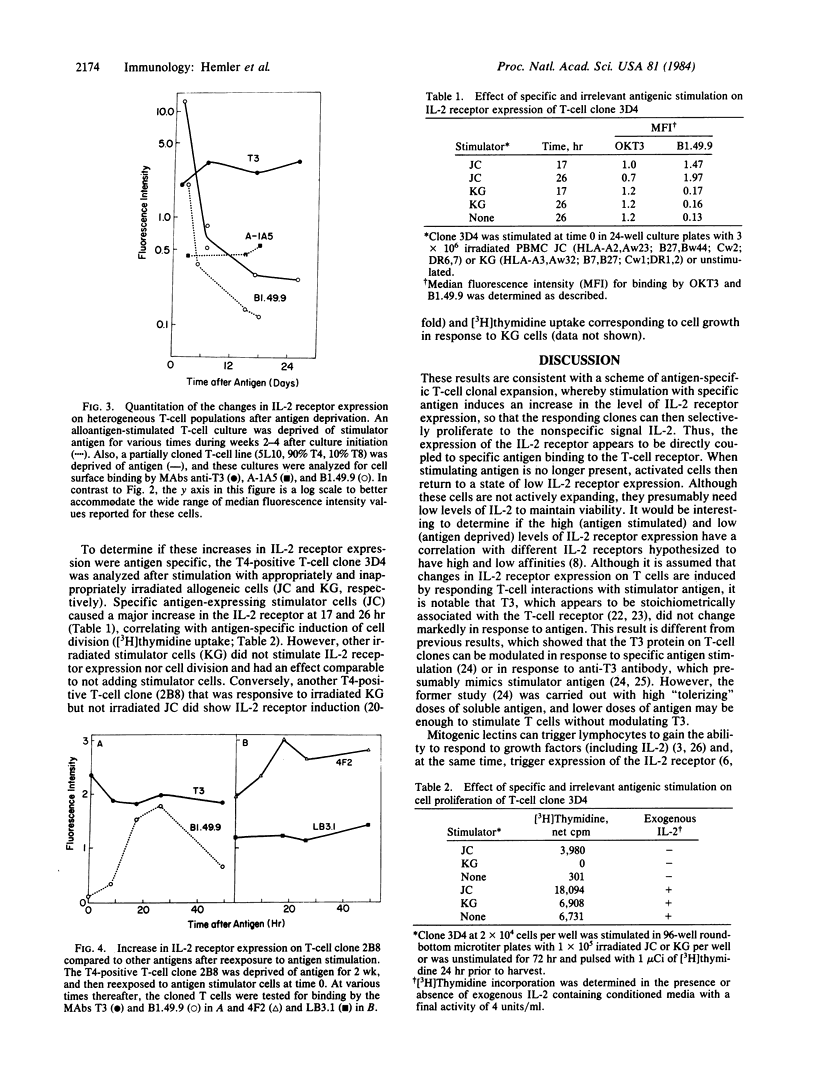
Antigen-specific, interleukin 2 (IL-2)-dependent human T-cell lines and clones were utilized to study the relationship between IL-2 receptor expression and antigenic stimulation. T cells that had not been exposed to antigen for 2 wk or more expressed a stable low level of the IL-2 receptor. After reexposure to antigen, a 10- to 30-fold increase in the level of the IL-2 receptor was rapidly induced, with the peak level of IL-2 receptor expression occurring at 15-30 hr. This peak preceded the peak in cell proliferation [( 3H]thymidine incorporation), which was at 48-72 hr. Within 2-14 days after peak IL-2 receptor expression, it returned to a low base-line level. The transient elevation in IL-2 receptor level was antigen specific because it occurred in response to specific allogeneic stimulator cells but not after exposure to cells expressing irrelevant HLA allotypes. The levels of other cell-surface proteins, including those related to T-cell activation (HLA-DR, T10, 4F2, A-1A5) as well as T3, which has been proposed to be a component of the T-cell receptor complex for antigen, did not change in response to antigen exposure or deprivation. Because IL-2 was maintained at a consistently high level throughout these experiments, the antigen-induced changes in the IL-2 receptor appear to be independent of changes induced by IL-2 itself. Both cloned T cells and mixed populations containing T4 and T8 subsets showed similar IL-2 receptor responsiveness, indicating that this finding is generalizable to most, if not to all, antigen-responsive T cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abo T., Miller C. A., Balch C. M., Cooper M. D. Interleukin 2 receptor expression by activated HNK-1+ granular lymphocytes: a requirement for their proliferation. J Immunol. 1983 Oct;131(4):1822–1826. [PubMed] [Google Scholar]
- Cotner T., Hemler M., Strominger J. L. Human T cell proteins recognized by rabbit heteroantisera and monoclonal antibodies. Int J Immunopharmacol. 1981;3(3):255–268. doi: 10.1016/0192-0561(81)90019-9. [DOI] [PubMed] [Google Scholar]
- Cotner T., Mashimo H., Kung P. C., Goldstein G., Strominger J. L. Human T cell surface antigens bearing a structural relationship to HLA antigens. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3858–3862. doi: 10.1073/pnas.78.6.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotner T., Williams J. M., Christenson L., Shapiro H. M., Strom T. B., Strominger J. Simultaneous flow cytometric analysis of human T cell activation antigen expression and DNA content. J Exp Med. 1983 Feb 1;157(2):461–472. doi: 10.1084/jem.157.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWolf W. C., Schlossman S. F., Yunis E. J. DRw antisera react with activated T cells. J Immunol. 1979 May;122(5):1780–1784. [PubMed] [Google Scholar]
- Evans R. L., Faldetta T. J., Humphreys R. E., Pratt D. M., Yunis E. J., Schlossman S. F. Peripheral human T cells sensitized in mixed leukocyte culture synthesize and express Ia-like antigens. J Exp Med. 1978 Nov 1;148(5):1440–1445. doi: 10.1084/jem.148.5.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis S., Watson J. Interleukin-2 dependent culture of cytolytic T cell lines. Immunol Rev. 1981;54:81–109. doi: 10.1111/j.1600-065x.1981.tb00435.x. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Verbi W., Festenstein H., Papasteriadis C., Jaraquemada D., Hayward A. "Ia-like" antigens on human T cells. Eur J Immunol. 1979 May;9(5):356–362. doi: 10.1002/eji.1830090504. [DOI] [PubMed] [Google Scholar]
- Grimm E. A., Robb R. J., Roth J. A., Neckers L. M., Lachman L. B., Wilson D. J., Rosenberg S. A. Lymphokine-activated killer cell phenomenon. III. Evidence that IL-2 is sufficient for direct activation of peripheral blood lymphocytes into lymphokine-activated killer cells. J Exp Med. 1983 Oct 1;158(4):1356–1361. doi: 10.1084/jem.158.4.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes B. F., Hemler M. E., Mann D. L., Eisenbarth G. S., Shelhamer J., Mostowski H. S., Thomas C. A., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (4F2) that binds to human monocytes and to a subset of activated lymphocytes. J Immunol. 1981 Apr;126(4):1409–1414. [PubMed] [Google Scholar]
- Haynes B. F., Hemler M., Cotner T., Mann D. L., Eisenbarth G. S., Strominger J. L., Fauci A. S. Characterization of a monoclonal antibody (5E9) that defines a human cell surface antigen of cell activation. J Immunol. 1981 Jul;127(1):347–351. [PubMed] [Google Scholar]
- Helderman J. H., Reynolds T. C., Strom T. B. The insulin receptor as a universal marker of activated lymphocytes. Eur J Immunol. 1978 Aug;8(8):589–595. doi: 10.1002/eji.1830080810. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Malissen B., Rebai N., Liabeuf A., Mawas C., Kourilsky F. M., Strominger J. L. A 55,000 Mr surface antigen on activated human T lymphocytes defined by a monoclonal antibody. Hum Immunol. 1983 Oct;8(2):153–165. doi: 10.1016/0198-8859(83)90010-1. [DOI] [PubMed] [Google Scholar]
- Hemler M. E., Strominger J. L. Characterization of antigen recognized by the monoclonal antibody (4F2): different molecular forms on human T and B lymphoblastoid cell lines. J Immunol. 1982 Aug;129(2):623–628. [PubMed] [Google Scholar]
- Hemler M. E., Ware C. F., Strominger J. L. Characterization of a novel differentiation antigen complex recognize by a monoclonal antibody (A-1A5): unique activation-specific molecular forms on stimulated T cells. J Immunol. 1983 Jul;131(1):334–340. [PubMed] [Google Scholar]
- Ko H. S., Fu S. M., Winchester R. J., Yu D. T., Kunkel H. G. Ia determinants on stimulated human T lymphocytes. Occurrence on mitogen- and antigen-activated T cells. J Exp Med. 1979 Aug 1;150(2):246–255. doi: 10.1084/jem.150.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung P., Goldstein G., Reinherz E. L., Schlossman S. F. Monoclonal antibodies defining distinctive human T cell surface antigens. Science. 1979 Oct 19;206(4416):347–349. doi: 10.1126/science.314668. [DOI] [PubMed] [Google Scholar]
- Kürzinger K., Reynolds T., Germain R. N., Davignon D., Martz E., Springer T. A. A novel lymphocyte function-associated antigen (LFA-1): cellular distribution, quantitative expression, and structure. J Immunol. 1981 Aug;127(2):596–602. [PubMed] [Google Scholar]
- Larsson E. L., Coutinho A., Martinez C. A suggested mechanism for T lymphocyte activation: implications on the acquisition of functional reactivities. Immunol Rev. 1980;51:61–91. doi: 10.1111/j.1600-065x.1980.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Malek T. R., Robb R. J., Shevach E. M. Identification and initial characterization of a rat monoclonal antibody reactive with the murine interleukin 2 receptor-ligand complex. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5694–5698. doi: 10.1073/pnas.80.18.5694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Acuto O., Hussey R. E., Hodgdon J. C., Fitzgerald K. A., Schlossman S. F., Reinherz E. L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature. 1983 Jun 30;303(5920):808–810. doi: 10.1038/303808a0. [DOI] [PubMed] [Google Scholar]
- Mier J. W., Gallo R. C. Purification and some characteristics of human T-cell growth factor from phytohemagglutinin-stimulated lymphocyte-conditioned media. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6134–6138. doi: 10.1073/pnas.77.10.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R. S., Rao P. E., Talle M. A., Look R., Goldstein G. Cell membrane perturbation of resting T cells and thymocytes causes display of activation antigens. J Exp Med. 1983 Jul 1;158(1):99–111. doi: 10.1084/jem.158.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan D. A., Ruscetti F. W., Gallo R. Selective in vitro growth of T lymphocytes from normal human bone marrows. Science. 1976 Sep 10;193(4257):1007–1008. doi: 10.1126/science.181845. [DOI] [PubMed] [Google Scholar]
- Popovic M., Lange-Wantzin G., Sarin P. S., Mann D., Gallo R. C. Transformation of human umbilical cord blood T cells by human T-cell leukemia/lymphoma virus. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5402–5406. doi: 10.1073/pnas.80.17.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Acuto O., Schlossman S. F. Comparison of T3-associated 49- and 43-kilodalton cell surface molecules on individual human T-cell clones: evidence for peptide variability in T-cell receptor structures. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4104–4108. doi: 10.1073/pnas.80.13.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Greene W. C. Direct demonstration of the identity of T cell growth factor binding protein and the Tac antigen. J Exp Med. 1983 Oct 1;158(4):1332–1337. doi: 10.1084/jem.158.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. A. T-cell growth factor. Immunol Rev. 1980;51:337–357. doi: 10.1111/j.1600-065x.1980.tb00327.x. [DOI] [PubMed] [Google Scholar]
- Terhorst C., van Agthoven A., LeClair K., Snow P., Reinherz E., Schlossman S. Biochemical studies of the human thymocyte cell-surface antigens T6, T9 and T10. Cell. 1981 Mar;23(3):771–780. doi: 10.1016/0092-8674(81)90441-4. [DOI] [PubMed] [Google Scholar]
- Trowbridge I. S., Omary M. B. Human cell surface glycoprotein related to cell proliferation is the receptor for transferrin. Proc Natl Acad Sci U S A. 1981 May;78(5):3039–3043. doi: 10.1073/pnas.78.5.3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama T., Broder S., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. I. Production of anti-Tac monoclonal antibody and distribution of Tac (+) cells. J Immunol. 1981 Apr;126(4):1393–1397. [PubMed] [Google Scholar]
- Uchiyama T., Nelson D. L., Fleisher T. A., Waldmann T. A. A monoclonal antibody (anti-Tac) reactive with activated and functionally mature human T cells. II. Expression of Tac antigen on activated cytotoxic killer T cells, suppressor cells, and on one of two types of helper T cells. J Immunol. 1981 Apr;126(4):1398–1403. [PubMed] [Google Scholar]
- Yachie A., Miyawaki T., Uwadana N., Ohzeki S., Taniguchi N. Sequential expression of T cell activation (Tac) antigen and Ia determinants on circulating human T cells after immunization with tetanus toxoid. J Immunol. 1983 Aug;131(2):731–735. [PubMed] [Google Scholar]
- Zanders E. D., Lamb J. R., Feldmann M., Green N., Beverley P. C. Tolerance of T-cell clones is associated with membrane antigen changes. Nature. 1983 Jun 16;303(5918):625–627. doi: 10.1038/303625a0. [DOI] [PubMed] [Google Scholar]
- van Agthoven A., Terhorst C., Reinherz E., Schlossman S. Characterization of T cell surface glycoproteins T 1 and T 3 present on all human peripheral T lymphocytes and functionally mature thymocytes. Eur J Immunol. 1981 Jan;11(1):18–21. doi: 10.1002/eji.1830110105. [DOI] [PubMed] [Google Scholar]


