Abstract
Objective
To evaluate the efficacy of emotional support and counselling combined with placebo or antidepressants with single or dual mechanism of action in the treatment of depression in primary care.
Design
Randomised double blind study.
Setting
Several locations in Norway.
Subjects
372 patients with depression.
Main outcome measures
Improvement (clinical remission) reported both by the patient (Montgomery Åsberg depression rating scale) and the physician (clinical global improvement and impression scales).
Results
Intention to treat analyses showed 47% remission in patients randomised to placebo compared with 61% remission in patients randomised to sertraline (odds ratio 0.56, 95% confidence interval 0.33 to 0.96) and 54% in patients randomised to mianserin (0.75, 0.44 to 1.27). Women responded better than men (1.86, 1.17 to 2.96). Subgroup analyses showed that subjects with recurrent depression (n=273) responded more frequently to sertraline than to placebo (0.43, 0.23 to 0.82) than those having their first episode of depression (1.18, 0.39 to 3.61). Statistically significant interactions between type of drug treatment and history of depression were not shown by logistic regression.
Conclusion
The combination of active drug and simple psychological treatment (counselling, emotional support, and close follow up over a 24 week period) was more effective than simple psychological treatment alone, in particular for those with recurrent depression. Overall, women may benefit more than men. If confirmed in future studies, the findings should lead to more differentiated treatment guidelines for depression in primary care.
Key messages
The effectiveness of simple psychological treatment and active drug provided by general practitioners is comparable to treatment results reported by psychiatrists and clinical psychologists
Treatment benefits women more than men
There may be differences in response to treatment depending on the nature of depression
A 6 month treatment period is necessary to evaluate effectiveness of treatments for depression in general practice
The development of more differentiated treatment guidelines for depression in primary care is needed
Introduction
None of the previously randomised controlled drug trials for the acute management of depression in primary care settings is representative of the heterogeneous patient populations in this setting.1,2 Of key concern for generalisability of these studies is their short duration; the exclusion of patients with concurrent medical disorders; the exclusion of patients responding to placebo before the study, the use of research criteria of a major depressive episode as used by American psychiatrists, and use of non-significant clinical symptom loads.3 Furthermore, the 50% reduction in rating scale scores used in most studies as a response criterion is very different from the response criteria used by general practitioners, which emphasise both patient reports and own clinical judgment. We aimed to overcome these limitations by applying a naturalistic design to a 24 week randomised double blind study of the efficacy of three different treatments for mild to moderate depression in patients in general practice.
Subjects and methods
Sixty one general practitioners with a caseload of 50-110 patients a week surveyed consecutive patients in their own practice for eligibility to the study (see box). If the patients were eligible, the general practitioners applied the Montgomery Åsberg depression rating scale4 and the clinical global impression of severity scale,5 completed a checklist for Diagnostic and Statistical Manual of Mental Disorders, third edition, revised (DSM-III-R) and ICD-10 (international classification of diseases, 10th revision) research criteria for depression.6,7 The general practitioners were trained to be reliable raters (12 test cases; intraclass correlation (1.1) ⩾0.70). Eligible patients were screened for clinical and biochemical abnormalities (box).
Criteria for eligibility of subjects to study
Inclusion criteria
Aged 18-79 years
Symptoms suggestive of a depressive disorder lasting for at last 2 weeks before consultation
Depression severe enough to require treatment beyond simple explanation and reassurance
Depression of at least mild severity (score of at least 3 on clinical global impression of severity scale of index episode)
Score of at least 20 on the Montgomery Åsberg depression rating scale
<25% reduction in score on the Montgomery Åsberg depression rating scale over a 1 week observation period
Exclusion criteria
Depressive symptoms due to:
Dementia
Schizophrenia
Bipolar disorder
Organic mental disorder (for example, hypothyroidism or anaemia)
Current episode:
Score of >40 on the Montgomery Åsberg depression rating scale
Psychotic symptoms (score of 5-6 on item 9 of the Montgomery Åsberg depression rating scale)
Severe suicidal ideation (score of 4-6 on item 10 of the Montgomery Åsberg depression rating scale)
Non-responding to adequate treatment (for example, amitriptyline 150 mg daily or equivalent for at least 8 weeks)
Condition exceeded 1 year
Psychiatric history:
Previously failed to respond to either a selective serotonin reuptake inhibitor or mianserin
Current alcoholism
Misuse of any of the active treatment drugs
Physical health:
Myocardial infarction within the past 3 months
Epilepsy treated with anticonvulsives known to have antidepressant effects
Clinically significant hypotension
Inability to provide informed consent:
Weak motivation or another serious emotional or intellectual problem that would invalidate informed consent or that would impair the patient's ability to follow the requirements of the study protocol
Unwillingness to use safe contraceptive measures
Assuming a type 1 error of α=0.05 and β=0.95 (statistical power) the sample size needed to detect a 25% difference in effect was estimated to be 98 patients in each group.8 Overall, 372 subjects met all study selection criteria (table 1). Two of the subjects did not attend the first assessment after randomisation (one patient each randomised to sertraline and to mianserin). These subjects were included in the intention to treat analysis as non-responders but excluded from all analyses looking at change from the baseline assessment. Table 2 shows the patients’ clinical characteristics. Only 65 (18%) subjects were considered profoundly depressed on the clinical global impression scale.
Table 1.
Sociodemographic and basic medical characteristics at randomisation in 372 patients with depression. Values are numbers (percentages) of patients unless stated otherwise
| Characteristic | Sertraline (n=122) | Mianserin (n=121) | Placebo (n=129) |
|---|---|---|---|
| Mean (SD) age in years | 48.6 (12.7) | 48.2 (12.9) | 47.8 (11.2) |
| Women | 94 (78) | 83 (70) | 92 (71) |
| Men | 27 (22) | 37 (30) | 37 (29) |
| Concurrent physical disorder | 61 (50) | 53 (44) | 64 (50) |
| Concomitant use of prescribed drugs at inclusion | 58 (48)* | 38 (32) | 46 (36) |
P<0.05 sertraline v placebo.
Table 2.
Psychiatric characteristics at randomisation in 372 patients with depression. Values are numbers (percentages) of patients unless stated otherwise
| Characteristic | Sertraline (n=122) | Mianserin (n=121) | Placebo (n=129) | P value |
|---|---|---|---|---|
| Mean (SD) Montgomery Åsberg depression rating scale | 26.8 (4.4) | 26.8 (4.5) | 26.5 (4.0) | 0.79 |
| Mean (SD) clinical global impression of severity scale | 4.1 (0.65) | 4.0 (0.60) | 4.0 (0.58) | 0.40 |
| Major depression (according to DSM-III-R*) | 107 (89) | 104 (87) | 111 (86) | 0.90 |
| Melancholia | 28 (23) | 25 (21) | 25 (20) | 0.78 |
| Double depression† | 16 (13) | 17 (14) | 24 (19) | 0.43 |
| Previous hospitalisations for depression | 12 (10) | 8 (8) | 10 (8) | 0.74 |
| Previous depressive episode | 94 (78) | 87 (73) | 92 (71) | 0.53 |
Diagnostic and Statistical Manual of Mental Disorders, third edition, revised.
Persistent dysthymic disorder and a current episode of depression.
Design
No placebo run-in took place. Psychotropic drugs were stopped 1-2 weeks before randomisation. Most general practitioners treated six patients: two patients with placebo, two with mianserin, and two with sertraline (five physicians treated 3, 9, or 12 patients). Within each block the three treatments occurred randomly. The general practitioner, the consultants, and the steering committee did not know the randomisation codes. After randomisation, patients were seen after 1, 2, 3, 4, 6, 8, 12, 16, 20, and 24 weeks of treatment. A modified version of a physician administered rating scale for side effects of drugs was applied at baseline and at weeks 8 and 24.9
Psychological treatment
The general practitioners were instructed to convey a sense of hope and optimism, establishing a positive relationship with the patient in the context of a thorough discussion of the course of the present as well as possible previous episodes of depressive illness.10 Patients were given the opportunity to describe their depressive feelings and share their fears and doubts. Simple suggestions such as advising increased physical activity were included. Specific organised systems of psychotherapy (for example, cognitive behavioural therapy) were not allowed.
Study drugs
Sertraline and mianserin have different receptor profiles. Sertraline is a potent and selective inhibitor of 5-hydroxytryptamine (5-HT or serotonin) reuptake. Mianserin, closely related to mirtazapine, affects serotonin metabolism through antagonism of postsynaptic 5-HT2 and 5-HT3 receptors and indirectly by antagonising the inhibitory effects of presynaptic β2 receptors on serotonergic neurotransmission.
Sertraline treatment was initiated at 50 mg/day with forced titration to 100 mg during the third week. If the patient did not show at least some improvement, the dose of sertraline was increased to 150 mg/day after 4 weeks and to a maximum of 200 mg/day at 6 weeks. Corresponding to guidelines for Norwegian general practice, the starting dose of mianserin was 30 mg/day, which was increased to 60 mg/day after 1 week in all patients. If the patient did not respond after 4 weeks, the dose was increased to 90 mg/day, with a further increase to 120 mg/day after 6 weeks if required. No psychoactive drug, with the exception of nitrazepam at night, was allowed.
Control of compliance
Compliance was assessed by counting pills and by measurement of active drug plasma concentrations (gas chromatography) at weeks 8 and 24 or at dropout. The mean doses for patients in the intention to treat group were 144.6 mg sertraline and 78.0 mg mianserin.
Attrition
Of the 372 patients randomised, 259 (70%) completed 16 weeks of treatment and 238 (64%) completed the whole study. Because of lack of efficacy, patients taking placebo dropped out more frequently (29%) than those patients taking sertraline (16%) or mianserin (14%) (P=0.008). The dropout rate as a result of side effects was 10% in patients taking sertraline, 15% in those taking mianserin, and 5% in those taking placebo (P=0.04). One patient committed suicide while being treated with sertraline.
Outcome criteria and statistical analyses
A clinically significant response to treatment was characterised by all three of the following variables: at least a 50% reduction of the total score on the Montgomery Åsberg depression rating scale compared with baseline; a clinical global impression rating of 1, 2, or 3 (no or mild illness), and clinical global impression improvement rating of 1 or 2 (much or very much improved). For statistical analyses we used spss (Release 6.0); odds ratios and Cornfield's confidence intervals for cross tabulations were calculated using Epi-Info (Version 5.01b). We chose as our end point the assessment 24 weeks after randomisation or the last observation after randomisation if the patient ended the treatment prematurely.
Quality assurance and ethics
All patients gave their written informed consent to participate in the study. No patient was paid for participation in the study, which followed the Norwegian guidelines for good clinical trial practice.11 Our study protocol was approved by the research committee of the general practitioner branch of the Norwegian Medical Association, the ethics committee, and the Norwegian data inspectorate.
Results
A significantly greater proportion of patients completely responded to sertraline than to either mianserin or placebo (table 3). Among those with at least one assessment after baseline, the mean change in scores on the Montgomery Åsberg depression rating scale (n=370) were –14.9 (SD 10.3), –15.5 (9.1), and –12.5 (10.0) for sertraline, mianserin, and placebo respectively (F=3.38; P=0.034). Of the 326 patients with major depression according to DSM-III-R, the efficacy of active treatments were less clear (sertraline versus placebo: odds ratio 0.63, 95% confidence interval 0.36 to 1.11; mianserin versus placebo: 0.83, 0.47 to 1.47).
Table 3.
Odd ratios with 95% confidence intervals for 372 patients with depression responding completely to treatment. All patients included in intention to treat analysis
| Treatment regimen | No (%) responding | Odds ratio
|
|
|---|---|---|---|
| Treatment v placebo (95% CI) |
Treatment v mianserin (95% CI) |
||
| Good clinical management plus | |||
| Sertraline (n=122) | 74 (61) | 0.56 (0.33 to 0.96) | 0.75 (0.44 to 1.29) |
| Mianserin (n=121) | 65 (54) | 0.75 (0.44 to 1.27) | — |
| Placebo (n=129) | 60 (47) | — | — |
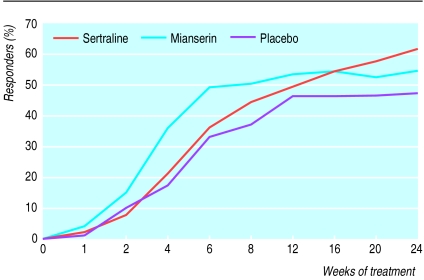
The figure shows a faster initial response to mianserin than to sertraline or placebo, but by week 8 this difference was no longer statistically significant and treatments were comparable. By week 12 a tendency for the superiority of sertraline emerged, which was statistically significant at the end of the study. The percentage of patients responding to sertraline continued to improve with prolonged treatment in contrast with a plateau effect seen with the other two treatments.
Subgroup analyses
Women responded better than men (1.86, 1.17 to 2.96). Although some significant results were found within other subgroups (table 4), logistic regression analyses failed to show any significant interaction between predictors and treatment group—that is, treatment group × sex, treatment group × previous depressive episode, and treatment group × melancholia.
Table 4.
Complete response to treatment and depression history in 370 patients*. Values are numbers (percentages) of patients unless stated otherwise
| Treatment regimen | First episode (n=97)
|
Recurrent episode (n=273)
|
|||
|---|---|---|---|---|---|
| Response | Odds ratio v placebo | Response | Odds ratio v placebo | ||
| Good clinical management plus | |||||
| Sertraline | 12 (44) | 1.18 (0.39 to 3.61) | 62 (66) | 0.43 (0.23 to 0.82) | |
| Mianserin | 21 (64) | 0.54 (0.19 to 1.56) | 44 (51) | 0.82 (0.44 to 1.54) | |
| Placebo | 18 (49) | — | 42 (46) | — | |
Two patients were excluded for not showing up for first assessment after randomisation.
Discussion
Our study has several unique features: a large number of subjects, reliable ratings, inclusion of patients with concomitant physical illness, no exclusion because of placebo response, low attrition rate, serum concentration measurements to control for compliance, duration of 6 months, a simple psychological treatment close to the reality of primary care, and clinical meaningful criterion for response.
Our findings challenge current guidelines2,12,13 that claim equality of effect between drug and psychological treatment in mild to moderate depression in primary care. Although the remission rate in patients given emotional support and counselling plus placebo (47%) was comparable with the intention to treat results in psychotherapy studies,2 the combination of active drug and psychological treatment yielded higher response rates.
The failure to show significant interaction between predictor and treatment variables, however, do not justify confirmative conclusions about preferred treatment. Future studies with a more equal sample size for assessing the prevalence of melancholia versus non-melancholia and first depression versus recurrent depression are needed to explore the predictive value of these variables in primary care.
Patients treated with sertraline continued to improve over the 24 week treatment period. In contrast, patients treated with mianserin showed a faster remission rate in the first 6 weeks, and in patients taking placebo a plateau of the recovery rate was seen after 12 weeks.Most guidelines are based on results from short term (for example, 6 weeks) trials despite the fact that studies on the natural course of depressive episodes suggest a duration of 12-20 weeks.14Our findings support the recommendation of a 6 month treatment period for evaluation of treatment efficacy.
Do all drugs have equal efficacy?
The lack of superiority of mianserin over placebo in this study is of note and not easily explained. The frequency of side effects did not suggest underdosage of mianserin compared with sertraline; moreover, serum concentrations of mianserin were in the treatment range (mean 156-160 ng/ml after 8 and 24 weeks respectively). The initial difference between mianserin and sertraline was mainly explained by better initial improvement of sleep and appetite in the patients treated with mianserin. Sertraline had better effects on pessimistic thoughts and worrying, which appeared later in the course of treatment. This raises the issue of different response to treatment depending on the nature of depression. More studies are needed in primary care to clarify possible differences in response over time between drugs with different mechanisms of action and their overall significance for patients.
Sex and response to treatment
Women responded better to treatment than men, suggesting sex differences in response to treatment. More women than men seem to suffer from depression and seek treatment,15 and there are important sex differences in the phenomenology and neurobiology of psychiatric disorders and stress.13 Sex differences in the response to treatment have, however, largely been neglected in clinical trials. It may be that men seeking treatment for depression differ neurobiologically from women with depression, although this has not been assessed. The number of men in our study, however, was too small to answer this conclusively.
Methodological issues and limitations of the study
Our study was designed to be as close to real practice as possible. Accordingly, several cases of depression may have gone undetected. In retrospect, we have roughly estimated that about 0.5% of the patients seen by the participating general practitioners were enrolled. Studies based on selected general practices suggest that 4.8%-8.6% of patients seen by general practitioners have depression,3 with a detection rate about one third to one half.16 How many of those would have fitted into our inclusion and exclusion criteria is unknown. However, the mean age of our patients and the ratio of women to men and first depressive episode to recurrent depressive episode correspond to other studies of depression in primary care, suggesting that our sample is representative of the type of patients actually treated by highly qualified general practitioners.15,16
Eleven local psychiatrists were consulted by the general practitioners. We do not think this has influenced the results. Consultations occurred infrequently and were close to what is regular practice in Scandinavia (for example, uncertainty about likelihood of committing suicide, uncertainty about side effects). It is likely, however, that the possibility to consult when in doubt had increased the compliance rate of both patients and general practitioners.
We excluded patients who had been non-responsive to the study drugs in the past. From a clinical point of view this makes sense as general practitioners do not treat patients with drugs previously known not to benefit the patients. By doing so, however, we may to some degree enhance drug effects. This should, however, affect the two active drug arms equally.
None of the general practitioners was able to identify the patient-drug combinations correctly, probably owing to the few and rather insignificant side effects in this sample of patients. We therefore do not think that our findings can be explained by expectations of general practitioners of differences in effect.
Our forced titration regimen, rigorous response criteria, and the good tolerability of sertraline may have favoured the higher sertraline doses.17 The rapid upward titration is not in line with the current recommendations for sertraline, however, which claim that patients should remain on the starting dose of 50 mg for 2-4 weeks before escalation of dose is considered.17
Clinical implications and conclusions
Our results suggest that the criticism towards doubtful quality of treatment of depression in general practice is not justified, at least for trained and motivated general practitioners. The effectiveness of simple psychological treatment and active drug over 24 weeks was comparable with treatment results reported by psychiatrists and clinical psychologists, and thus offers a good alternative to the more complicated cognitive behavioural therapies in the treatment of mild to moderate depression in primary care.2
With regard to the drug of choice for general practitioners in the treatment of depression, current guidelines suggest that all drugs have equal efficacy. Our results raise several important questions for future research. Though a sedative drug with a dual mechanism of action, such as mianserin, may facilitate faster remission in some patients,18 this advantage may be transient and not sustainable over a 6 month period. This surprising finding, and the observation of sex differences in remission rates, opposite to those reported in studies conducted in specialised psychiatric settings,13 emphasise the need for more studies in general practice not only comparing simple psychological treatments with drug treatments but also drugs with different mechanism of action over an appropriate period of time.
Figure.
Clinical remission of patients with depression treated with good clinical management and sertraline, mianserin, or placebo (intention to treat; n=372)
Acknowledgments
We thank all participating physicians and their patients, Pfizer Norway for sponsoring the study, and Trond Smedsrud for his continuous efforts to secure the safe progress and quality of the study.
Footnotes
Competing interests: UFM, OHR, HPM, and OB have been reimbursed by Organon (manufacturer of mianserin) and Pfizer (manufacturer of sertraline) for attending several conferences. UFM has been paid by Organon and Pfizer for running educational programmes and has received fees from both companies for consulting. Members of UFM’s staff have received research funds from both Organon and Pfizer.
References
- 1.Paykel ES, Priest RG. Recognition and management of depression in general practice: consensus statement. BMJ. 1992;305:1198–1202. doi: 10.1136/bmj.305.6863.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Depression guideline panel. Depression in primary care. Vol 2. Treatment of major depression. Rockville: US Department of Health and Human Services; 1993. [Google Scholar]
- 3.Salokangas RKR, Poutanen O, Stengård E, Jähi R, Palo-oja T. Prevalence of depression among patients seen in community health centres and community mental health centres. Acta Psychiatr Scand. 1996;93:427–433. doi: 10.1111/j.1600-0447.1996.tb10673.x. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery SA, Åsberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:322–329. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 5.Bech P, Malt UF, Dencker SJ, Ahlfors UG, Elgen K, Lewander T, et al. Scales for the assessment of diagnosis and severity of mental disorders. Acta Psychiatr Scand 1993;87(suppl 372).
- 6.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 3rd revision. Washington, DC: American Psychiatric Press; 1987. [Google Scholar]
- 7.World Health Organisation. The ICD-10 classification of mental and behavioural disorders. Clinical descriptions and diagnostic guidelines. Geneva: WHO; 1992. [Google Scholar]
- 8.Alltman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [Google Scholar]
- 9.Lingjærde O, Ahlfors UG, Bech P, Dencker SJ, Elgen K. The UKU side effect rating scale. A new comprehensive rating scale for psychotropic drugs and a cross-sectional study of side effects in neuroleptic-treated patients. Acta Psychiatr Scand 1987;76(suppl 334). [DOI] [PubMed]
- 10.Fawcett J, Epstein P, Fiester SJ, Elkin I, Autry JH. Clinical management imipramine/placebo administration manual. Psychopharmacol Bull. 1987;23:309–321. [PubMed] [Google Scholar]
- 11.Nordic Council on Medicines. Good clinical trial practice. NLN Publication No 28, Uppsala: Nordiska Läkemedelsnämden, 1989.
- 12.Mynors-Wallis LM, Gath DH, Lloyd-Thomas AR, Tomlison D. Randomised controlled trial comparing problem solving treatment with amitriptyline and placebo for major depression in primary care. BMJ. 1995;310:441–445. doi: 10.1136/bmj.310.6977.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thase ME, Greenhouse JB, Frank E, Reynolds CF, III, Pilkonis P, Hurley K, et al. Treatment of major depression with psychotherapy or psychotherapy-pharmacotherapy combinations. Arch Gen Psychiatry. 1997;54:1009–1015. doi: 10.1001/archpsyc.1997.01830230043006. [DOI] [PubMed] [Google Scholar]
- 14.Soloman DA, Keller MB, Leon AC, Mueller TI, Shea MT, Warshsaw M, et al. Recovery from major depression: a 10-year prospective follow-up across multiple episodes. Arch Gen Psychiatry. 1997;54:10001–10006. doi: 10.1001/archpsyc.1997.01830230033005. [DOI] [PubMed] [Google Scholar]
- 15.Ekselius L, von Knorring L, Eberhard G. A double-blind multicenter trial comparing sertraline and citalopram in patients with major depression treated in general practice. Int Clin Psychopharm. 1997;12:323–331. doi: 10.1097/00004850-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Coyne JC, Klinkman MS, Gallo SM, Schwenk T. Short-term outcomes of detected and undetected depressed primary care patients and depressed psychiatric patients. Gen Hosp Psychiatry. 1997;19:333–343. doi: 10.1016/s0163-8343(97)00055-8. [DOI] [PubMed] [Google Scholar]
- 17.Lane R, Baldwin D, Preskorn SH. The SSRIs: advantages, disadvantages and differences. J Psychopharmacol. 1995;9(suppl 2):163–178. doi: 10.1177/0269881195009002011. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery SA. Fast-onset antidepressants. Intern Clin Psychopharm. 1997;12(suppl 3):1–5S. doi: 10.1097/00004850-199707003-00001. [DOI] [PubMed] [Google Scholar]